CIN85 transduces B cell receptor signals to IKK-β, and its expression in B cells is essential for T cell–independent type II antibody responses in mice.
Abstract
CIN85, an adaptor protein which binds the C-terminal domain of tyrosine phosphorylated Cbl and Cbl-b, has been thought to be involved in the internalization and subsequent degradation of receptors. However, its physiological function remains unclear. To determine its role in B cells, we used Mb1-cre to generate mice with a B cell–specific deletion of CIN85. These mice had impaired T cell–independent type II antibody responses in vivo and diminished IKK-β activation and cellular responses to B cell receptor (BCR) cross-linking in vitro. Introduction of a constitutively active IKK-β construct corrected the defective antibody responses as well as cellular responses in the mutant mice. Together, our results suggest that CIN85 links the BCR to IKK-β activation, thereby contributing to T cell–independent immune responses.
By recruiting signaling proteins into complexes, adaptor molecules create nodes of regulation and activity. CD2AP (alternatively named CMS), the founding member of the CD2AP/CIN85 family of adaptor proteins, was initially isolated in a yeast interaction screen as a binding partner of CD2 expressed on T cells (Dustin et al., 1998). Subsequently, its mammalian homologue CIN85 (Cbl interacting protein of 85 kD) was identified as a partner of the E3 ubiquitin ligase Cbl (Take et al., 2000). CIN85 contains three Src homology 3 (SH3) domains at the N terminus that recognize an atypical proline-arginine motif (PX(P/A)XXR), a central proline-rich region acting as an interaction module for other SH3 domain–containing proteins, and a coiled-coil domain in the C terminus (Dikic, 2002; Fig. 1 A). CIN85 is expressed in almost all the tissues, where at least 10 different isoforms are differentially expressed in each tissue (Gout et al., 2000; Take et al., 2000). For instance, extra long and long isoforms (CIN85-xl, and CIN85-l) are expressed abundantly in nerve systems, whereas in immune systems CIN85-l and CIN85-ΔA isoforms are expressed dominantly (Fig. 1 C; Shimokawa et al., 2010).
Figure 1.
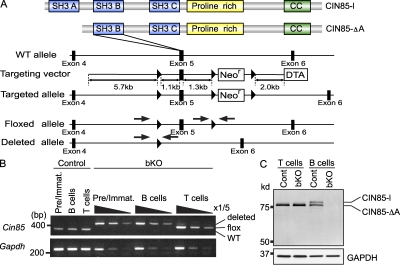
Generation of CIN85 bKO mice. (A) CIN85-l and CIN85-ΔA contain three or two SH3 domains, respectively. Both isoforms contain a proline-rich region and a coiled-coil (CC) domain. A schematic of CIN85 WT and floxed allele is shown. Exon 5 is flanked by two loxP sites. Arrows indicate primer position for PCR. (B) BM pre–B cells and immature B cells (Pre/Immat.) or spleen B cells or T cells from mb1-Cre; CIN85wt/Y (control) or mb1-Cre; CIN85fl/Y (CIN85 bKO) mice were sorted. CIN85 is located on the X chromosome and Y indicates Y chromosome. DNA was extracted from sorted cells and the deletion efficiency was assayed by PCR. The template DNA was serially diluted. (C) Inactivation of the CIN85 was confirmed by Western blotting in spleen B cells and T cells. Migration of molecular mass markers is shown on the left side. The membrane was stripped and reprobed with anti-GAPDH antibody. Representative data of three independent experiments are shown.
Based on coimmunoprecipitation experiments, colocalization studies, and in vitro protein–protein interaction assays using fibroblasts, it has been proposed that CIN85 primarily functions in endocytosis to down-regulate receptor tyrosine kinase activity (Dikic and Giordano, 2003). According to this model, CIN85 constitutively associates with endophilin and, on stimulation with growth factors such as epidermal growth factor, complexes with Cbl to mediate receptor down-regulation (Petrelli et al., 2002; Soubeyran et al., 2002). The same mechanism also appears to operate in immune cells. CIN85 overexpression in the RBL-2H3 rat mast cell line accelerated the redistribution of engaged FcεRI complexes, their sorting in early endosomes, and their delivery to a lysosomal compartment for degradation (Molfetta et al., 2005). Consequently, FcεRI-mediated degranulation was impaired. In addition to affecting endocytosis, overexpression of CIN85 in the RBL-2H3 was also reported to decrease the protein level of Syk, an effect presumably mediated through Cbl (Peruzzi et al., 2007). Overall, these data indicate that CIN85 plays a negative role in the context of FcεRI signaling, consistent with the model established in fibroblasts.
In contrast to the mast cell line data, a positive role for CIN85 in pre-TCR signaling has been recently suggested. The cytoplasmic tail of pre–TCR-α possesses a poly-proline-arginine sequence that interacts in vitro with SH3 domains of CD2AP, as well as CIN85, and deletion of the pre–TCR-α CD2AP/CIN85-binding motif impaired pre-TCR–mediated calcium mobilization in Jurkat T cells (Navarro et al., 2007). Because both CD2AP and CIN85 are recruited to the cytoplasmic domain of the pre–TCR-α chain, it is likely that both CIN85 and CD2AP act downstream of the pre-TCR to promote pre-TCR signaling. Thus, collectively with its functions in mast cells, CIN85 may mediate distinct biological outcomes that depend on the cell types and developmental stages of each cell type.
To test the physiological function of CIN85 in B lineage cells, we have generated B cell–specific CIN85 knockout mice. In this paper, we report that CIN85 links the BCR to IKK-β/NF-κB activation, thereby contributing to T cell–independent immune responses.
RESULTS
Expression of CIN85 in B lineage cells
During a yeast two-hybrid screen for BLNK (alternatively named SLP-65 or BASH) interacting proteins, we found that one of the clones isolated encoded CIN85. Using a reverse strategy with CIN85 as a bait, Watanabe et al. (2000) had previously identified BLNK as a CIN85 interacting protein. BLNK is a key adaptor molecule in BCR signaling and subsequent B cell responses (Fu et al., 1998; Goitsuka et al., 1998; Wienands et al., 1998), which prompted us to hypothesize that CIN85 could play a significant role upstream and/or downstream of BLNK in the BCR signaling context. Before addressing this issue, we first examined which CIN85 isoform is expressed in B cells because several isoforms of CIN85, generated by different promoter usage and alternative splicing, had already been reported (Buchman et al., 2002; Shimokawa et al., 2010). Western blotting analysis using an antibody raised against a C-terminal region of CIN85, common to all isoforms, detected two protein species in lysates of splenic B cells (Fig. 1 C). We concluded that the larger and smaller isoforms represent CIN85-l and CIN85-ΔA, respectively, because transcripts generating these two isoforms were detected in splenic B cells (Fig. S1 A) and because expression of cDNAs encoding the two isoforms in 293T cells gave rise to proteins that co-migrated with the endogenous CIN85 proteins in splenic B cells (Fig. S1 B).
In agreement with the previous data that both CIN85-l and CIN85-ΔA transcripts are expressed in various immune cell lineages, we found their expression not only in B cells but also in macrophages, DCs, and T cells in the spleen. Because B cells can be divided into several subsets based on their developmental stage or location, we examined expression of the two transcripts in each subpopulation. CIN85-l mRNA was higher in naive follicular (FO) and marginal zone (MZ) B cells in the spleen relative to developing B cells (pro–/pre–B and immature B cells) in the BM. We also observed significantly lower levels of CIN85-l and CIN85-ΔA transcripts in isolated germinal center (GC) B cells and in splenic B cells activated in vitro with anti-IgM, anti-CD40 antibody, IL-4, or toll-like receptor ligands (Fig. S1 A). CD2AP was also expressed in splenic B cells, as determined by quantitative (q) PCR and Western blotting analysis (Fig. S1, A and C).
