In caspase 8-deficient mouse T cells, necroptosis occurs via a Ripk3- and Ripk1-dependent pathway independent of autophagy and programmed necrosis.
Abstract
Cell populations are regulated in size by at least two forms of apoptosis. More recently, necroptosis, a parallel, nonapoptotic pathway of cell death, has been described, and this pathway is invoked in the absence of caspase 8. In caspase 8–deficient T cells, necroptosis occurs as the result of antigen receptor–mediated activation. Here, through a genetic analysis, we show that necroptosis in caspase 8–deficient T cells is related neither to the programmed necrosis as defined by the requirement for mitochondrial cyclophilin D nor to autophagy as defined by the requirement for autophagy-related protein 7. Rather, survival of caspase 8–defective T cells can be completely rescued by loss of receptor-interacting serine-threonine kinase (Ripk) 3. Additionally, complementation of a T cell–specific caspase 8 deficiency with a loss of Ripk3 gives rise to lymphoproliferative disease reminiscent of lpr or gld mice. In conjunction with previous work, we conclude that necroptosis in antigen-stimulated caspase 8–deficient T cells is the result of a novel Ripk1- and Ripk3-mediated pathway of cell death.
The maintenance of T cell population size is controlled by two forms of apoptosis, one that is initiated by permeabilization of the mitochondrial outer membrane and propagated by the release of cytochrome c and another that is initiated by death receptor ligation (Green, 2005). Engaged death receptors in turn bind Fas-associated protein with death domain (Fadd) and activate the initiator cysteine protease caspase 8. These interactions unleash the cascade of proteolytic events performed by executioner caspases. The manner in which these two forms of apoptosis regulate various aspects of T cell development and homeostasis is still being studied.
In the course of exploring a role for death receptor–mediated apoptosis in T cell population dynamics, another form of cell death emerged. T cells deficient for Fadd or caspase 8 might have been expected to expand to abnormally high levels in response to T cell antigen receptor (TCR)-mediated stimulation, and yet, such T cells proliferate poorly in culture and exhibit little expansion in vivo in response to viral infection (Hedrick et al., 2010). The cause of this defect has been controversial. One study characterized human and mouse T cells deficient for caspase 8 and concluded that they do not activate the prosurvival NF-κB pathway (Su et al., 2005), although this has been contested for mouse T cells and B cells deficient in either Fadd or caspase 8 (Salmena et al., 2003; Arechiga et al., 2005; Beisner et al., 2005; Imtiyaz et al., 2006; Ch’en et al., 2008). For example, TCR-stimulated mouse T cells with an inactivated Casp8 gene exhibit normal degradation of IκB, nuclear localization of RelA, normal induction of active NF-κB dimers as measured by electrophoretic mobility shift assay, and no differences in the induction of NF-κB target genes. Other studies have suggested that there is a cell cycle progression defect in Fadd- or caspase 8–deficient T cells (Zhang et al., 2001; Arechiga et al., 2007), and yet, by several criteria, caspase 8-deficient and wild-type T cells divide at the same rate, both in culture and in vivo (Salmena et al., 2003; Ch’en et al., 2008).
Experiments measuring the viability of stimulated T cells showed that the deficit in T cell expansion caused by a loss of caspase 8 was clearly explained by a continuous loss in cell viability; however, the death was not apoptotic. No DNA fragmentation was evident, as measured by DNA laddering or TdT-mediated dUTP-biotin nick end labeling (TUNEL; Ch’en et al., 2008). Other studies have suggested that this death occurred as a result of overexuberant autophagy (Yu et al., 2004; Bell et al., 2008), although an RNA interference screen for suppression of nonapoptotic death did not uncover autophagy genes (Hitomi et al., 2008). Instead of acting to preserve cell viability under conditions of starvation, this form of autophagy was proposed to give rise to the accumulation of reactive oxygen species (Yu et al., 2006).
Other investigations suggested that this death was related to that of cells signaled to die through TNFRI, but defective for either Fadd or caspase 8 (Schulze-Osthoff et al., 1994). This death has been termed necroptosis, and it can be blocked by the receptor-interacting serine/threonine-protein kinase (Ripk) 1 kinase inhibitor necrostatin-1 (Degterev et al., 2005, 2008). Consistent with these results, the expansion defect in caspase 8–deficient T cells was rescued by necrostatin-1 or a knockdown of Ripk1 (Ch’en et al., 2008). As such, it would appear that caspase 8 can function as both an initiator of apoptosis and an inhibitor of necroptosis; in its absence, perhaps a consequence of viral infection, T cells die via necroptosis.
Recent work has suggested that Ripk1 and Ripk3 function as a complex to induce programmed necrotic cell death through the synthesis of reactive oxygen species (Cho et al., 2009; He et al., 2009; Zhang et al., 2009). This suggests that in TCR-stimulated caspase 8–deficient T cells, necroptotic death is similarly mediated, although a recent work could find no evidence for the participation of Ripk3 in the death associated with the loss of Fadd in T cells (Osborn et al., 2010).
In this report, we have investigated T cell death associated with a loss of caspase 8 with respect to the role of programmed necrosis as defined by the requirement for cyclophilin D, the role of autophagy as defined by the requirement for autophagy-related protein 7 (Atg7), and Ripk3-dependent necroptosis. Genetic complementation experiments show that only a loss of Ripk3 is able to rescue the expansion of caspase 8–deficient T cells and reveal an abnormal CD3+CD4–CD8–B220+ population of T cells characteristic of human beings and mice with mutations in TNFRSF6 (Fas).
