Abstract
Hypoxia ischemia (HI; reduced blood oxygenation and/or flow to the brain) represents one of the most common injuries for both term and preterm/very low birth weight (VLBW) infants. These children experience elevated incidence of cognitive and/or sensory processing disabilities, including language based learning disabilities. Clinical data also indicate more substantial long-term deficits for HI injured male babies as compared to HI injured females. Previously, we reported significant deficits in rapid auditory processing and spatial learning in male rats with postnatal day 1 (P1), P7, or P10 HI injury. We also showed sex differences in HI injured animals, with more severe deficits in males as compared to females. Given these findings, combined with extant clinical data, the current study sought to assess a putative role for perinatal testosterone in modulating behavioral outcome following early hypoxic-ischemic injury in rats. Male, female, and testosterone-propionate (TP) treated females were subjected to P7 HI or sham surgery, and subsequently (P30+) underwent a battery of auditory testing and water maze assessment. Results confirm previous reports of sex differences following HI, and add new findings of significantly worse performance in TP-treated HI females compared to vehicle treated HI females. Post mortem anatomic analyses showed consistent effects, with significant brain weight decreases seen in HI male and TP-treated HI females but not female HI or sham groups. Further neuromorphometric analysis of brain structures showed that HI male animals exhibited increased pathology relative to HI females as reflected in ventricular enlargement. Findings suggest that neonatal testosterone may act to enhance the deleterious consequences of early HI brain injury, as measured by both neuropathology and behavior.
Keywords: HI, language impairment, prematurity, auditory processing
1. Introduction
Perinatal hypoxic-ischemic injury is a major cause of brain damage in the premature/very low birth weight (VLBW) infant (<1500g), as well as in infants suffering from birth trauma (see Fatemi et al., 2009 for review). In the premature brain, HI is often due to the vulnerability of the underdeveloped and extremely fragile nature of the neural vascular system (Boylan et al., 2000), which can lead to hemorrhagic injury (termed intraventricular (IVH) or periventricular hemorrhage (PVH); Volpe, 2001). As a result of intracranial bleeding, brain tissue is compressed, further limiting its blood and oxygen supply (see Scafidi et al., 2009 for review). Non-hemorrhagic HI injuries may also be seen, including re-perfusion failure and ischemic injury resulting in periventricular leukomalacia (PVL). Also prevalent in severely premature infants, PVL is associated with loss of white matter surrounding the cerebral ventricles (see Takashima et al., 2009 for review). Risk of HI injury and neurological impairment increase with decreasing gestational age at birth (Dudink et al., 2007; Nosarti et al., 2008;see Scafidi et al., 2009 for review).
In term infants, hypoxic-ischemic incidents related to birth complications and trauma result in a different pattern of neuropathology as compared to that seem in preterm children, due to the greater vulnerability of gray matter to excitotoxicity at this age (Jensen, 2002). Thus term HI more typically manifests as damage to gray matter structures such as the thalamus and basal ganglia (Sie et al., 2000; see McClean et al., 2004 and Fatemi et al., 2009 for review).
Hypoxic-ischemic insult to the developing brain also results in long-lasting behavioral consequences. Specifically, 25–50% of VLBW infants later show cognitive and behavioral deficits (Volpe, 2001; see van Handel et al, 2007 for review), including delayed language acquisition (Casiro et al., 1990) and deficits in verbal and language domains (Marlow et al., 2005). Term infants suffering from HI also show decreased language skills (Robertson and Finer, 1985), and display lower verbal and performance IQs (Steinman et al., 2009). Auditory processing deficits (suggested to be both predictive of and possibly causal to later language related impairments) have also been reported in premature babies (Benasich and Tallal, 2002; Benasich, 2002; Downie et al., 2002; Benasich et al., 2006; Choudhury et al., 2007). Specifically, rapid auditory processing (RAP; or the ability to detect brief changes in sound stimuli, speech, or non-speech stimuli changing within tens of milliseconds) is critical to accurate speech processing, and early impairments in RAP have been associated with cascading deleterious effects on subsequent language development (Sie et al., 2000; Benasich et al., 2002; see Fitch and Tallal, 2003 for review; Choudhury et al., 2007). Previous research has reliably measured this ability in animals, and tests of RAP in male rats have revealed deficits in subjects with induced neonatal brain injuries including perinatal hypoxia ischemia (McClure et al., 2005a, 2005b, 2006a, 2006b, 2007), microgyria (small induced cortical malformations (Peiffer et al., 2001, 2002, 2004; Threlkeld et al., 2006, 2007), and prenatal teratogenic exposure (Threlkeld et al., 2009). These findings suggest that RAP indices may serve as a marker of neural disruptions associated with language-based impairments.