CIN85 is indispensable for B-1a B cell development
Because CIN85 is expressed in many different immune cells, to examine its B cell–intrinsic functions, we generated B cell–specific knockout mice by crossing the mb1-cre mouse, which has a Cre recombinase transgene under the control of the mb1 promoter (mb1-cre; Hobeika et al., 2006), with a mouse in which Cin85 exon 5 is flanked by loxP sites (CIN85fl/Y; CIN85 is located on the X chromosome; Fig. 1 A). Exon 5 encodes an SH3 B region that is present in both CIN85-l and CIN85-ΔA isoforms (Fig. 1 A). As expected, in these mice (mb1-cre; CIN85fl/Y; shown as CIN85 bKO or bKO in figure legends or figures, respectively) efficient deletion of the targeted exon was seen in early B cells as well as peripheral splenic B cells (Fig. 1 B). Moreover, there was no detectable CIN85-l and CIN85-ΔA protein in B cell extracts from the spleen of mb1-cre; CIN85fl/Y mice (Fig. 1 C). In this study, mb1-cre; CIN85wt/Y mice were used as control mice. It was previously reported that mb1 is transcribed, at least to some extent, in thymic T cells. Probably because of this, ∼1% of the CIN85 allele in splenic T cells was deleted; however, this leaky deletion had almost no effect on the total number of splenic T cells (Table I). Moreover, overall T cell development in mb1-cre; CIN85fl/Y mice was normal, as assessed by expression of CD4 and CD8 of the thymocytes (Fig. 2).
Table I.
Lymphocyte population in CIN85 bKO mice
| Genotype | BM (×106) | Spleen (×106) | PerC (×105) | |||||||
| Pro/Pre | Immature | Recirculate | T1 | T2 | T3 | FO | MZ | T cells | B-1a | |
| Control | 8.9 ± 2.8 | 3.7 ± 1.4 | 3.4 ± 0.3 | 1.7 ± 0.3 | 3.6 ± 0.6 | 3.4 ± 1.0 | 24.6 ± 2.6 | 2.5 ± 0.5 | 21.5 ± 2.4 | 3.0 ± 0.5 |
| bKO | 8.9 ± 2.5 | 2.6 ± 1.0 | 2.3 ± 0.3 | 1.9 ± 0.3 | 2.8 ± 0.6 | 2.7 ± 0.8 | 19.5 ± 1.9 | 2.8 ± 0.5 | 19.4 ± 1.8 | 0.4 ± 0.2 |
Mean cell numbers of each population were calculated on the basis of total cell number and the percentages obtained from the flow cytometric analysis. Data are shown as mean ± SE (control, n = 7; bKO, n = 7). Pro, pro–B cells; Pre, pre–B cells, Immature, immature B cells; Recirculate, recirculating B cells; T1, T1 B cells; T2, T2 B cells; T3, T3 B cells; FO, FO B cells; MZ, MZ B cells; PerC, peritoneal cavity.
Figure 2.
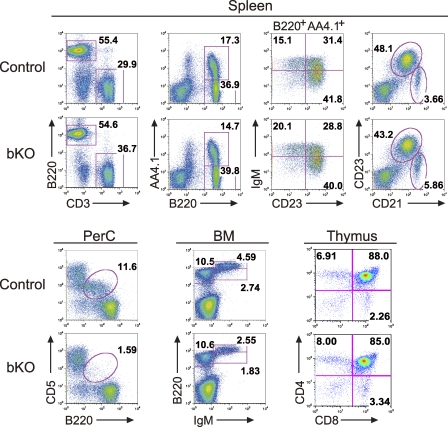
B-1a cells in the peritoneal cavity are reduced in CIN85 bKO mice. B and T cell development in the BM, spleen, thymus, and peritoneal cavity (PerC) of control and CIN85 bKO mice were analyzed by flow cytometry. Numbers represent the percentages of cells within the gates. Representative data of seven independent experiments are shown.
B cell development in the mutant mice was examined by multiparameter flow cytometry. In the BM of the mb1-cre; CIN85fl/Y mice, no gross developmental arrest was observed; however, the total number of immature and recirculating B cells was reduced by ∼30% (Table I). Reflecting this decrease, the number of B220+ B cells in the spleen was also reduced by ∼20% (Table I). Peripheral splenic B cells can be divided into immature (transitional [T] 1, T2, and T3), mature FO, and mature MZ B cells, based on the expression of AA4.1, IgM, B220, CD23, and CD21 as described previously (Hardy and Hayakawa, 2001). The numbers of MZ B cells and immature T1 B cells were similar between control and mb1-cre; CIN85fl/Y mice, whereas the numbers of immature T2, immature T3, and mature FO B cells were reduced by ∼20% in the mutant mice. The B cell subset most affected in the mutant mice was the peritoneal B-1a B cells, which were approximately eightfold reduced. Thus, we conclude that CIN85 is required for normal development or maintenance of the peritoneal B-1a B cell compartment.
CIN85 is essential for normal T cell–independent immune responses
To determine whether CIN85 is essential for B cell responses to antigen in vivo, we immunized mice with 4-hydroxy-3-nitrophenylacetyl (NP) coupled to chicken γ globulin (CGG; NP-CGG) or Ficoll (NP-Ficoll) to elicit T cell–dependent (TD) and T cell–independent type II (TI-II) immune responses, respectively. The NP-specific TD IgM response was somewhat decreased in mb1-cre; CIN85fl/Y mice, whereas the IgG1 response was slightly increased. Moreover, the amount of higher affinity anti-NP IgG1, as assessed by using NP1-BSA as a capture antigen in the ELISA assay, was not significantly altered in the mutant mice (Fig. S2 A). GC formation took place normally in the mutant mice (Fig. S2 B).
In contrast, NP-Ficoll responses were barely detectable in mb1-cre; CIN85fl/Y mice, both in terms of NP-specific IgM and IgG3 (Fig. 3 A). Having demonstrated the importance of CIN85 in TI-II responses, we wished to address the underlying mechanisms. In vivo, antigen accessibility and its recognition are critical for initiating immune responses and, in this regard, the importance of MZ B cells in inducing TI-II immune responses is well documented (Guinamard et al., 2000). Moreover, recent studies have demonstrated that MZ B cells rapidly capture complement-opsonized antigens, such as Ficoll, and deliver these antigens to the B cell follicles (Cinamon et al., 2008). Thus, one possible explanation for the failure of mb1-cre; CIN85fl/Y mice to elicit a TI-II response is that, despite the presence of MZ B cells, defined as CD21hiCD23lo (Fig. 2), these cells may not be able to capture Ficoll and subsequently transport it. However, our analysis renders this possibility unlikely; MZ B cells in mb1-cre; CIN85fl/Y mice bound TNP-Ficoll and transported it into the FO zones (Fig. 3, B and C).
Figure 3.
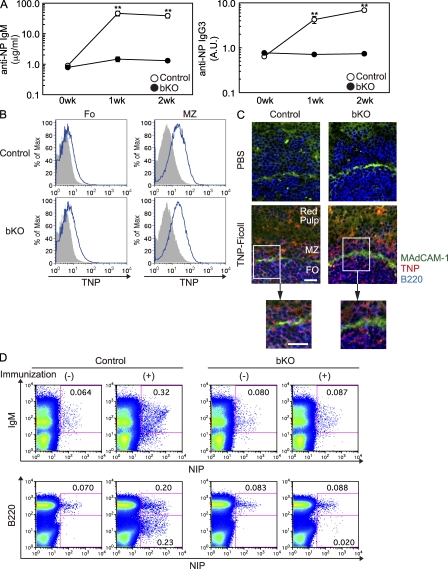
TI-II but not TD responses are impaired in CIN85 bKO mice. (A) Control or CIN85 bKO mice were injected i.p. with 50 µg NP-Ficoll. Sera from each mouse were collected before and every week after the immunization and the titers of anti-NP IgM (micrograms per milliliter) and IgG3 (arbitrary units [A.U.]) were measured by ELISA. Results are shown as the mean ± SD. **, P < 0.01. Each group consisted of at least four mice and the representative data of three repeated experiments are shown. (B and C) Mice were injected i.v. with PBS or 100 µg TNP-Ficoll. After 30 min, spleens were collected and TNP-binding B cells were analyzed by flow cytometry (B) or immunohistochemistry (C). In the flow cytometric analysis, FO B cells and MZ B cells were defined as CD21loCD23hi and CD21hiCD23lo cells, respectively. The gray histogram represents PBS-injected control mice. Frozen sections were stained with anti–MAdCAM-1 (green), TNP (red), and B220 (blue) antibodies. Bars, 20 µm. (D) Spleen cells were collected from control or CIN85 bKO mice 4 d after the i.p. injection with or without NP-Ficoll and analyzed by flow cytometry. NP-binding B cells were detected by staining with NIP-BSA-PE as described in the Materials and methods. Representative data of at least two independent experiments are shown (B–D).
