Abstract
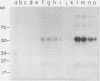
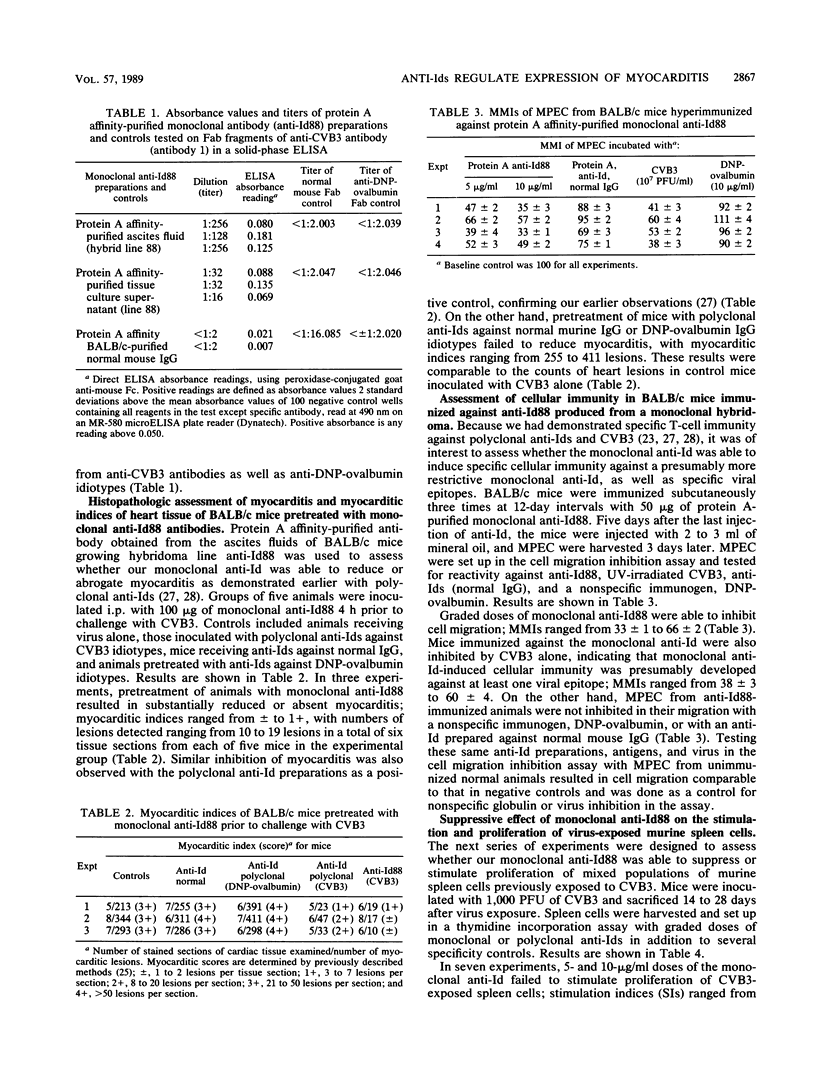
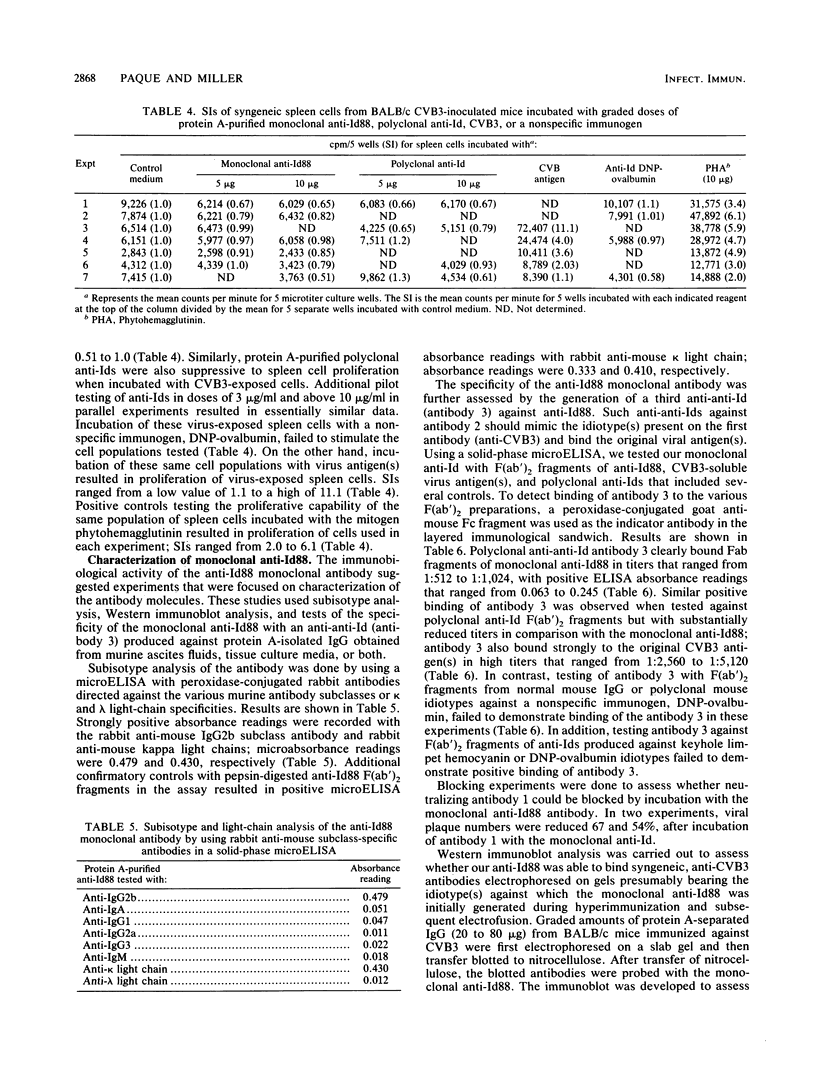
A monoclonal anti-idiotypic antibody (anti-Id), produced by electrofusion and designated anti-Id88, was able to modulate expression of murine autoimmune myocarditis mediated by coxsackievirus B3 (CVB3). The anti-Id was characterized as an immunoglobulin G2b species possessing kappa light chains and was able to reduce expression of inflammatory myocarditis in anti-Id-pretreated mice challenged with CVB3. Anti-Id88 was able to stimulate specific cell-mediated immunity against anti-Id88, as well as CVB3, and exerted a suppressive effect on the proliferation of mixed spleen cell populations from virus-exposed mice. Anti-Id stimulated an anti-anti-Id antibody 3 population able to bind antibody 2 F(ab')2 fragments or virus antigen in an indirect enzyme-linked immunosorbent assay. Western blot (immunoblot) analysis of anti-Id88 exhibited binding of syngeneic anti-Id antibody to idiotypes present on immunoglobulin G molecules from virus-immunized mice.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger U., Scheuber P. H., Sailer-Kramer B., Bartsch K., Hartmann A., Beck G., Hammer D. K. Anti-idiotypic antibodies that inhibit immediate-type skin reactions in unsensitized monkeys on challenge with staphylococcal enterotoxin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7054–7058. doi: 10.1073/pnas.83.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrand H., Källen B. On the statistical evaluation of the macrophage migration inhibition assay. Scand J Immunol. 1973;2(2):173–187. doi: 10.1111/j.1365-3083.1973.tb02028.x. [DOI] [PubMed] [Google Scholar]
- Bona C. A., Heber-Katz E., Paul W. E. Idiotype-anti-idiotype regulation. I. Immunization with a levan-binding myeloma protein leads to the appearance of auto-anti-(anti-idiotype) antibodies and to the activation of silent clones. J Exp Med. 1981 Apr 1;153(4):951–967. doi: 10.1084/jem.153.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Moran T. Idiotype vaccines. Ann Inst Pasteur Immunol. 1985 May-Jun;136C(3):299–312. doi: 10.1016/s0769-2625(85)80002-7. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Giles T. D. The role of viruses in the production of heart disease. Am J Cardiol. 1972 Feb;29(2):231–240. doi: 10.1016/0002-9149(72)90634-0. [DOI] [PubMed] [Google Scholar]