Clinical data also reveal sex differences in the incidences of several brain-based developmental disorders, with most having higher prevalence for males than females (Donders and Hoffman, 2002; Rutter et al., 2003; Tioseco et al., 2006; Lauterbach et al., 2008; Raz et al., 2010). For example, males show a higher incidence of prematurity, anoxia, intraventricular hemorrhage, and infection, as well as increased mortality from prematurity or stillbirth (Gualtieri and Hicks, 1985; Donders and Hoffman, 2002; Tioseco et al., 2006; Lauterbach et al., 2008; Raz et al., 2010), and evidence suggests female premature/VLBW babies may be at an advantage as compared to their male counterparts. Specifically, IQ measures taken at 4–5 years of age revealed that premature males with ICH fell significantly below matched female counterparts with comparable ICH injury on full scale, verbal, and performance IQ (although no differences in IQ measures were found between matched male and female premature control groups without ICH; Raz et al, 1995). Clinically, boys suffer more long-term cognitive deficits, and are more likely than girls to have speech and language disorders (including stutter and dyslexia), as well as learning disabilities (Gualtieri and Hicks, 1985; Donders and Hoffman, 2002; Lauterbach et al., 2008).
The causal factor(s) underlying clinical evidence of heightened vulnerability to cognitive impairments associated with early neurodevelopmental disruption in males remains unknown, but could relate to the substantially higher levels of plasma testosterone in neonatal males. In humans, fetal males show evidence of increased testosterone production relative to females as early as 8 weeks gestation, and plasma levels remain markedly elevated through the first year of life, after which levels drop until the start of puberty (Knickmeyer and Baron-Cohen, 2006). Substantial evidence has shown that this early —surge in testosterone production is tied to the masculinization of subsequent neural morphology and behavior (Knickmeyer and Baron-Cohen, 2006). To examine the question of whether poorer outcomes following early brain injury in infant males may relate to this early testosterone exposure, the current study examined well-studied and replicated deficits in behavioral performance caused by early HI injury in male rats, and assessed whether similar HI effects would be seen in females, as well as females treated with early testosterone propionate. This paradigm followed evidence that plasma testosterone in fetal male rats is significantly higher than female littermates beginning at embryonic day 18 (E18; until approximately postnatal day 5; Weisz and Ward, 1980), coupled with substantial evidence that a postnatal regimen of testosterone exposure in female rats leads to masculinization of numerous measures of brain and behavior (McCarthy, 1994; Clark and Goldman-Rakic, 1989; Rosen et al., 1999). We hypothesized that treating female rat pups with testosterone propionate (P1–5), prior to the induction of HI, would lead to poorer performance on behavioral tasks, as well as increased brain damage (comparable to that typically seen in male HI rats).