RESULTS AND DISCUSSION
Caspase 8–deficient T cells do not die by classical necrosis
The process of necroptosis, mediated by Ripk1, is thought to function through programmed necrosis involving the formation of a mitochondrial permeability transition pore (mPTP; Vandenabeele et al., 2010). For example, both necroptosis and ischemia reperfusion death can be blocked by necrostatin-1 (Degterev et al., 2005, 2008; Ch’en et al., 2008). Separate studies showed that mice lacking the Ppif gene encoding cyclophilin D, an essential component of the mPTP, were also protected from ischemia reperfusion (Baines et al., 2005; Nakagawa et al., 2005). We thus reasoned that Ripk1 and cyclophilin D might be important for necroptosis associated with the loss of caspase 8 (Vandenabeele et al., 2010).
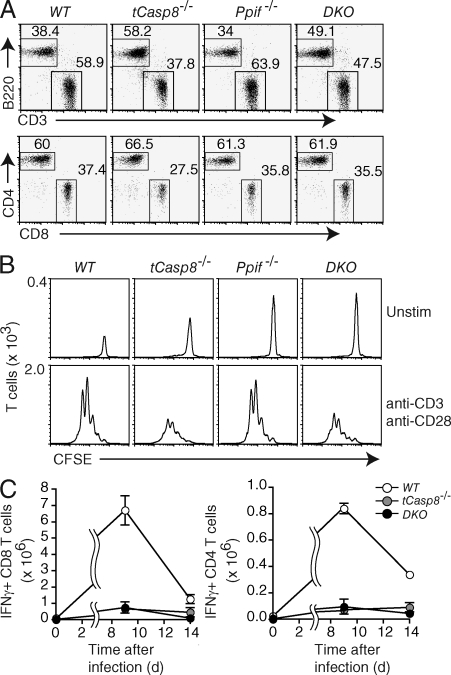
To test this possibility, we crossed Casp8f/f Cd4Cre (tCasp8−/−) mice with mice harboring a Ppif-null allele and generated four genotypes for analysis: Casp8f/f (WT), tCasp8−/−, Ppif−/−, and tCasp8−/− Ppif−/− (double KO [DKO]) mice. To confirm the deletion appropriate to each genotype, purified T cells were immunoblotted for caspase 8 and cyclophilin D (Fig. S1). As previously shown, tCasp8−/− mice have a reduced percentage of LN T cells (Salmena et al., 2003), whereas Ppif−/− mice showed no apparent changes in the proportion or number of T and B cells (Fig. 1 A and not depicted). The four genotypes showed no differences in the proportion of CD44+ memory-effector cells (unpublished data).
Figure 1.
Caspase 8–deficient T cells do not die by classical necrosis. (A) The percentages of live-gated T and B cells from the lymph nodes of WT (Casp8f/f), tCasp8−/− (Casp8f/f Cd4Cre), Ppif−/− (Casp8f/f Ppif−/−), and DKO (Casp8f/f Ppif−/− Cd4Cre) mice were determined by flow cytometry. Data are representative of seven independent experiments. (B) Purified T cells were labeled with CFSE, and then cultured in media alone or stimulated with anti-CD3 and anti-CD28 for 72 h. All cells were resuspended in an equal volume and collected for the same amount of time on the flow cytometer. The numbers on the ordinate indicate the number of T cells per interval of intensity, where the area under the curve equals the total number of T cells collected. Data are representative of seven independent experiments. (C) Cohorts of mice were infected with LCMV Armstrong. On days 9 and 14 after infection, mice were sacrificed, and splenocytes were stimulated with LCMV peptides for 5 h in vitro. Intracellular IFN-γ in gated CD4+ and CD8+ T cells was measured by flow cytometry. Error bars represent the SEM. Data are representative of two independent experiments.
To analyze the dynamics of T cell expansion, CFSE-labeled cells were cultured for 72 h, and collected such that the area under each curve is representative of the total accumulation of cells (Fig. 1 B). In accord with previous results (Ch’en et al., 2008), there was a diminished accumulation of tCasp8−/− CD4+ T cells, although the number of cell divisions was unchanged from wild-type. Ppif−/− T cells showed no difference from wild-type, but contrary to the prediction described above, the loss of Ppif did not rescue the decreased viability found in tCasp8−/− T cells. Similar results were found for CD8+ T cells (unpublished data).
To test this further, mice were infected with lymphocytic choriomeningitis virus (LCMV) Armstrong, and the number of LCMV-specific T cells measured at the peak of the response, day 9, and at day 14 after a marked contraction of the population. As depicted in Fig. 1 C and previously reported, there was little expansion in tCasp8−/− mice when compared with wild-type mice (Salmena et al., 2003; Ch’en et al., 2008). Again, the loss of Ppif did not rescue this diminished proliferation (Fig. 1 C). We have also found no defects in LCMV-mediated expansion in Ppif−/− mice (unpublished data).
Although the mPTP complex has been characterized as consisting of the adenosine nucleotide translocator, the voltage-dependent anion channel, and cyclophilin D among other molecules, only a mutation in Ppif is sufficient to protect against ischemia reperfusion injury in vivo (Kokoszka et al., 2004; Baines et al., 2007). Such programmed necrosis was thought to be synonymous with necroptosis, especially given the finding that TNF-induced adenosine nucleotide translocator inhibition leading to cell death was shown to be Ripk1 dependent (Temkin et al., 2006). Notwithstanding these strong connections, we conclude that Ripk1-dependent necroptosis induced in activated T cells does not rely on the activity of cyclophilin D. Either this is a form of necroptosis that is distinct from that initiated by TNF, or in general, necroptosis does not use the pathway involving mPTP formation.
