Abstract
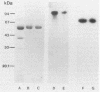
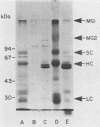
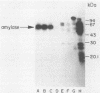
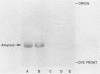
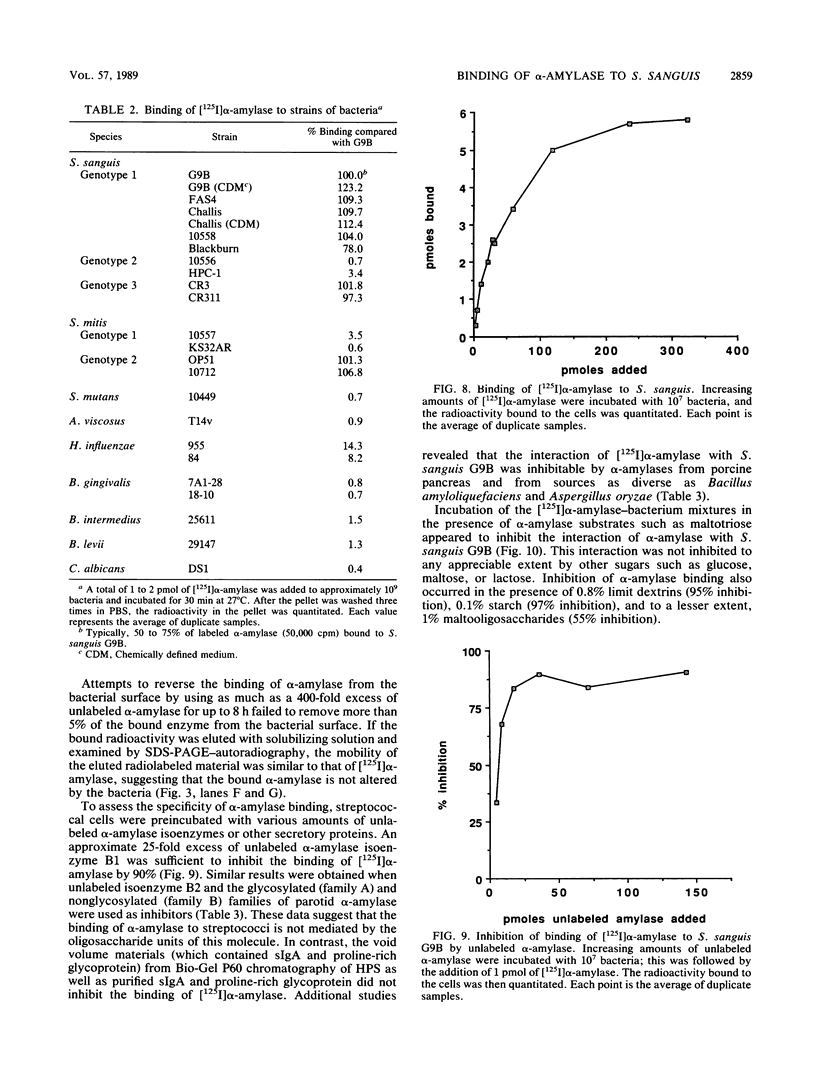
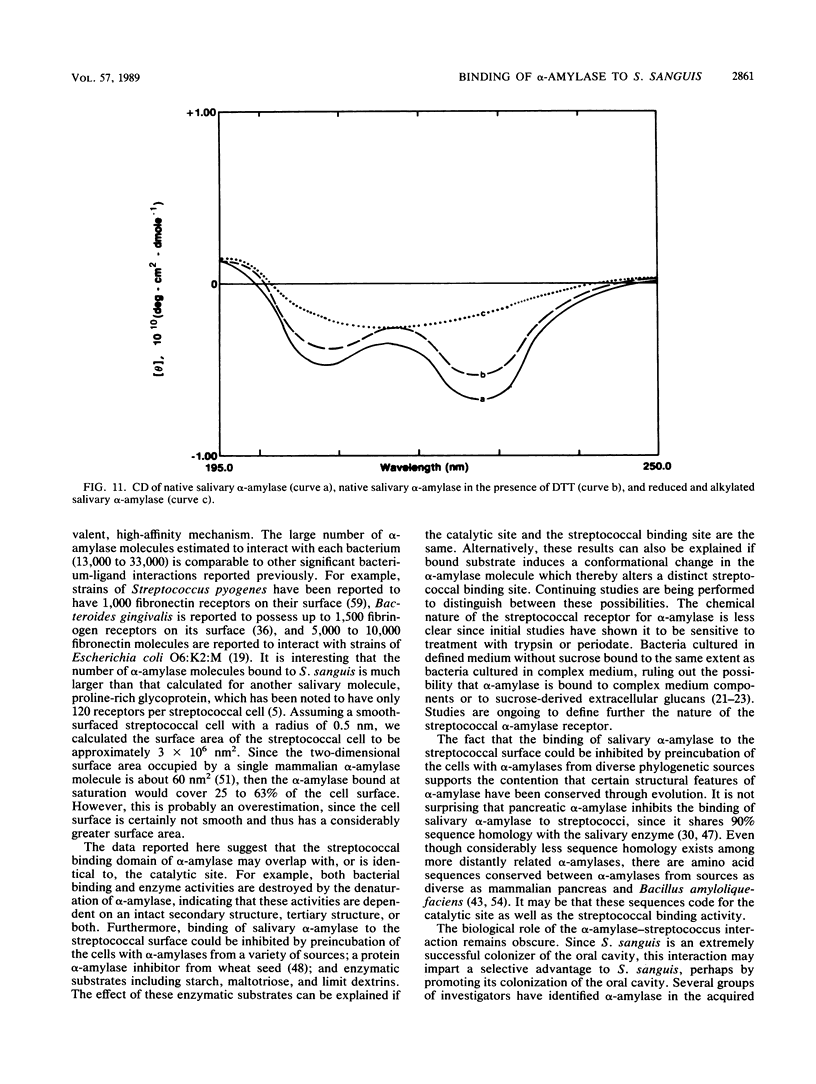
The purpose of this study was to identify the major salivary components which interact with oral bacteria and to determine the mechanism(s) responsible for their binding to the bacterial surface. Strains of Streptococcus sanguis, Streptococcus mitis, Streptococcus mutans, and Actinomyces viscosus were incubated for 2 h in freshly collected human submandibular-sublingual saliva (HSMSL) or parotid saliva (HPS), and bound salivary components were eluted with 2% sodium dodecyl sulfate. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western transfer, alpha-amylase (EC 3.2.1.1) was the prominent salivary component eluted from S. sanguis. Studies with 125I-labeled HSMSL or 125I-labeled HPS also demonstrated a component with an electrophoretic mobility identical to that of alpha-amylase which bound to S. sanguis. Purified alpha-amylase from human parotid saliva was radiolabeled and found to bind to strains of S. sanguis genotypes 1 and 3 and S. mitis genotype 2, but not to strains of other species of oral bacteria. Binding of [125I]alpha-amylase to streptococci was saturable, calcium independent, and inhibitable by excess unlabeled alpha-amylases from a variety of sources, but not by secretory immunoglobulin A and the proline-rich glycoprotein from HPS. Reduced and alkylated alpha-amylase lost enzymatic and bacterial binding activities. Binding was inhibited by incubation with maltotriose, maltooligosaccharides, limit dextrins, and starch.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre A., Levine M. J., Cohen R. E., Tabak L. A. Immunochemical quantitation of alpha-amylase and secretory IgA in parotid saliva from people of various ages. Arch Oral Biol. 1987;32(4):297–301. doi: 10.1016/0003-9969(87)90024-0. [DOI] [PubMed] [Google Scholar]
- Al-Hashimi I., Levine M. J. Characterization of in vivo salivary-derived enamel pellicle. Arch Oral Biol. 1989;34(4):289–295. doi: 10.1016/0003-9969(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Hasty D. L., Simpson W. A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986 Feb;51(2):405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Simpson W. A. Inhibition of the interaction of Streptococcus sanguis with hexadecane droplets by 55- and 60-kilodalton hydrophobic proteins of human saliva. Infect Immun. 