- Cazenave P. A. Idiotypic-anti-idiotypic regulation of antibody synthesis in rabbits. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5122–5125. doi: 10.1073/pnas.74.11.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci G., Beazer Y., Waksal S. D. Interactions between hepatitis B virus and polymeric human serum albumin. II. Development of syngeneic monoclonal anti-anti-idiotypes which mimic hepatitis B surface antigen in the induction of immune responsiveness. Eur J Immunol. 1987 Mar;17(3):371–374. doi: 10.1002/eji.1830170311. [DOI] [PubMed] [Google Scholar]
- Dreesman G. R., Kennedy R. C. Anti-idiotypic antibodies: implications of internal image-based vaccines for infectious diseases. J Infect Dis. 1985 May;151(5):761–765. doi: 10.1093/infdis/151.5.761. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fenner M., Siegmann K., Binz H. Monoclonal antibodies specific for Sendai virus. II. Production of monoclonal anti-idiotypic antibodies. Scand J Immunol. 1986 Sep;24(3):341–349. doi: 10.1111/j.1365-3083.1986.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Sharpe A. H., Chang D. W., Fields B. N., Greene M. I. Syngeneic monoclonal internal image anti-idiotopes as prophylactic vaccines. J Immunol. 1986 Nov 1;137(9):2930–2936. [PubMed] [Google Scholar]
- Gell P. G., Moss P. A. Production of cell-mediated immune response to herpes simplex virus by immunization with anti-idiotypic heteroantisera. J Gen Virol. 1985 Aug;66(Pt 8):1801–1804. doi: 10.1099/0022-1317-66-8-1801. [DOI] [PubMed] [Google Scholar]
- Hiernaux J. R. Idiotypic vaccines and infectious diseases. Infect Immun. 1988 Jun;56(6):1407–1413. doi: 10.1128/iai.56.6.1407-1413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiernaux J. R., Marvel J., Meyers P., Moser M., Leo O., Slaoui M., Urbain J. Study of idiotopic suppression induced by anti-cross-reactive idiotype monoclonal antibody in the anti-p-azophenylarsonate antibody response. J Immunol. 1986 Mar 15;136(6):1960–1967. [PubMed] [Google Scholar]