Autophagy and cell death in T cells
Experiments have shown that monocytic cell lines or primary macrophages treated with irreversible caspase inhibitors or with diminished caspase 8 expression spontaneously died. An analysis indicated that there was a corresponding increase in autophagic vacuoles and that death could be inhibited by RNA interference–mediated diminution of the autophagy pathway components Atg7 and Beclin-1 (Yu et al., 2004). Similar results were found for Fadd- or caspase 8–deficient T cells using pharmacologic inhibition of phospoinositide 3 kinase or an short hairpin RNA knockdown of Atg7 (Bell et al., 2008). As such, we sought to determine whether a targeted deletion of Atg7 could likewise rescue the death associated with the loss of caspase 8 in T cell activation.
Mice with a conditional Atg7 deletion (Komatsu et al., 2005) were crossed with mice bearing Casp8f/f. We generated the following four genotypes for analysis: Casp8f/f (WT), Casp8f/f Cd4Cre (tCasp8−/−), Atg7f/f Cd4Cre (tAtg7−/−), and Casp8f/f Atg7f/f Cd4Cre (DKO). To confirm the deletion appropriate to each genotype, purified T cells were immunoblotted for caspase 8 and Atg7 (Fig. S2 A). Analysis of the total T cells and B cells (Fig. S2 B), along with the number and proportion of memory–effector T cells (Fig. S2 C) verified previously published data showing a loss in T cell viability in tAtg7−/− mice because of abnormally high numbers of mitochondria. We found that the addition of a Casp8-null allele accentuated the loss of T cells (Fig. S2 B); at present we do not have an explanation for this genetic interaction.
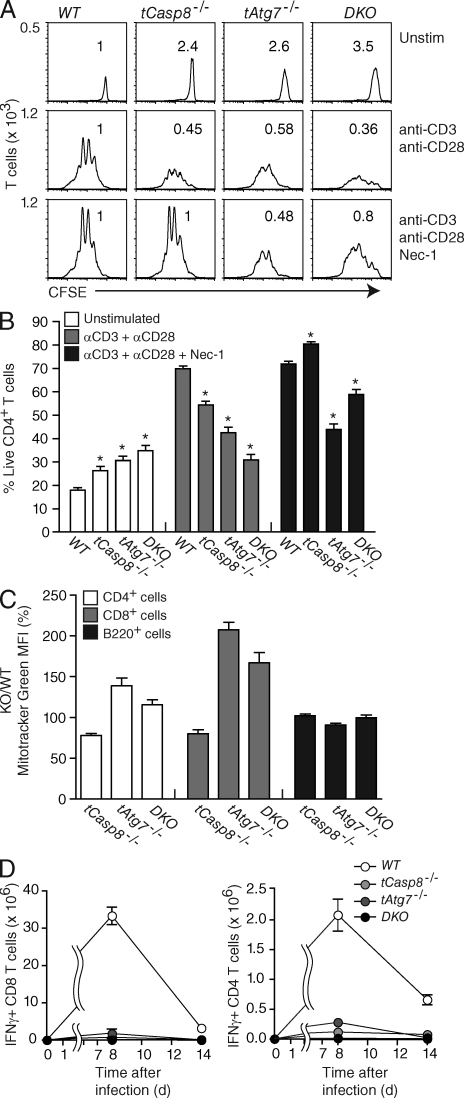
To determine if the proliferation defect in the absence of Casp8 is caused by an increase in autophagic death, we characterized proliferation and accumulation of T cells with or without the addition of necrostatin-1. Upon stimulation, a T cell–specific deficiency in either Casp8 or Atg7 caused reduced recovery (Fig. 2 A). The double-mutant T cells exhibited an additional decrease in accumulation, whereas there was no defect in the number of cell divisions. With the addition of necrostatin-1, the accumulation of tCasp8−/− T cells was rescued as expected, whereas the addition of necrostatin-1 to tAtg7−/− T cells had no effect. The addition of necrostatin-1 to double-mutant T cells partially restored accumulation, presumably overcoming the loss of Casp8 but not Atg7 (Fig. 2, A and B). These results show that the decrease in T cell accumulation caused by an Atg7 loss of function is not dependent on the kinase activity of Ripk1. More importantly, the presence of an Atg7 mutant allele did not rescue the necrostatin-sensitive cell death associated with a loss of caspase 8.
Figure 2.
Loss of Atg7 does not rescue caspase 8–deficient T cell proliferation. (A) Purified WT (Casp8f/f), tCasp8−/− (Casp8f/f Cd4Cre), tAtg7−/− (Atg7f/f Cd4Cre), and DKO (Casp8f/f Atg7f/f Cd4Cre) T cells were labeled with CFSE, and then cultured in media alone or stimulated with anti-CD3 and anti-CD28 in the absence or presence of necrostatin-1 (Nec-1) for 72 h. Flow cytometry analysis was performed as described in the legend to Fig. 1 B. Numbers above each curve represent the proportion of recovered cells relative to WT. Data are representative of eight independent experiments. (B) Percentage of live T cells was determined from proliferation described in A. Data are cumulative from eight independent experiments, and error bars indicate the SEM. Asterisks indicate a significant difference from the WT control; P < 0.01. (C) Mitochondrial mass was measured with MitoTracker Green and percent positive in tCasp8−/−, tAtg7−/−, and DKO T cells versus WT T cells was calculated. Data are cumulative from three independent experiments, and the bars indicate SEM. (D) Cohorts of mice were infected with LCMV Armstrong. On days 8 and 14 after infection, mice were sacrificed and splenocytes were stimulated with LCMV peptides for 5 h in vitro. Intracellular IFN-γ in gated CD4+ and CD8+ T cells was measured by flow cytometry. Data are representative of three independent experiments.