1986 Aug;53(2):278–284. doi: 10.1128/iai.53.2.278-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey E. J., Levine M. J., Reddy M. S., Bradway S. D., Al-Hashimi I. Use of the photoaffinity cross-linking agent N-hydroxysuccinimidyl-4-azidosalicylic acid to characterize salivary-glycoprotein-bacterial interactions. Biochem J. 1986 Feb 15;234(1):43–48. doi: 10.1042/bj2340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner C. A., Miller R. D., Arnold R. R. Effects of alpha-amylase on in vitro growth of Legionella pneumophila. Infect Immun. 1983 Jul;41(1):44–49. doi: 10.1128/iai.41.1.44-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- CURBY W. A. Device for collection of human parotid saliva. J Lab Clin Med. 1953 Mar;41(3):493–496. [PubMed] [Google Scholar]
- Carlsson J. Zooglea-forming streptococci, resembling Streptococcus sanguis, isolated from dental plaque in man. Odontol Revy. 1965;16(4):348–358. [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Classification and identification of the viridans streptococci. Clin Microbiol Rev. 1989 Jul;2(3):315–328. doi: 10.1128/cmr.2.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. W. The binding of human salivary alpha-amylase by oral strains of streptococcal bacteria. Arch Oral Biol. 1983;28(7):567–573. doi: 10.1016/0003-9969(83)90003-1. [DOI] [PubMed] [Google Scholar]
- Ericson D. Agglutination of Streptococcus mutans by low-molecular-weight salivary components: effect of beta 2-microglobulin. Infect Immun. 1984 Nov;46(2):526–530. doi: 10.1128/iai.46.2.526-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frank R. M., Brendel A. Ultrastructure of the approximal dental plaque and the underlying normal and carious enamel. Arch Oral Biol. 1966 Sep;11(9):883–912. doi: 10.1016/0003-9969(66)90080-x. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Gillard B. K. Quantitative gel-electrophoretic determination of serum amylase isoenzyme distributions. Clin Chem. 1979 Nov;25(11):1919–1923. [PubMed] [Google Scholar]
- Golub E. E., Cheruka J., Boosz B., Davis C., Malamud D. A comparison of bacterial aggregation induced by saliva, lysozyme, and zinc. Infect Immun. 