2. Methods
2.1 Subjects
Time-mated female Wistar rats were ordered from Charles River Laboratories and shipped on embryonic day 5 (E5) to minimize prenatal stress. Dams were housed in the University of Connecticut animal facility in a 12-h light/dark cycle where they gave birth. Pups were culled to litters of five males and five females on P1. All pups received subcutaneous injections of either 0.05ml sesame oil, or 0.1mg TP in 0.05ml sesame oil, on the mornings of postnatal days 1–5 and underwent HI or sham surgery on P7 (for N’s see Table 1). This regimen of TP administration is well-established as leading to masculinization of female morphology and behavior in rodents (Clark and Goldman-Rakic, 1989; Rosen et al., 1999; Knickmeyer and Baron-Cohen, 2006), with doses ranging from 100μg to 1mg on postnatal days 1 through 7 leading to male typical behavior (i.e., mounting; Zuloaga et al., 2009) and brain anatomy (i.e., SDN-POA size and SBN cell number and size; Reuben et al., 1990; Jacobson et al., 1981; Fishman and Breedlove, 1985), as well as a reduction in female typical behavior (i.e., lordodic response; Diaz et al., 1995) in TP-treated female rats. Due to concerns for maternal exposure and contamination of milk, treatment with TP or vehicle (oil) was assigned between litters.
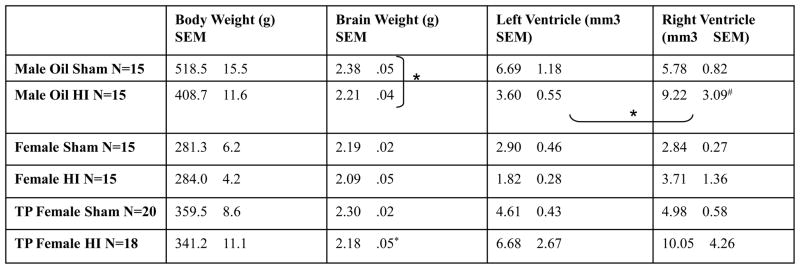
Table 1.
Mean body weight (g) at time of perfusion (~P110) is presented in column 2. Brain weight (g) was taken just after perfusion and is presented in column 3. Significant comparisons (p < .05) between HI and sham counterparts are marked with stars (*). Left and right ventricle size as measured using Cavalieri’s Estimator of volume is presented in column 4 and 5 respectively. Significant comparisons (p < .05) between left and right ventricular volumes are marked with stars (*).
|
2.2 Induction of hypoxia-ischemia
On P7, pups were randomly selected for sham or HI procedure (balanced within litter). At surgery, HI selected pups were anesthetized with isoflurane (2.5%), and a longitudinal midline incision was made in the neck. The right common carotid artery was located, separated from surrounding tissue, and completely cauterized. The incision was sutured, footpad marking injections were made, and pups were returned to dams after recovering from anesthesia under a warming lamp. Approximately two hours after recovery (allowing time to feed), pups were placed under a warming lamp in an air-tight chamber containing 8% humidified oxygen (balanced with nitrogen) for 120 minutes. Sham animals underwent the same procedure, excluding artery cauterization and hypoxia (shams were exposed to room air in an equivalent chamber for 120 minutes). All pups were returned to their mothers, where they remained housed until weaning on P21.
2.3 Behavioral testing: Startle Reduction
The startle reduction paradigm utilizes the subject’s acoustic startle reflex (ASR), a large motor reflex response to a startle eliciting stimulus (SES; 105dB white noise burst), coupled with a benign acoustic stimulus just prior to the SES on cued trials. Termed prepulse inhibition or startle reduction, this procedure provides an indirect measure of cue detectability based on the magnitude of startle attenuation elicited by the prepulse cue (see Fitch et al., 2008 for review). This procedure allows for analysis of the magnitude of the startle response on cued versus uncued trials as a function of cue properties (e.g., gap duration), thus providing a measure of detectability of the pre-SES cue.
2.3.1 Apparatus, auditory testing
During auditory testing, each subject was placed on a Med Associates PHM-252B load cell platform in an opaque polypropylene cage, in a quiet testing room. Output voltages from each platform were sent from a PHM-250-60 linear load cell amplifier to a Biopac MP100A-CE Acquisition system connected to a Power Macintosh G3. This apparatus recorded the amplitude of each subject’s startle reflex (150 ms) from the onset of the SES. The extracted peak value from this interval served as the subject’s response amplitude for that trial. Auditory stimuli were generated on a Pentium III Dell PC with custom programmed software and a Tucker Davis Technologies (RP2) real time processor, amplified by a Niles SI-1260 Systems Integration Amplifier and delivered through 10 Cambridge Soundworks MC100 loudspeakers placed 53 cm above the platforms. The SES was always a 105dB, 50 ms burst of white noise.