Next, we analyzed the frequency of NP-specific B cells in the spleen on day 4 after the NP-Ficoll immunization. Before immunization, we found almost equal numbers of NP-reactive precursor B cells at frequencies consistent with previous results (Anderson et al., 2007). The percentage of NP-specific B cells in control mice increased from 0.06 to 0.32% after immunization, whereas there was only a barely detectable increase in mb1-cre; CIN85fl/Y mice (Fig. 3 D). Thus, CIN85 is most likely to be required for expansion of B cells in response to TI-II antigens in vivo.
CIN85 mediates BCR-induced B cell proliferation and survival in vitro
To further elucidate the function of CIN85, we directly assessed the survival and proliferative response of mb1-cre; CIN85fl/Y B cells to various stimuli in vitro. These mutant B cells exhibited defective survival after anti-IgM stimulation but not after other stimuli such as anti-CD40, LPS, IL-4, and BAFF (Fig. 4 A). We also assessed proliferation in response to anti-IgM stimulation with CFSE-labeled B cells from mutant and control mice. As shown in Fig. 4 B, the mb1-cre; CIN85fl/Y B cells essentially failed to divide at doses of 1 and 3 µg/ml anti-IgM. At saturating doses of 10 µg/ml anti-IgM, the mutant B cells began to divide but to a lesser extent than control B cells. No defects in proliferation were observed after stimulation with LPS, CpG, and anti-CD40 + IL-4. The proliferation of mb1-cre; CIN85fl/Y B cells in response to anti-CD40 ligation alone was somewhat decreased (Fig. 4 B).
Figure 4.
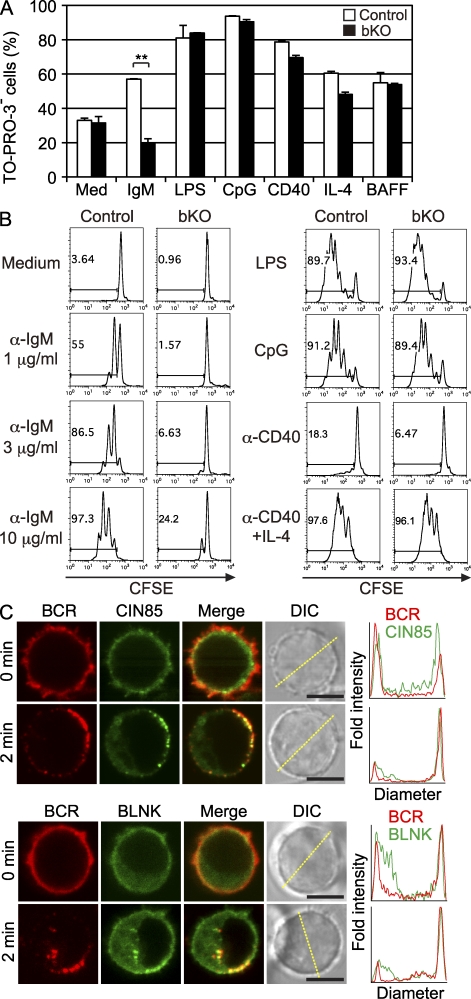
BCR-induced proliferation and survival in vitro are impaired in CIN85 bKO mice. (A) Spleen B cells from each mouse were negatively purified with anti-CD43 magnetic beads and were cultured with 10 µg/ml anti-IgM F(ab’)2 fragment, 10 µg/ml LPS, 100 nM CpG, 2 µg/ml anti-CD40 antibody, 10 ng/ml IL-4, or 50 ng/ml BAFF. After 48 h of culture, the proportion of live cells was enumerated as percentage of TO-PRO-3–excluding cells. Results represent the mean ± SD. n = 3. **, P < 0.01. (B) Purified spleen B cells were labeled with 5 µM CFSE. These CFSE-labeled cells were then cultured with 1, 3, or 10 µg/ml anti-IgM F(ab’)2 fragment, LPS, CpG, anti-CD40 antibody, or anti-CD40 plus IL-4 as indicated. After the culture for 72 h, cell division was measured by flow cytometry. Representative data from three independent experiments are shown (A and B). (C) Purified spleen B cells were stimulated with LPS for 1 d, and cells were transduced with retroviruses encoding CIN85-GFP or BLNK-YFP. 2 d after the infection, microscopic analysis was performed. Top, CIN85 bKO B cells expressing CIN85-GFP (green) were stained by rat anti-IgM antibody (red), fixed before (top) or 2 min after (bottom) cross-linking by anti–rat IgG antibody, and imaged by confocal microscopy. Histograms show fold fluorescent intensities of BCR (red) and CIN85-GFP (green) measured on the diagonal yellow line shown in the DIC images. Bars, 5 µm. Bottom, splenic B cells expressing BLNK-YFP (green) were stained by rat anti-IgM antibody (red), fixed before (top) or 2 min after (bottom) cross-linking by anti–rat IgG antibody, and imaged by confocal microscopy. More than 70% of BCR clusters were colocalized by BLNK or CIN85 on the cell surface (BLNK, 74.9 ± 11.6%; CIN85, 71.9 ± 19.9%; n = 20). Histograms show fold fluorescent intensities of BCR (red) and BLNK-YFP (green) measured on the diagonal yellow line shown in the DIC images. Bars, 5 µm. n = 50.
Involvement of CIN85 in BCR signaling was further substantiated by imaging analysis using confocal microscopy. CIN85-GFP or BLNK-YFP was transduced to LPS-stimulated B cells, and BCR stimulation was performed by in vitro antibody cross-linking. Upon BCR stimulation, 90% of B cells demonstrated dense CIN85-GFP clusters colocalizing with BCR (n = 50, a representative of three independent experiments; Fig. 4 C, top). This clustering was similar to that of BLNK translocation to the plasma membrane. 88% of the cells formed BLNK-YFP clusters with the BCR (n = 50, a representative of three independent experiments; Fig. 4 C, bottom). Thus, CIN85 participates specifically in BCR-mediated proliferation and survival.
CIN85 links the BCR to IKK
Because BCR stimulation induces multiple signaling pathways that culminate in cellular responses such as proliferation and survival, we wished to address which pathways are perturbed in mb1-cre; CIN85fl/Y B cells. As proximal events, we first examined the status of overall tyrosine phosphorylation and calcium mobilization but detected no significant differences in either readout (Fig. S3 A). These results suggest that CIN85 is not essential for overall activation of proximal events of the BCR signaling cascade. Indeed, tyrosine phosphorylation status of Syk, BLNK, and PLC-γ2 was also not significantly changed between control and mutant cells (Fig. S3 B).