1985 Apr;48(1):204–210. doi: 10.1128/iai.48.1.204-210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Embery G. The isolation and partial characterization of a sulphated glycoprotein from human whole saliva which aggregates strains of Streptococcus sanguis but not Streptococcus mutans. Arch Oral Biol. 1979;24(10-11):791–797. doi: 10.1016/0003-9969(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Numerical taxonomy and laboratory identification of Bacterionema matruchotii, Rothia dentocariosa, Actinomyces naeslundii, Actinomyces viscosus, and some related bacteria. J Gen Microbiol. 1973 May;76(1):43–63. doi: 10.1099/00221287-76-1-43. [DOI] [PubMed] [Google Scholar]
- Horii A., Emi M., Tomita N., Nishide T., Ogawa M., Mori T., Matsubara K. Primary structure of human pancreatic alpha-amylase gene: its comparison with human salivary alpha-amylase gene. Gene. 1987;60(1):57–64. doi: 10.1016/0378-1119(87)90213-7. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Shulkin J. D., Merritt A. D., Newell R. C. Evidence for post-transcriptional modification of human salivary amylase (amyl) isozymes. Biochem Genet. 1973 Dec;10(4):341–350. doi: 10.1007/BF00485989. [DOI] [PubMed] [Google Scholar]
- Karn R. C. The comparative biochemistry, physiology, and genetics of animal alpha-amylases. Adv Comp Physiol Biochem. 1978;7:1–103. doi: 10.1016/b978-0-12-011507-5.50007-0. [DOI] [PubMed] [Google Scholar]
- Kauffman D. L., Zager N. I., Cohen E., Keller P. J. The isoenzymes of human parotid amylase. Arch Biochem Biophys. 1970 Apr;137(2):325–339. doi: 10.1016/0003-9861(70)90446-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Rowland R. W., Switalski L. M., Hök M. Interactions of Bacteroides gingivalis with fibrinogen. Infect Immun. 1986 Dec;54(3):654–658. doi: 10.1128/iai.54.3.654-658.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. M., Virolle M. J., Chang S. Y., Chang S., Bibb M. J. alpha-Amylase gene of Streptomyces limosus: nucleotide sequence, expression motifs, and amino acid sequence homology to mammalian and invertebrate alpha-amylases. J Bacteriol. 1987 Dec;169(12):5745–5754. doi: 10.1128/jb.169.12.5745-5754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellersh A., Clark A., Hafiz S. Inhibition of Neisseria gonorrhoeae by normal human saliva. Br J Vener Dis. 1979 Feb;55(1):20–23. doi: 10.1136/sti.55.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Neuraminidase activity: a biochemical marker to distinguish Streptococcus mitis from Streptococcus sanguis. J Dent Res. 1984 Feb;63(2):111–113. doi: 10.1177/00220345840630020201. [DOI] [PubMed] [Google Scholar]