The loss of viability in T cells deficient for Atg7 was shown to originate from a build-up of mitochondria and overproduction of reactive oxygen species (Pua et al., 2009). Compared with tAtg7−/− T cells, DKO T cells were rescued at a substantially greater level with the addition of necrostatin-1, and one possible implication of this result is that a caspase 8 deficiency partially attenuates the loss of Atg7 and the build-up of mitochondria. To test this, we measured mitochondrial mass in T cells using MitoTracker (Fig. 2 C). Indeed, there was a decrease in the fluorescence corresponding to the mitochondrial mass in T cells with mutations in both Casp8 and Atg7 when compared with Atg7-defective T cells.
We next determined if the loss of Atg7 could complement the loss of Casp8 in the proliferation associated with LCMV infection. Mice were infected with LCMV, and spleens were collected and analyzed 8 and 14 d after infection. As shown in Fig. 2 D, there was little accumulation of LCMV-specific CD8+ or CD4+ T cells from either tCasp8−/− or tAtg7−/− mice. In addition, T cells from DKO mice also did not expand. We note that a hemizygous loss of Atg7 also did not complement the Casp8 defect (unpublished data).
Increased apoptotic death in the absence of Atg7
The analysis of the Atg7 mutation as a means of complementing a loss of caspase 8 is complicated by the diminished viability of T cells associated with a defect in autophagy. We therefore sought to characterize the form of death occurring as a consequence of a loss of caspase 8, Atg7, or both. As a first analysis, we measured the proportion of viable versus dead or dying T cells by double staining for Annexin V and 7-aminoactinomycin D (7AAD). Annexin V detects a loss of membrane asymmetry associated with apoptosis, and 7AAD fluoresces after DNA intercalation in cells with a compromised plasma membrane characteristic of the early phases of necrosis. Both of these measurements are time-dependent, and all dying cells eventually become positive for both Annexin V and 7AAD. Cells that do not stain with either dye are viable.
T cells cultured in the absence of stimulation undergo Bim-dependent apoptosis at a high rate (Marrack and Kappler, 2004). This apoptosis is greatly diminished in cultures optimally stimulated with agonistic antibodies specific for CD3 and CD28, and this is illustrated by the analysis of wild-type T cells from Casp8f/f mice (Fig. 3 A and Fig. S3 A, WT). As previously shown, similarly stimulated caspase 8–deficient T cells undergo cell death at a higher rate (Fig. 3 A and Fig. S3 A, tCasp8−/− vs. WT). A previous study showed that T cells directly explanted from Atg7f/f LckCre mice showed diminished viability (Pua et al., 2009); however, in this study we found that after 72 h of culture, tAtg7−/− T cells displayed a similar or increased viability compared with wild-type T cells. With stimulation through CD3 and CD28, the viability was substantially reduced compared with WT T cells (Fig. 2 A and Fig. 3 A, tAtg7−/−), and interestingly, the viability of stimulated DKO T cells was further reduced such that they did not display increased viability over the unstimulated controls (Fig. 3 A).
Figure 3.
Increased apoptotic death in the absence of Atg7. Purified T cells were cultured in media alone (Unstim) or stimulated with anti-CD3 and anti-CD28 in the absence or presence of necrostatin-1 (Nec-1), qVD, or both for 72 h. Dead cells were detected by staining for Annexin V and 7AAD (A) or TUNEL and active caspase 3 (B). Genotypes are abbreviated as listed in legend to Figure 2. Data are cumulative from eight (A) or five (B) independent experiments, bars indicate the SEM, and asterisks indicate a significant difference from the WT control; P < 0.01.
To directly compare the form of death for T cells from each genotype, a similar analysis was performed with the addition of necrostatin-1, or qVD, which is a pan-caspase inhibitor highly effective in blocking apoptosis. The addition of necrostatin-1 diminished death in tCasp8−/− T cells, as previously described, but had no effect on tAtg7−/− T cells (Fig. 3 A). In contrast, the addition of qVD rescued tAtg7−/− T cells, but had no effect on tCasp8−/− T cells. Double-mutant T cells exhibited the highest death of all, and this death was partially diminished with necrostatin-1, but not with qVD. These results indicate that the loss of the autophagy pathway did not affect the necroptosis associated with a loss of caspase 8, and the DKO mice maintained a component of death that was eliminated with the addition of necrostatin-1.
To further characterize the form of death displayed by Atg7 mutant T cells, we measured DNA fragmentation, a defining characteristic of apoptosis. The presence of DNA fragmentation, as measured by TUNEL, is accompanied by the activation of caspases, most notably, caspase 3. All of the death associated with a loss of Atg7 showed hallmarks of apoptosis—the cells were positive for TUNEL staining and active caspase 3 and the death was completely inhibited with the addition of qVD (Fig. 3 B and Fig. S3, A and B). These experiments reveal that in the absence of autophagy, stimulated T cells are highly sensitive to death by apoptosis, and we presume this sensitivity corresponds with earlier studies showing an abnormal increase in mitochondrial mass with corresponding increases in oxidative stress (Pua et al., 2009). Because we previously showed that tCasp8−/− T cells do not exhibit hallmarks of apoptosis, mutations in Atg7 or Casp8 are clearly affecting different pathways.
Previous studies on TNF signaling in the absence of Fadd or caspase 8 revealed the induction of autophagy, although inhibition of the autophagic signaling components did not prevent necroptotic cell death (Degterev et al., 2005). These results contrasted with other studies on caspase 8–deficient cells that demonstrated a form of cell death dependent on components of autophagy (Yu et al., 2004; Bell et al., 2008); however, the experiments presented here show that the loss of Atg7 does not diminish the necroptotic death associated with a loss of caspase 8 in T cells.