2.3.2 Normal Single Tone (NST, P25)
On cued trials, subjects were presented with a single 75dB, 7 ms, 2300Hz tone followed 50 ms later by a 105dB, 50 ms SES. On uncued trials, only the 105dB SES was presented. The attenuated response (ATT; cued score/uncued score × 100) served as a measure of detection of the cue, with higher scores indicating poorer detection (100% = chance). NST measures thus provide a baseline measure of startle attenuation, which can be used to confirm intact hearing and startle circuitry.
2.3.3 Silent Gap (SG, P27–30)
The gap detection task involved 300 trials of randomly presented silent gaps embedded in a continuous broadband white noise 75dB background. The Silent Gap 0–100 task featured gaps of 2, 5, 10, 20, 20, 40, 50, 75, and 100 ms. The gap, serving as the cue, was presented 50 ms prior to the SES on cued trials, while there were no gaps (gap duration = 0 ms) on uncued trials. ITIs of 16, 18, 22, or 24 seconds randomly separated each trial.
2.4 Behavioral Testing, water maze, water escape
Water maze testing began with a water escape task on Day 1 (P86). Each rat was released at the end of an oval water filled tub and had to swim to the opposite end where an escape platform was visible. If the rat did not swim to the platform after 45 seconds, it was guided to the platform, where it remained for 10 seconds. This procedure was followed for each animal in order to acclimate to water tests, as well as to assess any group differences in baseline motor (swimming) behavior.
For all remaining water maze tasks, a Sony Handycam DCR-TRV280 camera mounted above the pool recorded and translated the path taken, as well as the time to the platform (latency), using a Smart Video Tracking System Version 2.5 on a Dell Dimension E521 computer.
2.4.1 Morris water maze (MWM, P89–93)
The MWM is designed to assess spatial reference memory. This task was performed in a round tub filled with water at room temperature, with a submerged platform that remained in a fixed location. Extra-maze cues around the room included large black shapes painted on the surrounding walls, and the doorframe. To begin, each rat was placed into the maze at one of four start positions (N, S, E, or W quadrant wall), where it was required to swim until it found the hidden platform (or until 45 seconds elapsed). It was then returned to its cage under a warming lamp for 2–3 minutes before the next trial. On the 3 remaining trials, the rat was placed into the maze from one of the remaining starting points. This procedure was repeated over 5 days, and employs spatial navigation and memory.
2.4.2 Non-spatial water maze (NSM, P96–100)
Non-spatial maze testing was similar to MWM, however, the location where the rat was placed into the pool remained constant whereas the escape platform was paired with one of four intra-maze visual cues (vertical lines, horizontal lines, white dots on a black background, or black dots on a white background) painted on a circular insert placed just inside the outer wall of the maze tub. The location of the platform, along with the position of the associated cue, were randomly varied (NW, NE, SW, SE quadrants). The escape platform was consistently paired with the same intra-maze cue (vertical lines), and both the platform and the cue changed locations together throughout the 4 trials per day, for 5 days. The center of the platform was approximately 10 inches from the perimeter of the maze wall where the associated cue was located, and animals had 45 seconds to find the platform before being guided to its location. This task was repeated over 5 days, and employs associative learning ability and memory (i.e., associating the platform with a specific cue regardless of spatial cues).
2.5 Statistical Analysis
Based on specific a priori hypotheses, planned comparisons were performed between specific groups. This includes separate analyses for sham and HI males, sham and HI females, and sham and TP-treated HI females. Planned comparisons between TP-treated HI females vs. male HI animals, and TP-treated HI females vs. oil-treated female HI animals were also conducted to specifically examine the effects of TP on response to HI injury.