- Nishide T., Nakamura Y., Emi M., Yamamoto T., Ogawa M., Mori T., Matsubara K. Primary structure of human salivary alpha-amylase gene. Gene. 1986;41(2-3):299–304. doi: 10.1016/0378-1119(86)90110-1. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. D., McGeeney K. F. Purification and properties of an alpha-amylase inhibitor from wheat. Biochim Biophys Acta. 1976 Jan 23;422(1):159–169. doi: 10.1016/0005-2744(76)90016-4. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W. The acquired pellicle: immunofluorescent demonstration of specific proteins. J Oral Pathol. 1973;2(1):68–76. doi: 10.1111/j.1600-0714.1973.tb01675.x. [DOI] [PubMed] [Google Scholar]
- Pierrot M., Astier J. P., Abadie B., Marchis-Mouren G. Preliminary x-ray investigation on a new crystalline variety of porcine pancreatic alpha-amylase. FEBS Lett. 1977 Jul 1;79(1):105–108. doi: 10.1016/0014-5793(77)80360-8. [DOI] [PubMed] [Google Scholar]
- Prakobphol A., Levine M. J., Tabak L. A., Reddy M. S. Purification of a low-molecular-weight, mucin-type glycoprotein from human submandibular-sublingual saliva. Carbohydr Res. 1982 Oct 1;108(1):111–122. doi: 10.1016/s0008-6215(00)81896-0. [DOI] [PubMed] [Google Scholar]
- Rogers J. C. Conserved amino acid sequence domains in alpha-amylases from plants, mammals, and bacteria. Biochem Biophys Res Commun. 1985 Apr 16;128(1):470–476. doi: 10.1016/0006-291x(85)91702-4. [DOI] [PubMed] [Google Scholar]
- Rosan B., Malamud D., Appelbaum B., Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Nagata K., Nakamura R., Tsunemitsu A., Misaki A. Interaction of parotid saliva basic glycoprotein with Streptococcus sanguis ATCC 10557. J Periodontol. 1980 Sep;51(9):499–504. doi: 10.1902/jop.1980.51.9.499. [DOI] [PubMed] [Google Scholar]
- Shomers J. P., Tabak L. A., Levine M. J., Mandel I. D., Hay D. I. Properties of cysteine-containing phosphoproteins from human submandibular-sublingual saliva. J Dent Res. 1982 Feb;61(2):397–399. doi: 10.1177/00220345820610020601. [DOI] [PubMed] [Google Scholar]
- Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977 May;85(4):247–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanberg M., Krasse B. Oral implantation of saliva-treated Streptococcus mutans in man. Arch Oral Biol. 1981;26(3):197–201. doi: 10.1016/0003-9969(81)90130-8. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Nakayama T., Kitamura M., Endo Y., Kobata A. Structural studies of the sugar chains of human parotid alpha-amylase. J Biol Chem. 1980 Jun 25;255(12):5635–5642. [PubMed] [Google Scholar]