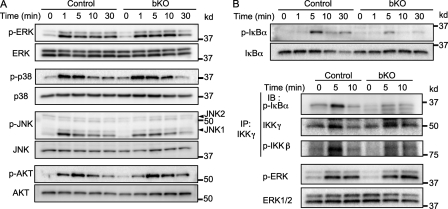
NF-κB, mitogen-activated protein kinase, and Akt pathways have been implicated in regulating B cell proliferation and survival; however, the phosphorylation status of Akt, Erk1, Erk2, and p38 after anti-IgM stimulation was not significantly altered in mb1-cre; CIN85fl/Y B cells (Fig. 5 A). In contrast, the level of IκBα phosphorylation was significantly decreased. The phosphorylation of JNK1 at 1 min was also decreased (Fig. 5, A and B). A defect in the canonical NF-κB pathway in mb1-cre; CIN85fl/Y B cells was also confirmed by an in vitro kinase assay of the IKK complex. After a 5-min stimulation of BCR, the in vitro kinase activity of the IKK complex toward IκBα was significantly impaired in mb1-cre; CIN85fl/Y B cells (Fig. 5 B). In contrast to the canonical NF-κB pathway, the BAFF-mediated noncanonical NF-κB pathway functioned normally in mb1-cre; CIN85fl/Y B cells, as assessed by nuclear translocation of the p52 subunit of NF-κB2 (Fig. S4). Thus, mutant B cells display a selective partial defect in BCR coupling to IKK signaling pathway.
Figure 5.
BCR-dependent activation of the canonical NF-κB pathway is impaired in CIN85 bKO B cells. (A) Purified spleen B cells were stimulated with 10 µg/ml anti-IgM F(ab’)2 for indicated times (minutes). The cells were lysed and subjected to SDS-PAGE. Transferred membranes were probed with the indicated antibodies. (B) Spleen B cells from control or CIN85 bKO mice were stimulated with 10 µg/ml anti-IgM F(ab’)2 fragment for the indicated times. After stimulation, some of the cells were subjected to SDS-PAGE to measure the phosphorylation status of IκBα (top). Other cells were collected, precipitated by anti–IKK-γ antibody and subjected to an IKK kinase assay using GST-IκBα as a substrate. The amount of phosphorylated GST-IκBα was detected by Western blotting as described in the Materials and methods (bottom). Phosphorylation status of IKK-β was also analyzed by Western blotting. p-ERK was examined with whole cell lysate. Representative data of at least three independent experiments are shown.
Previous studies with the transformed RBL-2H3 mast cell line showed that overexpression of CIN85 decreases the Syk protein level and enhances FcεRI-mediated endocytosis (Peruzzi et al., 2007). Thus, we made a point of carefully checking the Syk protein level in mutant and control B cells but detected no significant difference (Fig. S5 A). To examine BCR-mediated endocytosis, we took two approaches: (1) in vitro BCR-mediated endocytosis; and (2) in vivo antigen-mediated endocytosis and subsequent antigen presentation by B cells. No significant differences between mutant and control B cells were detected in these two assays (Fig. S5, B and C).
Expression of constitutively active IKK-β in B cells can substitute for some functions of CIN85
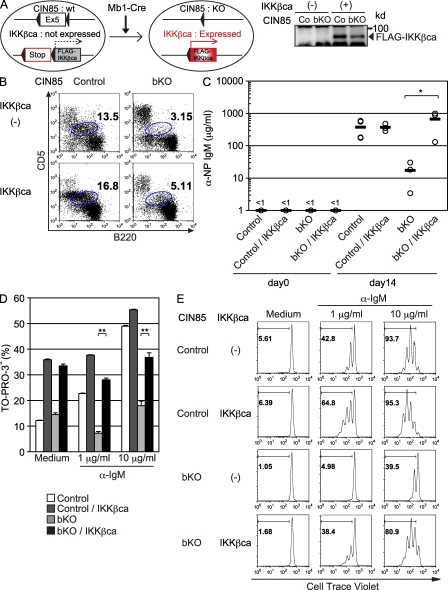
Having demonstrated the importance of CIN85 in BCR-mediated canonical NF-κB activation, we reasoned that the defects in mb1-cre; CIN85fl/Y mice should be restored by manipulations that enhance canonical NF-κB signaling. To test this hypothesis, we crossed mb1-cre; CIN85fl/Y mice with mice in which a cDNA encoding constitutively active IKK-β, preceded by a loxP-flanked STOP cassette, is knocked into the ubiquitously expressed ROSA26 locus (mb1-cre; CIN85fl/Y; R26StopflIKK-βca; Sasaki et al., 2006). As shown in Fig. 6 A, mb1-cre; CIN85fl/Y B cells from these mice express constitutively active IKK-β; however, the defect in B-1a cell development was not corrected (Fig. 6 B). In contrast, TI-II immune responses, as judged by anti-NP IgM titers, were almost completely restored by introduction of constitutively active IKK-β (Fig. 6 C). Well correlated with restoration of TI-II immune response, in vitro BCR-mediated proliferation and survival were also restored (Fig. 6, D and E). Together, these results suggest that defective T cell–independent immune responses could be, at least partly, explained by defective BCR-mediated canonical NF-κB activation in mb1-cre; CIN85fl/Y mice.
Figure 6.
Impaired TI-II responses in CIN85 bKO mice are restored by introduction of constitutively active IKK-β. (A) Schematic design of the experiment. In the presence of Cre, exon 5 of Cin85 is deleted and Flag-IKK-βca is expressed. To confirm the expression of FLAG-tagged IKK-βca, spleen B cells from control (Co) and CIN85 bKO were subjected to Western blotting. (B) Peritoneal cells from control, CIN85 bKO, CIN85 control; R26IKKβcaKI/wt, and CIN85 bKO; R26IKKβcaKI/wt mice were analyzed by flow cytometry. Numbers indicate the percentages of B-1a cells within the gate. (C) 3 × 106 purified spleen B cells from mice of indicated genotypes were injected i.v. into Rag1−/− mice. On the next day, 50 µg NP-Ficoll was injected i.p. Sera were collected 14 d later and the anti-NP IgM titer was measured by ELISA. Circles represent each titer and black bars represent the means. *, P < 0.05 (D) Purified spleen B cells from each genotype were cultured with medium alone or anti-IgM F(ab’)2 fragment for 48 h and the proportion of live cells were enumerated using TO-PRO-3. Data are shown as the mean ± SD. **, P < 0.01 (E) Spleen B cells of indicated genotypes were labeled with 5 µM CellTrace violet and cultured with medium alone or anti-IgM F(ab’)2 fragment for 72 h and fluorescence intensity was measured by flow cytometry. Each group consisted of three mice (B and C) and representative data of three independent experiments are shown (D and E).
DISCUSSION
Although a slightly decreased number of splenic T2, T3, and mature B cells was observed in the absence of CIN85, overall B-2 B cell development took place normally. In contrast, there was a deficiency in the peritoneal B-1 cell compartment. The selective B-1 deficiency fits with the idea that BCR cross-linking by self-antigen is required for maintenance of this self-renewing B cell compartment (Hayakawa et al., 1999). In this regard, MZ B cells are thought to be similarly selected by self-antigen to the B-1 population (Wen et al., 2005), as there are shared characteristics between B-1 and MZ B cells, raising the question of why MZ B cells develop normally in the mutant mice. There are several, not necessarily mutually exclusive, possibilities. First, the decreased signaling resulting from loss of CIN85 is still sufficient to promote the maturation and/or survival of the MZ subset but not the B-1 subset. Given the requirement for BAFF in development of the MZ, but not the B-1 subset (Schiemann et al., 2001), an alternative possibility is that the BAFF-R signal might compensate for the decreased BCR-mediated signal in MZ B cells, thereby promoting their development. Indeed, the BAFF-R–mediated survival signal is normal in mb1-cre; CIN85fl/Y B cells (Fig. 4 A and Fig. S4).
TI-II antigens, which are typically derived from polysaccharides and consist of complex repeating units, drive B cell responses by extensive cross-linking of cognate BCRs. Although B-1 and MZ B cells have been thought to be responsible for the initial TI-II response, FO B-2 B cells also participate in the overall TI-II antibody responses (Swanson et al., 2010). Indeed, mice devoid of B-1 and MZ B cells, such as CD19−/− mice, can still evoke TI-II responses (Rickert et al., 1995; Shih et al., 2002). Thus, the almost complete failure of mb1-cre; CIN85fl/Y mice to respond to TI-II antigens (Fig. 3 A) cannot be simply accounted for by the B-1 deficiency in the mutant mice.