A recent work showed that there is an etoposide-generated autophagy that is Atg5 and Atg7 independent (Nishida et al., 2009). It does not include lipidation of LC3 (the mammalian Atg8 orthologue), but does require Beclin-1 and a Rab9-dependent fusion of the phagophore with vesicles derived from the trans-Golgi and late endosomes. Although it was shown to be operative in the clearance of mitochondria from developing erythrocytes, it may be rare under physiological conditions, and it is not redundant with the many examples of defective autophagy found in Atg7-null cells (Komatsu and Ichimura, 2010). Whether this alternative pathway is involved in cell death is presently unknown. We think it is unlikely that this process is involved in the cell death studied here, and conclude that caspase 8–deficient T cells die by a process that does not require autophagy.
Targeted deletion of Ripk3 rescues caspase 8–dependent necroptosis
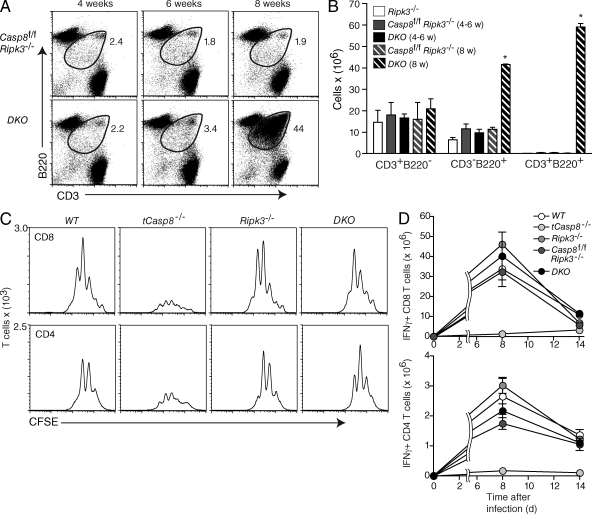
The mechanism by which Ripk1 mediates cell death is not understood. The process includes neither DNA fragmentation nor caspase activation, but as described in the introduction, in cells signaled through TNF, a programmed necrosis occurs that requires Ripk1 complexed with Ripk3. The proximal cause of death may involve the overproduction of reactive oxygen species (Cho et al., 2009; He et al., 2009; Zhang et al., 2009). To determine whether this applies to T cells stimulated to divide through the antigen receptor, we crossed a targeted mutation in the Ripk3 gene into the Casp8f/f Cd4Cre strain to generate Casp8f/f (WT), Casp8f/f Cd4Cre (tCasp8−/−), Ripk3−/−, and Casp8f/f Ripk3−/− Cd4Cre (DKO) mice (Newton et al., 2004). To confirm the deletion appropriate to each genotype, purified T cells were immunoblotted for caspase 8 and Ripk3 (Fig. S4). The first indication that the Ripk3 loss of function allele complemented tCasp8−/− was that DKO mice exhibited lymphadenopathy caused by the expansion of a population of abnormal CD3+CD4−CD8− T cells that also expressed B220—a cell type found in mice with a Fas or FasL deficiency (Cohen and Eisenberg, 1991). This population accrued in mice with a double mutation, but not in either single KO alone (Fig. 4, A and B), implicating that Ripk3-mediated cell death can replace Fas-mediated clearance of naturally occurring B220+ T cells (Mohamood et al., 2008). In addition, the presence of the CD3+CD4−CD8−B220+ T cell population was not observed until mice were 8 wk of age.
Figure 4.
Targeted deletion of Ripk3 rescues caspase 8–dependent necroptosis. (A) Live-gated Casp8f/f Ripk3−/− and DKO (Casp8f/f Ripk3−/− Cd4Cre) lymphocytes from mice of the indicated ages were stained with CD3 and B220. Data are representative of three independent experiments. (B) The number of live-gated CD3+B220−, CD3−B220+, and CD3+B220+ cells from the lymph nodes of Ripk3−/−, Casp8f/f Ripk3−/−, and DKO mice were determined by flow cytometry. Data are cumulative from three independent experiments. Bars indicate the SEM, and asterisks indicate a significant difference from the WT control; P < 0.01. (C) Purified T cells were labeled with CFSE and stimulated with anti-CD3 and anti-CD28 for 72 h. Flow cytometry analysis was performed as described in the legend to Fig. 1 B. Data are representative of three independent experiments. (D) Cohorts of mice were infected with LCMV Armstrong. On days 8 and 14 after infection, mice were sacrificed and splenocytes were stimulated with LCMV peptides for 5 h in vitro. Intracellular IFN-γ in gated CD4+ and CD8+ T cells was measured by flow cytometry. Data for days 8 and 14 after infection are representative of three and one independent experiments, respectively.
To determine directly whether the Ripk3 KO can complement a caspase 8 deficiency, T cells were stimulated through CD3 and CD28, and the division and accumulation in culture was recorded as described in Fig. 1. As shown, the paucity of T cell accumulation of T cells deficient in caspase 8 was rescued by the additional genetic deletion of Ripk3 (Fig. 4 C). No changes in T cell expansion were noted in T cells with a mutant version of Ripk3 alone. Finally, we wished to confirm the rescue by determining the expansion of T cells in response to LCMV infection. As shown, there was little T cell accumulation in the absence of caspase 8, but this phenotype was rescued at the peak of the immune response by the additional deletion of Ripk3. By 14 d after infection, the contraction of the T cell population with defects in either Rip3−/− or both tCasp8−/− and Rip3−/− was similar to WT.
The cellular pathways of necroptosis, autophagy, and apoptosis appear to be highly interconnected, and thus identifying a specific pathway can be complicated in different experimental models in which cell death occurs (Vandenabeele et al., 2010). We have shown that neither programmed necrosis, as defined by a requirement for cyclophilin D, nor autophagy, as defined by the requirement for Atg7, is operative in the necroptosis observed in caspase 8–deficient T cells. Rather, necroptosis is a unique death pathway mediated by Ripk1 and Ripk3 (Cho et al., 2009; He et al., 2009; Zhang et al., 2009), and here we show that this is the unique cell death pathway initiated by TCR-mediated T cell activation in the absence of caspase 8. We can deduce that caspase 8 prevents the activation of the Ripk1–Ripk3 axis, and in its absence, necroptosis results. As previously discussed, the physiological significance of such signaling circuitry may be that the absence of caspase 8 activity serves as a pathogen-associated molecular pattern indicating the presence of a virally expressed caspase inhibitor (Ch’en et al., 2008).