The pre-pulse inhibition paradigm was used in this context to assess complex acoustic processing of cues in intact and injured animals. As such, it was important to ascertain that baseline differences in hearing, startle response, or simple pre-pulse inhibition did not contribute to reported differences between groups. Such effects would confound our interpretation regarding the effects of HI injury on more complex sound processing, and associated extension of the results to human clinical data from infants with early injuries and language problems. To specifically address the issue of complex acoustic processing — without the confounds of hearing, startle, or PPI differences — baseline single-tone ATT scores were used as a covariate in analyses of gap detection data. This means that any differences in gap processing as measured that were attributable to underlying differences in hearing, startle, or baseline PPI were removed from effects as reported. Although use of the covariate leads to a more conservative statistical test of group differences (by eliminating some of the between-group variance), we feel this procedure is critical to the interpretability of results — particularly since a Sex × Treatment effect on NST was found (possibly reflecting sex differences in hearing, startle, body weight/strength, or PPI). Although such effects may be of interest in another context, they are not the measure of interest in the current study. Thus, baseline differences in simple PPI were removed from further analyses through use of the NST as a covariate.
Multivariate analyses of variance (ANOVA) were used to analyze both auditory ATT scores and latency to the platform for water maze tasks. Variables presented in the Results section include: Sex (2 levels; male, female); Treatment (2 levels; HI, sham); Hormone (2 levels; TP, oil); Day (4 levels for auditory testing and 5 levels for maze testing); Gap (9 levels); and Task (2 levels; MWM, NSM). All analyses were conducted using SPSS 15.0 with an alpha criterion of .05. Data presented in auditory task graphs is presented as mean attenuation score (cued response/uncued response *100) ± SEM, with higher scores indicating poorer performance (100% = chance levels). Data presented in water maze task graphs is presented as mean latency to the platform ± SEM in seconds.
2.6 Histological Analysis
Upon the completion of behavioral testing, all animals were weighed and deeply anesthetized with an IP injection of a mix of ketamine and xylazine (100mg/Kg and 15mg/Kg), then transcardially perfused with .9% saline followed by 10% buffered formalin. Brains were removed from the skull, and post-fixed in 10% buffered formalin before being sent to Beth Israel Deaconess Medical Center, where they were weighed, embedded in celloidin, cut on a sliding microtome at 40 microns, stained with cresyl violet, and mounted on glass slides (every 10th section). The slides were returned to the University of Connecticut, where each slice was photographed under 1.3x magnification on a Fisher Scientific Micromaster digital microscope using Micron software, and analyzed (blind to treatment or sex) for damage and structural 3D volume indices for the cerebral ventricles. Measures were derived using a grid overlay and ImageJ software. Cavalieri’s point counting estimator of volume was used to estimate total volume of the left and right ventricles separately (see Mouton, 2002). One sham male was removed from the study following anatomical indication of hydrocephalus, and this is reflected in total N’s for Table 1.
3. Results
3.1 Normal Single Tone
Results of a univariate ANOVA performed for male and female oil-treated animals revealed no significant differences of Sex or Treatment. However, a significant Sex × Treatment interaction was found, [F (1,58) = 7.56, p < .01] (data not shown). In addition, a second univariate ANOVA for TP and oil-treated females revealed a nearly significant Treatment effect, [F (1,67) = 3.99, p = .05] (data not shown). These scores were used as a covariate in all further acoustic analyses.