In the case of MZ B cells, their importance for delivering complement-opsonized antigens such as Ficoll has been recently appreciated (Cinamon et al., 2008). According to this model, MZ B cells shuttle between MZ and the B cell follicles, thereby capturing blood-borne large antigens and subsequently transporting them from the blood to splenic FO DCs, which then display the antigen to B cells. As shown in Fig. 3 C, this process appears to occur normally in the mutant mice, suggesting that antigen-specific B cells in the spleen are capable of recognizing cognate antigens. Therefore, the inability of the mutant mice to induce antibody responses is most likely explained by poor expansion and survival of antigen-specific MZ and FO B cells, as demonstrated in Fig. 3 D. In fact, BCR-mediated proliferation/survival and in vivo TI-II responses were restored by introduction of a constitutively active B cell–specific IKK-β in the mutant mice. Despite restoration of TI-II responses by active IKK-β, B-1 B cell was not rescued. Assuming that an optimal intensity of NF-κB signal is required for B-1 B cell development, it is possible that the intensity of NF-κB signaling induced by our IKK-β mutant might be too strong, thereby interfering with B-1 B cell development. An alternative possibility is that the canonical NF-κB pathway is necessary, but not sufficient, for the development of B-1 B cells and that other pathways, such as JNK1, are also required.
Based on studies of 293T cells, after receptor tyrosine kinase activation, Cbl-CIN85 interaction is thought to be essential for the promotion of the initial steps of endocytosis and pinching off of the endocytic vesicles (Dikic, 2002). Cbl, one of the RING-type E3 ubiquitin ligases, is recruited to active tyrosine phosphorylated PTKs through its SH2 domain and ubiquitinated the receptors, which causes their endocytic-dependent degradation (Joazeiro et al., 1999). In addition, the interaction of Cbl with PTKs also induces tyrosine phosphorylation of Cbl, which unmasks its proline-rich determinants that can then interact with SH3 domains of CIN85 (Szymkiewicz et al., 2002). The association between Cbl and CIN85 is required for ligand-induced mono-ubiquitination of CIN85, which has been thought to be important for recognition of proteins of the endocytic machinery. This mechanism has been shown to operate in physiological settings as well. By using neuron-specific CIN85 knockout mice, Shimokawa et al. (2010) have recently demonstrated the involvement of CIN85 in endocytosis of the D2 dopamine receptor (D2DR), which belongs to the seven-transmembrane G protein–coupled receptor superfamily. But, in contrast to the D2DR, endocytosis of the D1 dopamine receptor was not affected by the loss of CIN85, suggesting the coexistence of CIN85-dependent and -independent mechanisms. Thus, considering the previous evidence that BCR down-modulation and Igα ubiquitination are inhibited in Cbl−/−Cbl-b−/− B cells (Kitaura et al., 2007), one possible interpretation for the apparently normal in vitro BCR internalization in mb1-cre; CIN85fl/Y B cells is that the BCR utilizes the Cbl-dependent but CIN85-independent mechanism. As discussed later in this section, this might be caused by the preferential association of CIN85 to BLNK rather than to Cbl/Cbl-b in the BCR signaling context. Our results nevertheless allow for the alternative possibility that CIN85 and CD2AP participate, redundantly, in BCR endocytosis.
Our studies indicate that CIN85 has a positive role in BCR-mediated survival and proliferation by participating in canonical NF-κB signaling. Although PLC-γ2 and subsequent PKC-β activation are involved in BCR-mediated NF-κB activation (Saijo et al., 2002; Su et al., 2002), the apparently normal tyrosine phosphorylation status of PLC-γ2 and subsequent calcium mobilization in mb1-cre; CIN85fl/Y B cells suggest that CIN85 affects canonical NF-κB activation independently of PLC-γ2/PKC-β. Thus, given the previous evidence that defects in BCR-mediated NF-κB signaling pathways are induced by mutations in CARMA1, Bcl10, MALT1, and TAK1 (Shinohara and Kurosaki, 2009), it is reasonable to speculate that CIN85 interacts with such complexes, directly or indirectly.
The precise biochemical mechanism by which CIN85 activates IKK-β remains unclear. One possibility is that the protein–protein interaction motifs of CIN85 might allow it to recruit additional signaling molecules, such as CARMA1, Bcl10, and MALT1, that could mediate IKK-β activation directly or indirectly. Considering that CIN85 was recruited to the plasma membrane quite rapidly after BCR cross-linking, these interactions might have two consequences: membrane recruitment of CIN85 partners and their conformational changes. With regard to membrane recruitment, a role for the SH3 domain of CARMA1 was strongly implicated by studies using a lack-of-function CARMA1 mutant in the Jurkat T cell line (Wang et al., 2004). Thus, the proline-rich region of CIN85 might contribute to its association with the CARMA1 SH3 domain and its subsequent membrane recruitment. When we used a lower concentration of anti-IgM compared with Fig. 4 C, we could not observe significant association between BCR and CIN85 (not depicted). This suggests that only strong BCR signal, such as in the case of TI-II responses, utilizes CIN85 for membrane recruitment of its partner molecules, whereas the weak signal, such as tonic BCR signal, might not necessarily require CIN85 (Lam et al., 1997; Monroe, 2006), providing the potential explanation of why development of mature FO B cells was only marginally affected in the absence of CIN85.
In addition to the conventional protein–protein interactions mentioned in the previous paragraph, given the recent evidence that the SH3 C domain of CIN85 can function as an ubiquitin-binding domain (UBD; Bezsonova et al., 2008), CIN85 might exert its function through binding to ubiquitinated proteins. Indeed, ubiquitination of IKK-γ and TRAF6 has already been reported in the context of antigen receptor signaling being important for NF-κB activation (Sun et al., 2004). In addition, several NF-κB components, for instance, TAK1-binding protein TAB2, are known to contain the UBD (Kanayama et al., 2004). Thus, it is possible that the CIN85 UBD contributes to the assembly of complexes with targets such as IKK-γ and TRAF6, which are reversibly and covalently modified by specific ubiquitin chains in the BCR signaling context. It is also important to note that CIN85, like CD2AP, is capable of facilitating actin polymerization by inhibiting actin-capping proteins (Hutchings et al., 2003). Given the importance of actin cytoskeleton dynamics in NF-κB signaling (Legrand-Poels et al., 2007), deregulation of this process might occur in mb1-cre; CIN85fl/Y B cells, possibly leading to defective NF-κB signaling.
Assuming that CIN85 and CD2AP have redundant functions, the question arises of why T cells lacking CD2AP show enhanced TCR-mediated proliferation. It has been observed that both CIN85 and CD2AP can bind to BLNK but not SLP-76 (Wienands, J., personal communication). Because BCR and TCR use BLNK or SLP-76, respectively, as a critical signaling molecule, it is possible that there is a preferential association of CIN85/CD2AP with BLNK in the BCR signaling context, thereby allowing both CIN85 and CD2AP to participate in NF-κB activation, whereas in the case of TCR signaling, CIN85 and CD2AP associate with Cbl, thereby coupling them to an endocytotic pathway. Thus, depending on their binding partners, CIN85 and CD2AP could induce distinct biological outcomes. As an extension of this hypothesis, skewing the association of CIN85 and CD2AP toward Cbl in anergic anti-HEL B cells could explain why these cells shut off NF-κB and JNK signaling and display an enhancement of endocytosis (Jun and Goodnow, 2003; Bléry et al., 2006).
MATERIALS AND METHODS
Mice.