The lymphoproliferative disease found in mice or human beings with a germline Fas mutation, lpr disease, is characterized by an accumulation of CD3+CD4−CD8−B220+ cells in the peripheral lymphoid organs (Cohen and Eisenberg, 1991; Bidère et al., 2006). In a direct comparison with a germline deletion of Fas, this disease was not found in C57BL/6 mice with a T cell–specific deletion of Fas (Fasf/f Cd4Cre), at least up to 8 mo of age (Hao et al., 2004); rather, there was a gradual decline in T and B cell numbers in secondary lymphoid organs eventually resulting in complete lymphopenia. The genetic background can be determinative because (C57BL/6 x MRL)F1 hybrid mice with a T cell–specific deletion of Fas did manifest lpr-like lymphoproliferation. We have found that the tCasp8−/− C57BL/6 mice also do not manifest lpr disease, or any type of lymphoproliferation, up to at least 1 yr of age (unpublished data); however, Salmena and Hakem (2005) found an obvious, generalized lymphoproliferation in aged Casp8f/f Lck Cre mice from a mixed 129 and C57BL/6 genetic background. In contrast, tCasp8−/− Ripk3−/− C57BL/6 mice displayed a typical lpr-like lymphoproliferative disease, which was consistently manifested at 8 wk of age and included the expansion of CD3+CD4−CD8−B220+ cells. We interpret this to indicate that, at least in C57BL/6 mice, a loss of the extrinsic apoptosis death pathway confined to T cells, along with a loss of the necroptosis pathway, is sufficient to invoke lpr disease.
Experiments with MRL.Faslpr mice appear to reveal a requirement for dendritic cells in the expansion of T cells leading to lymphoproliferation (Teichmann et al., 2010), although there are substantial complexities to these studies (Platt and Randolph, 2010). Clearly, the role of other cell types and strain-specific background genes in lymphoproliferation require further investigation, including the role of necroptosis in lymphocyte homeostasis.
In mice, there is a single caspase that is activated via Fas, caspase 8, whereas in human beings there is a second downstream caspase, caspase 10. Interestingly, human beings deficient in caspase 10, but not caspase 8, exhibit lymphoproliferative disease (Bidère et al., 2006). This correlation implies that caspase 8 and caspase 10 are not entirely redundant, and specifically, caspase 8, but not caspase 10, exerts negative control on the necroptosis pathway.
MATERIALS AND METHODS
Mice.
Ppif−/− (encoding cyclophilin D), Casp8f/f, Atg7f/f, and Ripk3−/− mice were previously described (Newton et al., 2004; Baines et al., 2005; Beisner et al., 2005; Komatsu et al., 2005). Ripk3−/− mice were provided by V. Dixit (Genentech, Inc., South San Francisco, CA). Strains were crossed and backcrossed to obtain mice with single and double mutations with or without Cd4Cre (Taconic). Ppif−/−, Casp8f/f, and Atg7f/f strains were at least 5, 10, and 5 generations backcrossed to C57BL/6, respectively, whereas Ripk3−/− mice were generated on a C57BL/6 background. All mice were analyzed between 4 and 12 wk of age. Mice were bred and maintained in the animal care facilities at the University of California San Diego. Animal protocols were approved by the Institutional Animal Care and Use Committee.
Isolation of T cells.
Single-cell suspensions were prepared from lymph nodes and spleen. Resting T cells were purified by magnetic separation with an autoMACS (Miltenyi Biotec). Biotinylated antibodies specific for B220, MHCII, CD11b, and DX5 (eBioscience) were added to the cells, followed by the addition of streptavidin microbeads (Miltenyi Biotec). The purity of these negatively selected cells was determined to be >95% CD3+ as verified by flow cytometry.
In vitro proliferation.
Purified T cells were labeled with 0.5 µM CFSE (Invitrogen) for 10 min in PBS/0.1% BSA at 37°C and stimulated with 100 ng plate-bound anti-CD3 (145-2C11) and 1 µg/ml purified anti-CD28 (eBioscience). All cells were resuspended at 106 cells/ml in a 24-well plate. Where indicated, 30 µM 7-Cl-O-necrostatin (provided by A. Degterev, Tufts University, Medford, MA) or 20 µM Q-VD-OPH (SM Biochemicals LLC) was added to the culture.
Flow cytometry.
Cells were incubated with the appropriate antibodies for 15–20 min in FACS buffer (1x PBS, 1% FBS, 10 mM EDTA, and 0.1% Azide). FITC-, PE-, and allophycocyanin-conjugated antibodies specific for B220, CD3, CD4, CD8, CD44, CD62L, and IFN-γ were purchased from eBioscience. CD8 conjugated to PerCP was purchased from BioLegend. Cultured CFSE-labeled cells were resuspended in an equal volume of FACS buffer and collected for a constant length of time on the FACSCalibur (BD). Flow cytometric analysis was performed using FlowJo software (Tree Star). To stain for mitochondrial mass, lymphocytes were incubated for 30 min at 37°C with MitoTracker Green (Invitrogen) in RPMI 1640 complete media.
LCMV infection.