3.2 Silent Gap 0–100
3.2.1 Sex Effects
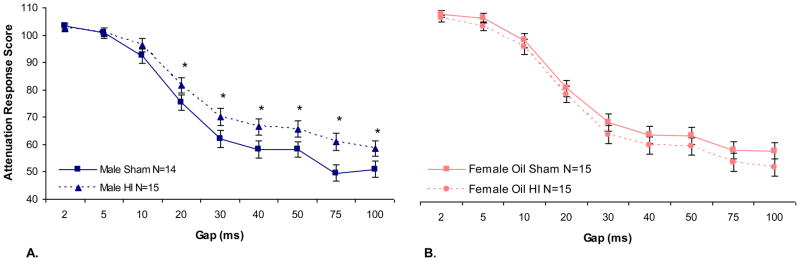
A repeated measures ANOVA performed on ATT scores across four days of testing for oil-treated male and female animals revealed no significant Sex or Treatment main effects. However, a Sex × Treatment interaction was found, [F (1,54) = 5.94, p < .05]. Separate analysis of male and female groups revealed a significant Treatment effect for males [F (1,26) = 6.47, p < .05], indicating poorer performance by HI males relative to sham males, but no effect of HI on females (see Figure 1, panels A and B respectively).
Figure 1. SG 0–100.
Panel A: No significant effects of Sex or Treatment were found on the SG 0–100 task (P27–30), [F (1,55) < 1, NS] and [F (1,55) < 1, NS] respectively. However, a significant Sex × Treatment interaction was found, [F (1,54) = 5.94, p < .05]. Separate analysis of male and female groups individually revealed an overall significant effect of Treatment for male animals, [F (1,26) = 6.47, p < .05] (HI worse than sham), significant differences between HI and sham males on gaps of 20–100 ms (*, p < .05) were also found. Panel B: No effect of Treatment was found for females animals, [F (1,27) < 1, NS].
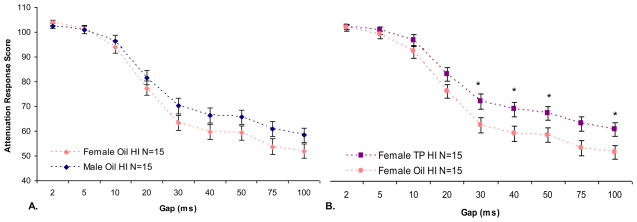
Next, a repeated measures ANOVA was performed on ATT scores across four days of testing for oil-treated male and female HI animals only. This analysis revealed a significant Gap × Sex interaction, [F (8,216) = 2.27, p < .05], indicating that early HI injury had a less detrimental effect on female animals (Figure 2A).
Figure 2. SG 0–100.
Panel A: Female HI animals performed significantly better than male HI animals (p < .05). Panel B: TP-treated HI females performed worse overall than oil-treated HI females (p < .05). Significant differences between oil and TP treated HI females on gaps of 30–50 ms and 100 ms (*p < .05) were also found.
3.2.2 Hormone Effects
A repeated measures ANOVA performed on ATT scores across four days of testing for TP and oil-treated females revealed no main effect of Treatment, Hormone, or a Treatment × Hormone interaction. However, a repeated measures ANOVA performed on ATT scores across four days of testing for TP and oil-treated HI females did reveal a main effect of Hormone, [F (1,27) = 4.92, p < .05] (TP-treated HI females worse than oil-treated HI females), and a significant Gap × Hormone interaction [F (8,216) = 2.70, p < .01] (Figure 2B). Results indicate that oil-treated HI female animals were better than TP-treated HI females at detecting gaps over 10 ms. Thus, early treatment with TP increased deleterious HI effects on females.
Finally, a repeated measures ANOVA performed on ATT scores across four days of testing for oil-treated HI males and TP-treated HI females revealed no effect of Treatment and no Treatment × Gap interaction. Results thus indicate that TP-treated female HI animals performed comparably to oil-treated male HI animals.
3.3 Water Maze Results
A pseudo-random subset of animals (balanced so that mean Silent Gap ATT scores reflected means from full groups) was taken from the total N for water maze testing. This use of a sub-sample was required due to time limitations. All individual groups (male sham, male HI, female sham, female HI, female TP HI, and female TP sham) had an N of 8, with a total N of 48 for maze tasks.
3.3.1 Water Escape
A univariate ANOVA performed on latency scores for male and female oil-treated animals revealed no significant effects of Sex or Treatment. Similarly, results of a one-way ANOVA performed on latency scores for TP-treated females revealed no significant effect of Treatment. These results indicate that all animals had intact and equivalent visual abilities and swimming capabilities.