To generate Flox-CIN85 mice, a PCR-amplified Cin85 exon 5 containing genomic fragment was flanked with two loxP sites and subcloned into a vector that contained a neomycin resistance gene, a diphtheria toxin A gene, and a 5.7-kbp 5′ and a 2.0-kbp 3′ homology Cin85 arm. The targeting vector was electroporated into C57BL/6 background Bruce4 embryonic stem cells (Köntgen et al., 1993) and selection was performed with G418 (Invitrogen). Correctly targeted clones were identified by Southern blotting. The neomycin resistant gene was removed by transiently transfecting a Cre expression vector as described elsewhere (Yamada et al., 2008). Targeted ES clones were injected into blastocysts from BALB/c mice to obtain chimeric mice. These chimeric mice were then crossed with C57BL/6 mice to obtain germline transmitted animals. To generate B cell–specific CIN85 knockout mice, floxed mice were crossed with mb1-Cre knockin (KI) mice (Hobeika et al., 2006). Mb1-Cre knockin mice were provided by E. Hobeika and M. Reth (Max Planck Institute for Immunobiology, Freiburg, Germany). Rosa26-stop-FLAG-IKK-βca mice have been described previously (Sasaki et al., 2006). Mice were maintained under specific pathogen-free conditions. All the protocols for animal experiments were approved by the RIKEN Animal Research Committee.
Flow cytometric analysis.
Single cell suspensions lysed of red blood cells were stained with fluorochrome-conjugated antibodies. Stained cells were analyzed with a FACSCalibur or FACSCanto II (BD). Anti-AA4.1, B220, Fas, GL7, IgD, IgM, MHC class II, CD3, CD4, CD5, CD8, CD21, CD23, and CD86 antibodies were purchased from BD, eBioscience, or BioLegend. NP-binding B cells were detected using (4-hydroxy-5-indo-3-nitrophenyl)acetyl (NIP)–BSA-PE.
For the detection of TNP-Ficoll binding cells, spleen cells were recovered from the mice injected with 100 µg TNP-Ficoll i.v. These cells were then stained with biotin-conjugated anti-TNP antibody (BD) in combination with streptavidin-FITC and analyzed using a FACSCanto II.
Immunohistology.
Small pieces of spleen were embedded in OCT compound (Sakura) and frozen at −80°C. Frozen sections (8 µm) were fixed and stained with Alexa Fluor 488–conjugated anti–MAdCAM-1 (BioLegend), biotin-conjugated anti-TNP, Alexa Fluor 647–conjugated B220 antibodies (BD), and Alexa Fluor 546–conjugated streptavidin (Invitrogen). Images were obtained using BIOREVO (Keyence).
Induction of immune responses.
For measuring TI-II antigen responses, mice were immunized with 50 µg NP28-Ficoll (Biosearch Technologies) i.p. To examine the TD antigen response, 100 µg NP-CGG precipitated with Imject Alum (Thermo Fisher Scientific) was injected i.p. 6 wk later, 50 µg of soluble NP-CGG was injected i.p. to induce recall responses. TI-II response in ROSA26-stop-FLAG-IKK-βca mice was examined by an adoptive transfer experiment. In brief, B cells were purified from the spleen of these mice by depletion of activated B cells and non–B cells with anti-CD43 microbeads using autoMACS (Miltenyi Biotec) and 3 × 106 of purified cells were injected into Rag1−/− mice i.v. On the next day, the recipient mice were immunized i.p. with 50 µg NP-Ficoll. Sera were collected before and every week after the immunization and anti-NP IgM titers were measured by ELISA.
ELISA.
96-well flat-bottom plates were coated with 2 µg/ml NP10-BSA followed by blocking with 0.5% BSA in PBS. Serially diluted samples were incubated at 4°C for 1 d. After washing with PBS/0.5% Tween-20, horseradish peroxidase–conjugated anti–mouse IgM or IgG3 antibodies (SouthernBiotech) were appropriately diluted and added to the wells. SureBlue (KPL) was used as the substrate and absorbance at 450 nm was measured using a microplate reader (Bio-Rad Laboratories). For detecting high-affinity anti-NP IgG1 antibody, NP1-BSA was used as a capture antigen.
In vitro B cell culture.
B cells were purified from the splenocytes using anti-CD43 microbeads and AutoMACS (Miltenyi Biotec). Collected cells were labeled with 5 µM CFSE (Dojindo) or CellTrace violet (Invitrogen) at 37°C for 20 min. Labeled cells were cultured in RPMI1640 supplemented with 10% FBS, 50 µM 2-ME, and penicillin/streptomycin. Anti-IgM F(ab’)2 (Jackson ImmunoResearch Laboratories), LPS (Sigma-Aldrich), CpG, anti-CD40 (BD), and IL-4 (R&D Systems) were added as indicated. After 72 h of culture, cell division was measured by FACSCanto II. For analyzing cell viability, negatively purified spleen B cells were cultured with anti-IgM F(ab’)2, LPS, CpG, anti-CD40, IL-4, or BAFF (R&D Systems). After 48 h of culture, the dead cells were labeled with TO-PRO-3 (Invitrogen) and the proportion of the live cells was enumerated as the percentage of TO-PRO-3–negative cells.
Western blotting.
Negatively purified spleen B cells were stimulated with 10 µg/ml anti-IgM F(ab’)2 for the indicated periods and lysed with RIPA buffer. Cell lysates were boiled with SDS sample buffer and subjected to SDS-PAGE followed by transfer to PVDF membranes (Millipore). The membranes were blocked and incubated with the following antibodies to the following antigens: phospho IκBα, phospho ERK, phospho p38, phospho AKT (Ser473), phospho BLNK, BLNK, and phospho Syk (Cell Signaling Technology); IκBα, ERK1, ERK2, p38, JNK1/3, AKT, Syk, PLC-γ2, and phospho IKK-β (Santa Cruz Biotechnology, Inc.); phospho JNK (Promega); IKK-γ (BD); and GAPDH and phosphotyrosine (Millipore). Anti-CIN85 polyclonal antibody was obtained by immunizing rabbits with CIN85 peptide-conjugated glutathione S-transferase (GST) as described previously (Oh-hora et al., 2003). Anti-CD2AP antibody was provided by A. Shaw (Washington University School of Medicine, St. Louis, MO). Then the membranes were incubated with the appropriate alkaline phosphatase-conjugated secondary antibody, followed by detection with a chemiluminescent substrate (Millipore). The IKK kinase assay was performed as previously described (Shinohara et al., 2007). In brief, spleen B cells from the control or CIN85 bKO mice were stimulated with 10 µg/ml anti-IgM F(ab’)2 fragment for the indicated times. After stimulation, cells were lysed and the IKK complex was precipitated with anti–IKK-γ antibody. IKK kinase activity was measured using GST-IκBα as a substrate. The amount of phosphorylated GST-IκBα was detected by Western blotting.
Quantitative RT-PCR.
Total RNA was extracted using TRIZOL (Invitrogen) according to the manufacturer’s instructions. DNase I (Invitrogen)–treated RNA was reverse transcribed using Super Script III (Invitrogen). Quantitative PCR was performed using SYBR Green (Invitrogen) and ABI Prism 7000 (Applied Biosystems). The following primers were used: 5′-GTGGAGGCCATAGTGGAGTT-3′ and 5′-CACGCTGATTGTCAGCTCAT-3′ (Cin85-l); 5′-CCCACAGACTTGCCTGAGA-3′ and 5′-ACTGGATACATCGTGCATGG-3′ (Cin85-ΔA); and 5′-GCTCTGTGAAACTTCGGACA-3′ and 5′-CCCAGAGGCTGTAGGATTAAAG-3′ (Cd2ap).
Analysis of B cell function.
To examine the BCR-mediated calcium response, purified B cells were loaded with Indo-1 AM (Dojindo) and Pluronic F-127 (Invitrogen) at 37°C for 45 min. Cells were stimulated with 10 µg/ml anti-IgM F(ab’)2 and the fluorescence was detected using LSR (BD) as described previously (Baba et al., 2008).
