Abstract
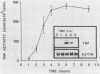
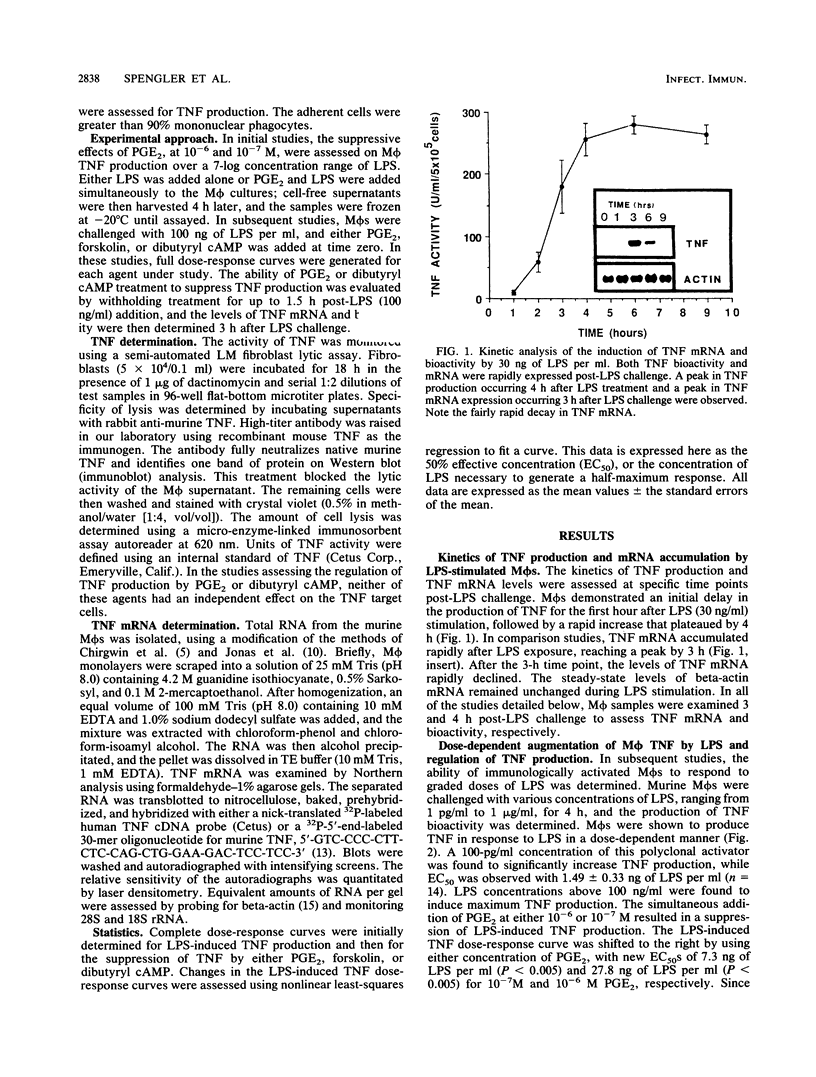
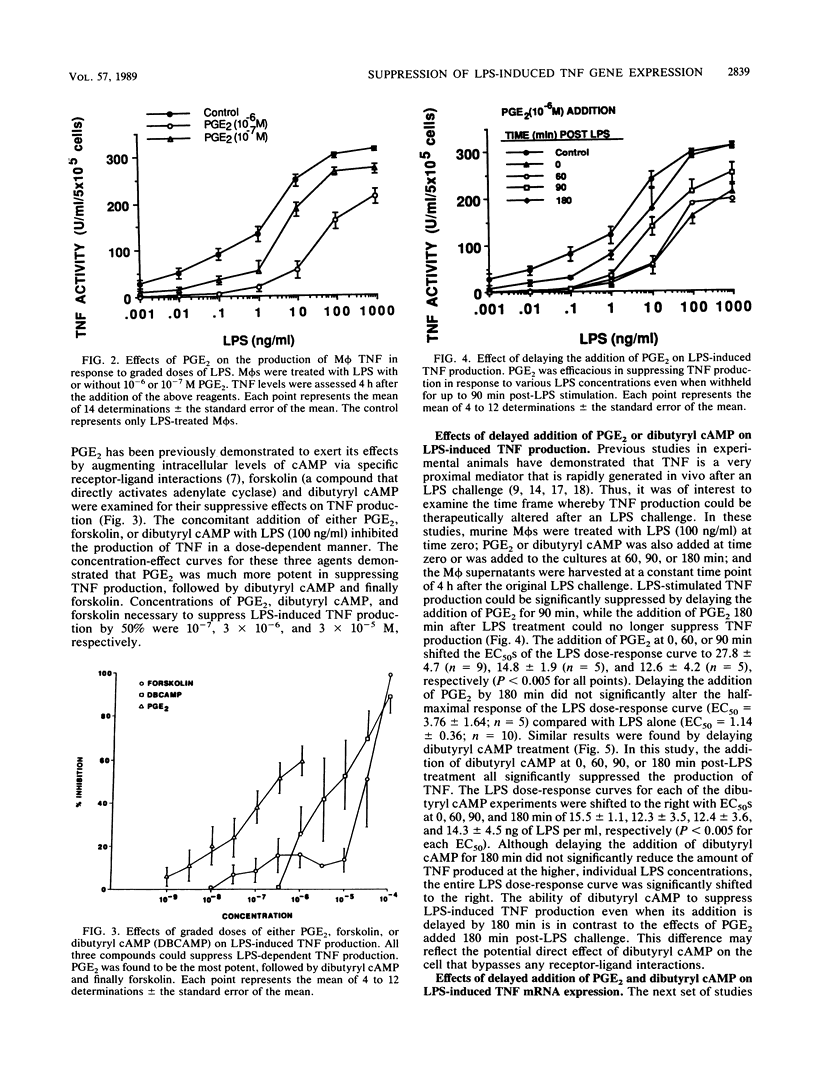
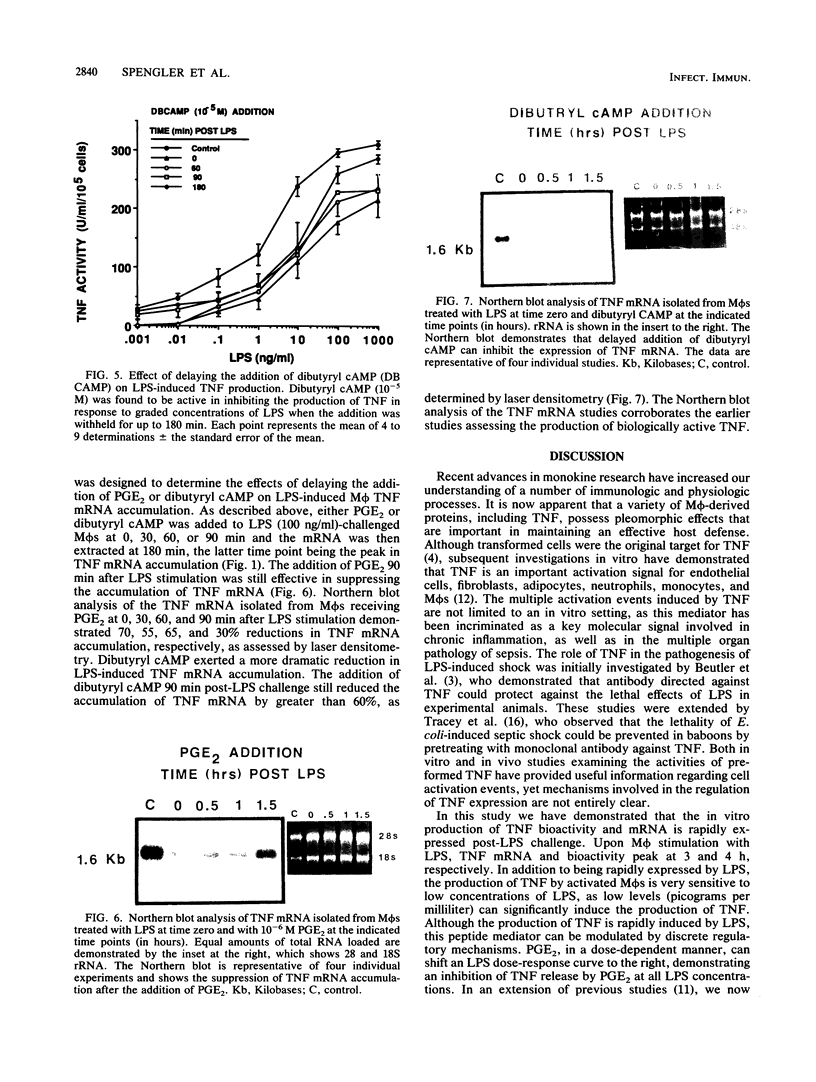
The regulation of lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF) production by prostaglandin E2 (PGE2), forskolin, and dibutyryl cyclic AMP (cAMP) was examined at the cellular and molecular levels. The above three agents could suppress LPS (100 ng/ml)-stimulated TNF production by immunologically activated murine macrophages (M phi s) in a dose-dependent manner. The concomitant addition of PGE2, dibutyryl cAMP, or forskolin to LPS-challenged M phi s resulted in 50% inhibition of TNF production at 10(-7), 3 X 10(-6), and 3 X 10(-5) M, respectively. Interestingly, delaying the addition of PGE2 or dibutyryl cAMP by 1.5 h post-LPS stimulation was also effective in suppressing the production of TNF bioactivity, but only dibutyryl cAMP was effective when its addition was delayed by 3 h. Northern (RNA) blot analysis of mRNA isolated from LPS-challenged M phi s treated with PGE2 or dibutyryl cAMP corroborated the bioactivity data. The delayed addition of PGE2 or dibutyryl cAMP by 1.5 h post-LPS stimulation resulted in a suppression of TNF mRNA accumulation by 50 to 70%. These data support the concept that LPS is a potent stimulus for M phi-derived TNF production and that this mediator is a very proximal signal in LPS-mediated disease states. Thus, therapeutic approaches that target the suppression of TNF in LPS-dependent disease states may be limited by the rapid expression of this mediator.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A., Hunt N. H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987 Oct;129(1):192–199. [PMC free article] [PubMed] [Google Scholar]
- Garrity M. J., Andreasen T. J., Storm D. R., Robertson R. P. Prostaglandin E-induced heterologous desensitization of hepatic adenylate cyclase. Consequences on the guanyl nucleotide regulatory complex. J Biol Chem. 1983 Jul 25;258(14):8692–8697. [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Ha D. K., Leung S. W., Fung K. P., Choy Y. M., Lee C. Y. Production of tumour necrosis factor in Listeria monocytogenes-infected animals. Int J Immunopharmacol. 1985;7(1):1–6. doi: 10.1016/0192-0561(85)90002-5. [DOI] [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Waage A. Production and clearance of tumor necrosis factor in rats exposed to endotoxin and dexamethasone. Clin Immunol Immunopathol. 1987 Dec;45(3):348–355. doi: 10.1016/0090-1229(87)90087-0. [DOI] [PubMed] [Google Scholar]