4–12-wk-old mice were infected by intraperitoneal injection with 2 × 105 plaque-forming units of LCMV Armstrong in 0.5 ml of PBS. At the indicated time points, spleens were harvested from infected and noninfected mice. Splenocytes were stimulated for 5 h in vitro with gp33 and NP396 peptides, MHC class I–restricted LCMV epitopes or gp61 and NP309 peptides, MHC class II–restricted LCMV epitopes, and Brefeldin A (GolgiStop; BD). Cells were labeled with CD4, CD8, and CD44 surface markers, fixed and permeabilized with the Cytofix/Cytoperm kit (BD), and then stained for intracellular IFN-γ. Standard error was calculated for each time point.
Cell death assays.
Cells were incubated with Annexin V (Invitrogen) in Annexin V–binding buffer (140 mM NaCl, 2.5 mM CaCl2, and 10 mM Hepes) for 15 min at room temperature. Staining with 7AAD was done on unpermeabilized cells in FACS buffer. TUNEL was performed using the In situ Cell Death Detection kit TMR red from Roche. To measure intracellular active caspase-3, cells were fixed, permeabilized, and stained according to manufacturer’s protocol and measured by flow cytometry (BD).
Western blotting.
Purified T cells were lysed on ice with Cell Lysis Buffer (Cell Signaling Technology) supplemented with protease and phosphatase inhibitors, and spun for 10 min at 4°C. Protein concentrations were determined using a Bio-Rad protein assay (Bio-Rad Laboratories). 10-15 µg of protein was separated on 4–12% or 12% Bis-tris NuPage Gels (Invitrogen), transferred to Immobilon-P PVDF membranes (Millipore), and blotted with antibodies specific for caspase 8, clone 1G12 (Enzo Life Sciences); cyclophilin D, clone E11AE12BD4 (MitoSciences Inc.); ATG7 (Cell Signaling Technology); Ripk3 (ProSci Inc.); and PLCγ (Santa Cruz Biotechnology). Membranes were incubated with a horseradish peroxidase–conjugated antibody to the appropriate species and visualized on Hyblot CL Autoradiography Film (Denville Scientific Inc.) using the ECL system.
Statistics.
Statistical analyses were performed using Prism 4 for Mac (GraphPad Software, Inc.). Error bars represent SEM, and p-values were calculated with a two-tailed Student’s t test.
Online supplemental material.
Fig. S1 shows the efficiency of deletion in WT, tCasp8−/−, Ppif−/−, and DKO (tCasp8−/− Ppif−/−) purified T cells. Fig. S2 shows the efficiency of deletion in WT, tCasp8−/−, tAtg7−/−, and DKO (tCasp8−/− tAtg7−/−) purified T cells, and the accumulated phenotypic data describing peripheral lymphocytes and splenocytes. Fig. S3 shows example profiles measuring the increased apoptotic death observed in tAtg7−/− and DKO (tCasp8−/− tAtg7−/−) stimulated cell cultures when compared with WT and tCasp8−/− (accumulated data shown in Fig. 3). Fig. S4 shows the efficiency of deletion in WT, tCasp8−/−, Ripk3−/−, and DKO (tCasp8−/− Ripk3−/−) purified T cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110251/DC1.
Acknowledgments
7-Cl-O-necrostatin-1 was generously provided by A. Degterev. Ripk3−/− mice were graciously provided by V. Dixit.
ILC was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease Training Grant T32DK007233. This work was supported by National Institutes of Health Grant AI037988 to SMH. SMH holds the Chancellor’s Endowed Chair in the Biological Sciences. The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- 7AAD
- aminoactinomycin D
- Atg7
- autophagy-related protein 7
- DKO
- double KO
- Fadd
- Fas-associated protein with death domain
- mPTP
- mitochondrial permeability transition pore
- Ripk
- receptor-interacting serine-threonine-protein kinase
- LCMV
- lymphocytic choriomeningitis virus
- TUNEL
- TdT-mediated dUTP-biotin nick end labeling
References
- Arechiga A.F., Bell B.D., Solomon J.C., Chu I.H., Dubois C.L., Hall B.E., George T.C., Coder D.M., Walsh C.M. 2005. Cutting edge: FADD is not required for antigen receptor-mediated NF-kappaB activation. J. Immunol. 175:7800–7804 [DOI] [PubMed] [Google Scholar]
- Arechiga A.F., Bell B.D., Leverrier S., Weist B.M., Porter M., Wu Z., Kanno Y., Ramos S.J., Ong S.T., Siegel R., Walsh C.M. 2007. A Fas-associated death domain protein/caspase-8-signaling axis promotes S-phase entry and maintains S6 kinase activity in T cells responding to IL-2. J. Immunol. 179:5291–5300 [DOI] [PubMed] [Google Scholar]
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., et al. 2005. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 434:658–662 10.1038/nature03434 [DOI] [PubMed] [Google Scholar]
- Baines C.P., Kaiser R.A., Sheiko T., Craigen W.J., Molkentin J.D. 2007. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 9:550–555 10.1038/ncb1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner D.R., Ch’en I.L., Kolla R.V., Hoffmann A., Hedrick S.M. 2005. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J. Immunol. 175:3469–3473 [DOI] [PubMed] [Google Scholar]
- Bell B.D., Leverrier S., Weist B.M., Newton R.H., Arechiga A.F., Luhrs K.A., Morrissette N.S., Walsh C.M. 2008. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc. Natl. Acad. Sci. USA. 105:16677–16682 10.1073/pnas.0808597105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidère N., Su H.C., Lenardo M.J. 2006. Genetic disorders of programmed cell death in the immune system. Annu. Rev. Immunol. 24:321–352 10.1146/annurev.immunol.24.021605.090513 [DOI] [PubMed] [Google Scholar]
- Ch’en I.L., Beisner D.R., Degterev A., Lynch C., Yuan J., Hoffmann A., Hedrick S.M. 2008. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc. Natl. Acad. Sci. USA. 105:17463–17468 10.1073/pnas.0808043105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.S., Challa S., Moquin D., Genga R., Ray T.D., Guildford M., Chan F.K. 2009. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 137:1112–1123 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P.L., Eisenberg R.A. 1991. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9:243–269 10.1146/annurev.iy.09.040191.001331 [DOI] [PubMed] [Google Scholar]
- Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. 2005. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1:112–119 10.1038/nchembio711 [DOI] [PubMed] [Google Scholar]
- Degterev A., Hitomi J., Germscheid M., Ch’en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G., et al. 2008. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 5:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R. 2005. Apoptotic pathways: ten minutes to dead. Cell. 121:671–674 10.1016/j.cell.2005.05.019 [DOI] [PubMed] [Google Scholar]
- Hao Z., Hampel B., Yagita H., Rajewsky K. 2004. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J. Exp. Med. 199:1355–1365 10.1084/jem.20032196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. 2009. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 137:1100–1111 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- Hedrick S.M., Ch’en I.L., Alves B.N. 2010. Intertwined pathways of programmed cell death in immunity. Immunol. Rev. 236:41–53 10.1111/j.1600-065X.2010.00918.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J., Christofferson D.E., Ng A., Yao J., Degterev A., Xavier R.J., Yuan J. 2008. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 135:1311–1323 10.1016/j.cell.2008.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiyaz H.Z., Rosenberg S., Zhang Y., Rahman Z.S., Hou Y.J., Manser T., Zhang J. 2006. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J. Immunol. 176:6852–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka J.E., Waymire K.G., Levy S.E., Sligh J.E., Cai J., Jones D.P., MacGregor G.R., Wallace D.C. 2004. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 427:461–465 10.1038/nature02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Ichimura Y. 2010. Selective autophagy regulates various cellular functions. Genes Cells. 15:923–933 10.1111/j.1365-2443.2010.01433.x [DOI] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., et al. 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169:425–434 10.1083/jcb.200412022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. 2004. Control of T cell viability. Annu. Rev. Immunol. 22:765–787 10.1146/annurev.immunol.22.012703.104554 [DOI] [PubMed] [Google Scholar]
- Mohamood A.S., Bargatze D., Xiao Z., Jie C., Yagita H., Ruben D., Watson J., Chakravarti S., Schneck J.P., Hamad A.R. 2008. Fas-mediated apoptosis regulates the composition of peripheral alphabeta T cell repertoire by constitutively purging out double negative T cells. PLoS ONE. 3:e3465 10.1371/journal.pone.0003465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T., Tsujimoto Y. 2005. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 434:652–658 10.1038/nature03317 [DOI] [PubMed] [Google Scholar]
- Newton K., Sun X., Dixit V.M. 2004. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24:1464–1469 10.1128/MCB.24.4.1464-1469.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Arakawa S., Fujitani K., Yamaguchi H., Mizuta T., Kanaseki T., Komatsu M., Otsu K., Tsujimoto Y., Shimizu S. 2009. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 461:654–658 10.1038/nature08455 [DOI] [PubMed] [Google Scholar]
- Osborn S.L., Diehl G., Han S.J., Xue L., Kurd N., Hsieh K., Cado D., Robey E.A., Winoto A. 2010. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc. Natl. Acad. Sci. USA. 107:13034–13039 10.1073/pnas.1005997107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt A.M., Randolph G.J. 2010. Does deleting dendritic cells delete autoimmunity? Immunity. 33:840–842 10.1016/j.immuni.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua H.H., Guo J., Komatsu M., He Y.W. 2009. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J. Immunol. 182:4046–4055 10.4049/jimmunol.0801143 [DOI] [PubMed] [Google Scholar]
- Salmena L., Hakem R. 2005. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J. Exp. Med. 202:727–732 10.1084/jem.20050683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L., Lemmers B., Hakem A., Matysiak-Zablocki E., Murakami K., Au P.Y., Berry D.M., Tamblyn L., Shehabeldin A., Migon E., et al. 2003. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17:883–895 10.1101/gad.1063703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Krammer P.H., Dröge W. 1994. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 13:4587–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Bidère N., Zheng L., Cubre A., Sakai K., Dale J., Salmena L., Hakem R., Straus S., Lenardo M. 2005. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 307:1465–1468 10.1126/science.1104765 [DOI] [PubMed] [Google Scholar]
- Teichmann L.L., Ols M.L., Kashgarian M., Reizis B., Kaplan D.H., Shlomchik M.J. 2010. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 33:967–978 10.1016/j.immuni.2010.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin V., Huang Q., Liu H., Osada H., Pope R.M. 2006. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol. Cell. Biol. 26:2215–2225 10.1128/MCB.26.6.2215-2225.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. 2010. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11:700–714 10.1038/nrm2970 [DOI] [PubMed] [Google Scholar]
- Yu L., Alva A., Su H., Dutt P., Freundt E., Welsh S., Baehrecke E.H., Lenardo M.J. 2004. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 304:1500–1502 10.1126/science.1096645 [DOI] [PubMed] [Google Scholar]
- Yu L., Wan F., Dutta S., Welsh S., Liu Z., Freundt E., Baehrecke E.H., Lenardo M. 2006. Autophagic programmed cell death by selective catalase degradation. Proc. Natl. Acad. Sci. USA. 103:4952–4957 10.1073/pnas.0511288103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kabra N.H., Cado D., Kang C., Winoto A. 2001. FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J. Biol. Chem. 276:29815–29818 10.1074/jbc.M103838200 [DOI] [PubMed] [Google Scholar]
- Zhang D.W., Shao J., Lin J., Zhang N., Lu B.J., Lin S.C., Dong M.Q., Han J. 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 325:332–336 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]