For measuring BCR internalization, B cells were incubated with 0.5, 1, or 5 µg/ml biotin-conjugated anti-IgM antibody (BD) on ice for 20 min. After washing with ice cold PBS, the cells were kept on ice or incubated at 37°C for indicated times followed by incubation with streptavidin-allophycocyanin (APC; BD) and analyzed using a FACSCanto II.
To examine in vivo antigen presentation by B cells, 100 µg NP-Eα-GFP (Itano et al., 2003; Pape et al., 2007) was injected i.p. into the mice. This construct was generated by K.A. Pape and M.K. Jenkins (University of Minnesota Medical School, Minneapolis, MN). After 12 h, splenocytes were collected and antigen-presenting B cells were detected by incubation with biotin-conjugated YAe antibody (eBioscience), followed by staining with NIP-BSA-PE, anti-B220, and streptavidin-APC. The cells were analyzed using a FACSCanto II.
Subcellular localization of CIN85.
Mouse CIN85 was fused to the N terminus of GFP or CFP and subcloned to pMXs retroviral vector. pMXs vector was provided by T. Kitamura (University of Tokyo, Tokyo, Japan). Purified B cells were cultured with 10 µg/ml LPS for 1 d, and GFP-fused CIN85 or YFP-fused BLNK was retrovirally transduced into LPS-stimulated B cells. 2 d later after the infection, the cells were incubated with 2 µg/ml anti-IgM antibody followed by cross-linking with fluorochrome-conjugated anti–rat IgG antibody. The cells were fixed and imaged by confocal microscopy (Leica).
Statistical analysis.
Statistical analyses were performed using two-tailed unpaired Student’s t test.
Online supplemental material.
Fig. S1 shows the expression of CIN85 and CD2AP in immune cells. Fig. S2 shows TD immune responses in CIN85 bKO mice. Fig. S3 shows normal activation of BCR-proximal signaling molecules in CIN85 bKO mice. Fig. S4 shows BAFF-induced noncanonical NF-κB activation in CIN85 bKO mice. Fig. S5 shows the effects of CIN85 deletion on protein degradation and internalization. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20102665/DC1.
Acknowledgments
We thank Dr. E. Hobeika and Dr. M. Reth for mb1-Cre mice, Dr. K.A. Pape and Dr. M.K. Jenkins for providing us with the EαGFP construct, Dr. T. Kitamura for pMXs vector, Dr. A. Shaw for anti-CD2AP antibody, Dr. H. Shinohara for technical advice, Dr. J. Wienands for reagents and critical discussion, and Dr. T. Oellerich and Dr. J. Wienands for communicating unpublished results.
This work was supported by grant-in-Aid to T. Kurosaki from the Ministry of Education, Culture, Sports, Science, and Technology in Japan and by a grant to T. Kurosaki from Japan Science and Technology Agency, Core Research of Evolutional Science and Technology. This work was also supported by RIKEN Special Postdoctoral Researchers Program to K. Kometani.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- APC
- allophycocyanin
- CGG
- chicken γ globulin
- FO
- follicular
- GC
- germinal center
- GST
- glutathione S-transferase
- MZ
- marginal zone
- NIP
- (4-hydroxy-5-indo-3-nitrophenyl)acetyl
- NP
- 4-hydroxy-3-nitrophenylacetyl
- SH3
- Src homology 3
- TD
- T cell–dependent
- TI-II
- T cell–independent type II
- UBD
- ubiquitin-binding domain
References
- Anderson S.M., Tomayko M.M., Ahuja A., Haberman A.M., Shlomchik M.J. 2007. New markers for murine memory B cells that define mutated and unmutated subsets. J. Exp. Med. 204:2103–2114 10.1084/jem.20062571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y., Nishida K., Fujii Y., Hirano T., Hikida M., Kurosaki T. 2008. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat. Immunol. 9:81–88 10.1038/ni1546 [DOI] [PubMed] [Google Scholar]
- Bezsonova I., Bruce M.C., Wiesner S., Lin H., Rotin D., Forman-Kay J.D. 2008. Interactions between the three CIN85 SH3 domains and ubiquitin: implications for CIN85 ubiquitination. Biochemistry. 47:8937–8949 10.1021/bi800439t [DOI] [PubMed] [Google Scholar]
- Bléry M., Tze L., Miosge L.A., Jun J.E., Goodnow C.C. 2006. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-κB in B cell clonal anergy. J. Exp. Med. 203:1773–1783 10.1084/jem.20060552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman V.L., Luke C., Borthwick E.B., Gout I., Ninkina N. 2002. Organization of the mouse Ruk locus and expression of isoforms in mouse tissues. Gene. 295:13–17 10.1016/S0378-1119(02)00821-1 [DOI] [PubMed] [Google Scholar]
- Cinamon G., Zachariah M.A., Lam O.M., Foss F.W., Jr, Cyster J.G. 2008. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 9:54–62 10.1038/ni1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I. 2002. CIN85/CMS family of adaptor molecules. FEBS Lett. 529:110–115 10.1016/S0014-5793(02)03188-5 [DOI] [PubMed] [Google Scholar]
- Dikic I., Giordano S. 2003. Negative receptor signalling. Curr. Opin. Cell Biol. 15:128–135 10.1016/S0955-0674(03)00004-8 [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Olszowy M.W., Holdorf A.D., Li J., Bromley S., Desai N., Widder P., Rosenberger F., van der Merwe P.A., Allen P.M., Shaw A.S. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 94:667–677 10.1016/S0092-8674(00)81608-6 [DOI] [PubMed] [Google Scholar]
- Fu C., Turck C.W., Kurosaki T., Chan A.C. 1998. BLNK: a central linker protein in B cell activation. Immunity. 9:93–103 10.1016/S1074-7613(00)80591-9 [DOI] [PubMed] [Google Scholar]
- Goitsuka R., Fujimura Y., Mamada H., Umeda A., Morimura T., Uetsuka K., Doi K., Tsuji S., Kitamura D. 1998. BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of Fabricius. J. Immunol. 161:5804–5808 [PubMed] [Google Scholar]
- Gout I., Middleton G., Adu J., Ninkina N.N., Drobot L.B., Filonenko V., Matsuka G., Davies A.M., Waterfield M., Buchman V.L. 2000. Negative regulation of PI 3-kinase by Ruk, a novel adaptor protein. EMBO J. 19:4015–4025 10.1093/emboj/19.15.4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R., Okigaki M., Schlessinger J., Ravetch J.V. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31–36 [DOI] [PubMed] [Google Scholar]
- Hardy R.R., Hayakawa K. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595–621 10.1146/annurev.immunol.19.1.595 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Asano M., Shinton S.A., Gui M., Allman D., Stewart C.L., Silver J., Hardy R.R. 1999. Positive selection of natural autoreactive B cells. Science. 285:113–116 10.1126/science.285.5424.113 [DOI] [PubMed] [Google Scholar]
- Hobeika E., Thiemann S., Storch B., Jumaa H., Nielsen P.J., Pelanda R., Reth M. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. USA. 103:13789–13794 10.1073/pnas.0605944103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings N.J., Clarkson N., Chalkley R., Barclay A.N., Brown M.H. 2003. Linking the T cell surface protein CD2 to the actin-capping protein CAPZ via CMS and CIN85. J. Biol. Chem. 278:22396–22403 10.1074/jbc.M302540200 [DOI] [PubMed] [Google Scholar]
- Itano A.A., McSorley S.J., Reinhardt R.L., Ehst B.D., Ingulli E., Rudensky A.Y., Jenkins M.K. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 19:47–57 10.1016/S1074-7613(03)00175-4 [DOI] [PubMed] [Google Scholar]
- Joazeiro C.A., Wing S.S., Huang H., Leverson J.D., Hunter T., Liu Y.C. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 286:309–312 10.1126/science.286.5438.309 [DOI] [PubMed] [Google Scholar]