- Jerne N. K., Roland J., Cazenave P. A. Recurrent idiotopes and internal images. EMBO J. 1982;1(2):243–247. doi: 10.1002/j.1460-2075.1982.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kawai C., Matsumori A., Kumagai N., Tokuda M. Experimental Coxsackie virus B-3 and B-4 myocarditis in mice. Jpn Circ J. 1978 Jan;42(1):43–47. doi: 10.1253/jcj.42.43. [DOI] [PubMed] [Google Scholar]
- Kennedy R. C., Adler-Storthz K., Burns J. W., Sr, Henkel R. D., Dreesman G. R. Antiidiotype modulation of herpes simplex virus infection leading to increased pathogenicity. J Virol. 1984 Jun;50(3):951–953. doi: 10.1128/jvi.50.3.951-953.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. C., Eichberg J. W., Lanford R. E., Dreesman G. R. Anti-idiotypic antibody vaccine for type B viral hepatitis in chimpanzees. Science. 1986 Apr 11;232(4747):220–223. doi: 10.1126/science.3952505. [DOI] [PubMed] [Google Scholar]
- McNamara M. K., Ward R. E., Kohler H. Monoclonal idiotope vaccine against Streptococcus pneumoniae infection. Science. 1984 Dec 14;226(4680):1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- Nisonoff A., Lamoyi E. Implications of the presence of an internal image of the antigen in anti-idiotypic antibodies: possible application to vaccine production. Clin Immunol Immunopathol. 1981 Dec;21(3):397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J. Assessment of cell-mediated immunity against coxsackievirus B3-induced myocarditis in a primate model (Papio papio). Infect Immun. 1981 Jan;31(1):470–479. doi: 10.1128/iai.31.1.470-479.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J., Trousdale M. D. Assessment of cell-mediated hypersensitivity against coxsackievirus B3 viral-induced myocarditis utilizing hypertonic salt extracts of cardiac tissue. J Immunol. 1978 May;120(5):1672–1678. [PubMed] [Google Scholar]
- Paque R. E., Kniskern P. J., Dray S., Baram P. In vitro studies with "transfer factor": transfer of the cell-migration inhibition correlate of delayed hypersensitivity in humans with cell lysates from humans sensitized to histoplasmin, coccidioidin, or PPD. J Immunol. 1969 Nov;103(5):1014–1021. [PubMed] [Google Scholar]
- Paque R. E., Miller R. Modulation of murine coxsackievirus-induced myocarditis utilizing anti-idiotypes. Viral Immunol. 1987;1(3):207–224. doi: 10.1089/vim.1987.1.207. [DOI] [PubMed] [Google Scholar]