- Jun J.E., Goodnow C.C. 2003. Scaffolding of antigen receptors for immunogenic versus tolerogenic signaling. Nat. Immunol. 4:1057–1064 10.1038/ni1001 [DOI] [PubMed] [Google Scholar]
- Kanayama A., Seth R.B., Sun L., Ea C.K., Hong M., Shaito A., Chiu Y.H., Deng L., Chen Z.J. 2004. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell. 15:535–548 10.1016/j.molcel.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Kitaura Y., Jang I.K., Wang Y., Han Y.C., Inazu T., Cadera E.J., Schlissel M., Hardy R.R., Gu H. 2007. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 26:567–578 10.1016/j.immuni.2007.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köntgen F., Süss G., Stewart C., Steinmetz M., Bluethmann H. 1993. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int. Immunol. 5:957–964 10.1093/intimm/5.8.957 [DOI] [PubMed] [Google Scholar]
- Lam K.P., Kühn R., Rajewsky K. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 90:1073–1083 10.1016/S0092-8674(00)80373-6 [DOI] [PubMed] [Google Scholar]
- Legrand-Poels S., Kustermans G., Bex F., Kremmer E., Kufer T.A., Piette J. 2007. Modulation of Nod2-dependent NF-kappaB signaling by the actin cytoskeleton. J. Cell Sci. 120:1299–1310 10.1242/jcs.03424 [DOI] [PubMed] [Google Scholar]
- Molfetta R., Belleudi F., Peruzzi G., Morrone S., Leone L., Dikic I., Piccoli M., Frati L., Torrisi M.R., Santoni A., Paolini R. 2005. CIN85 regulates the ligand-dependent endocytosis of the IgE receptor: a new molecular mechanism to dampen mast cell function. J. Immunol. 175:4208–4216 [DOI] [PubMed] [Google Scholar]
- Monroe J.G. 2006. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat. Rev. Immunol. 6:283–294 10.1038/nri1808 [DOI] [PubMed] [Google Scholar]
- Navarro M.N., Nusspaumer G., Fuentes P., González-García S., Alcain J., Toribio M.L. 2007. Identification of CMS as a cytosolic adaptor of the human pTalpha chain involved in pre-TCR function. Blood. 110:4331–4340 10.1182/blood-2007-06-094938 [DOI] [PubMed] [Google Scholar]
- Oh-hora M., Johmura S., Hashimoto A., Hikida M., Kurosaki T. 2003. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-γ2 to Ras in B cell receptor signaling. J. Exp. Med. 198:1841–1851 10.1084/jem.20031547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape K.A., Catron D.M., Itano A.A., Jenkins M.K. 2007. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 26:491–502 10.1016/j.immuni.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Peruzzi G., Molfetta R., Gasparrini F., Vian L., Morrone S., Piccoli M., Frati L., Santoni A., Paolini R. 2007. The adaptor molecule CIN85 regulates Syk tyrosine kinase level by activating the ubiquitin-proteasome degradation pathway. J. Immunol. 179:2089–2096 [DOI] [PubMed] [Google Scholar]
- Petrelli A., Gilestro G.F., Lanzardo S., Comoglio P.M., Migone N., Giordano S. 2002. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 416:187–190 10.1038/416187a [DOI] [PubMed] [Google Scholar]
- Rickert R.C., Rajewsky K., Roes J. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 376:352–355 10.1038/376352a0 [DOI] [PubMed] [Google Scholar]
- Saijo K., Mecklenbräuker I., Santana A., Leitger M., Schmedt C., Tarakhovsky A. 2002. Protein kinase C β controls nuclear factor κB activation in B cells through selective regulation of the IκB kinase α. J. Exp. Med. 195:1647–1652 10.1084/jem.20020408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Derudder E., Hobeika E., Pelanda R., Reth M., Rajewsky K., Schmidt-Supprian M. 2006. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 24:729–739 10.1016/j.immuni.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Schiemann B., Gommerman J.L., Vora K., Cachero T.G., Shulga-Morskaya S., Dobles M., Frew E., Scott M.L. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 293:2111–2114 10.1126/science.1061964 [DOI] [PubMed] [Google Scholar]
- Shih T.A., Meffre E., Roederer M., Nussenzweig M.C. 2002. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat. Immunol. 3:570–575 10.1038/ni803 [DOI] [PubMed] [Google Scholar]
- Shimokawa N., Haglund K., Hölter S.M., Grabbe C., Kirkin V., Koibuchi N., Schultz C., Rozman J., Hoeller D., Qiu C.H., et al. 2010. CIN85 regulates dopamine receptor endocytosis and governs behaviour in mice. EMBO J. 29:2421–2432 10.1038/emboj.2010.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara H., Kurosaki T. 2009. Comprehending the complex connection between PKCbeta, TAK1, and IKK in BCR signaling. Immunol. Rev. 232:300–318 10.1111/j.1600-065X.2009.00836.x [DOI] [PubMed] [Google Scholar]
- Shinohara H., Maeda S., Watarai H., Kurosaki T. 2007. IκB kinase β–induced phosphorylation of CARMA1 contributes to CARMA1–Bcl10–MALT1 complex formation in B cells. J. Exp. Med. 204:3285–3293 10.1084/jem.20070379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran P., Kowanetz K., Szymkiewicz I., Langdon W.Y., Dikic I. 2002. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 416:183–187 10.1038/416183a [DOI] [PubMed] [Google Scholar]
- Su T.T., Guo B., Kawakami Y., Sommer K., Chae K., Humphries L.A., Kato R.M., Kang S., Patrone L., Wall R., et al. 2002. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 3:780–786 [DOI] [PubMed] [Google Scholar]
- Sun L., Deng L., Ea C.K., Xia Z.P., Chen Z.J. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell. 14:289–301 10.1016/S1097-2765(04)00236-9 [DOI] [PubMed] [Google Scholar]
- Swanson C.L., Wilson T.J., Strauch P., Colonna M., Pelanda R., Torres R.M. 2010. Type I IFN enhances follicular B cell contribution to the T cell–independent antibody response. J. Exp. Med. 207:1485–1500 10.1084/jem.20092695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkiewicz I., Kowanetz K., Soubeyran P., Dinarina A., Lipkowitz S., Dikic I. 2002. CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J. Biol. Chem. 277:39666–39672 10.1074/jbc.M205535200 [DOI] [PubMed] [Google Scholar]
- Take H., Watanabe S., Takeda K., Yu Z.X., Iwata N., Kajigaya S. 2000. Cloning and characterization of a novel adaptor protein, CIN85, that interacts with c-Cbl. Biochem. Biophys. Res. Commun. 268:321–328 10.1006/bbrc.2000.2147 [DOI] [PubMed] [Google Scholar]
- Wang D., Matsumoto R., You Y., Che T., Lin X.Y., Gaffen S.L., Lin X. 2004. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol. Cell. Biol. 24:164–171 10.1128/MCB.24.1.164-171.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Take H., Takeda K., Yu Z.X., Iwata N., Kajigaya S. 2000. Characterization of the CIN85 adaptor protein and identification of components involved in CIN85 complexes. Biochem. Biophys. Res. Commun. 278:167–174 10.1006/bbrc.2000.3760 [DOI] [PubMed] [Google Scholar]
- Wen L., Brill-Dashoff J., Shinton S.A., Asano M., Hardy R.R., Hayakawa K. 2005. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 23:297–308 10.1016/j.immuni.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Wienands J., Schweikert J., Wollscheid B., Jumaa H., Nielsen P.J., Reth M. 1998. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J. Exp. Med. 188:791–795 10.1084/jem.188.4.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Kurosaki T., Hikida M. 2008. Essential roles of mgcRacGAP in multilineage differentiation and survival of murine hematopoietic cells. Biochem. Biophys. Res. Commun. 372:941–946 10.1016/j.bbrc.2008.05.170 [DOI] [PubMed] [Google Scholar]