- Paque R. E., Miller R. Polyclonal anti-idiotypes influence macrophage chemotaxis in coxsackievirus-induced murine myocarditis. J Leukoc Biol. 1989 Jan;45(1):79–86. doi: 10.1002/jlb.45.1.79. [DOI] [PubMed] [Google Scholar]
- Paque R. E., Straus D. C., Nealon T. J., Gauntt C. J. Fractionation and immunologic assessment of KCl-extracted cardiac antigens in coxsackievirus B3 virus-induced myocarditis. J Immunol. 1979 Jul;123(1):358–364. [PubMed] [Google Scholar]
- Reagan K. J. Modulation of immunity to rabies virus induced by anti-idiotypic antibodies. Curr Top Microbiol Immunol. 1985;119:15–30. doi: 10.1007/978-3-642-70675-2_2. [DOI] [PubMed] [Google Scholar]
- Rees A. D., Praputpittaya K., Scoging A., Dobson N., Ivanyi J., Young D., Lamb J. R. T-cell activation by anti-idiotypic antibody: evidence for the internal image. Immunology. 1987 Mar;60(3):389–393. [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Kirchhoff L. V., Hieny S., Sher A. Molecular mimicry of a carbohydrate epitope on a major surface glycoprotein of Trypanosoma cruzi by using anti-idiotypic antibodies. J Immunol. 1985 Dec;135(6):4155–4159. [PubMed] [Google Scholar]
- Sharpe A. H., Gaulton G. N., McDade K. K., Fields B. N., Greene M. I. Syngeneic monoclonal antiidiotype can induce cellular immunity to reovirus. J Exp Med. 1984 Oct 1;160(4):1195–1205. doi: 10.1084/jem.160.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Nealon T., Gauntt C. J. Assessment of coxsackievirus B3 ts mutants for induction of myocarditis in a murine model. Infect Immun. 1979 Feb;23(2):486–495. doi: 10.1128/iai.23.2.486-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Voltage modulation of membrane permeability and energy utilization in cells. Biosci Rep. 1983 Jun;3(6):487–505. doi: 10.1007/BF01120693. [DOI] [PubMed] [Google Scholar]
- Urbain J., Wikler M., Franssen J. D., Collignon C. Idiotypic regulation of the immune system by the induction of antibodies against anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5126–5130. doi: 10.1073/pnas.74.11.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. M., Ward R. E., Huang J. H., Kohler H. Idiotope vaccine against Streptococcus pneumoniae. A precursor study. J Immunol. 1987 Oct 15;139(8):2775–2780. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. III. Role of sex. J Immunol. 1977 Aug;119(2):591–597. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]