3.3.2 Morris Water Maze
A repeated measures ANOVA performed on latency scores across five days of testing for male and female oil-treated animals revealed a significant main effect of Sex, [F (1,28) = 5.98, p < .05, (with males performing better, consistent with literature reports; see Jonasson, 2005 for review)], Treatment, [F (1,28) = 5.17, p < .05], and Day, [F (4,112) = 13.34, p < .001] (data not shown). Therefore, analyses were performed for male, female, and TP-treated female animals separately.
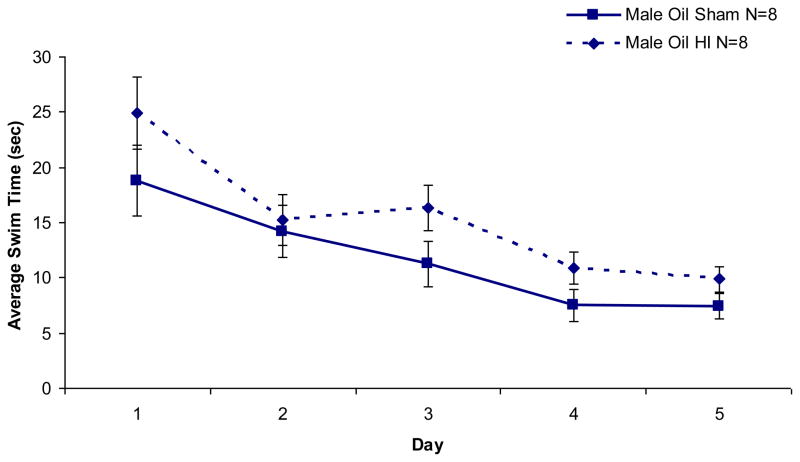
A repeated measures ANOVA of latency scores across five days of testing for male animals revealed a main effect of Treatment [F (1,14) = 3.81, p < .05 (one-tailed)] and Day [F (4,56) = 14.30, p < .001], indicating that male subjects performed better on each consecutive day of testing, with HI males significantly impaired as compared to sham males (see Figure 3). This same analysis performed for oil-treated female animals revealed a significant effect of Day [F (4,56) = 3.02, p < .05], but no effect of Treatment — indicating that both sham and HI female animals performed better on each consecutive day, but neither group performed better than the other (data not shown).
Figure 3. Morris Water Maze.
Male subjects performed better on each consecutive day of testing (p < .001), but HI males were significantly impaired overall as compared to sham males (p < .05, one-tailed). No effects of HI were seen for female oil-treated animals, although a p-level of .2 (with worse performance in TP-treated HI females) indicates a larger N may be needed to pull out any effect. Average latency (mean seconds ± SEM) across 5 days of testing follows: male HI 16.47 ± 1.50, male sham 12.95 ± 1.00, female HI 19.69 ± 1.83, female sham 16.72 ± 1.25, TP female HI 21.76 ± 3.01, TP female sham 16.42 ± 2.60.
A repeated measures ANOVA performed on latency scores across five days of testing for female TP and oil-treated animals revealed a significant effect of Day, [F (4,112) = 3.84, p < .01] (data not shown), indicating subjects performed better over days. Analyses were thus performed for oil and TP-treated female animals separately.
A repeated measures ANOVA performed on latency scores across five days of testing for TP-treated female animals revealed no significant effect for Treatment (although a p = .2 indicates that more subjects may be required to pull out any effect, if present). No Treatment effect was seen for oil-treated females.
3.3.3 Non-Spatial Water Maze
A repeated measures ANOVA performed on latency scores across five days of testing for male and female oil-treated animals revealed a significant main effect of Sex, [F (1,28) = 15.42, p = .001, (consistent with literature reports; see Jonasson, 2005 for review)] and Day, [F (4,112) = 4.65, p < .05] (data not shown). Therefore, analyses were performed for male, female, and TP-treated female animals separately.
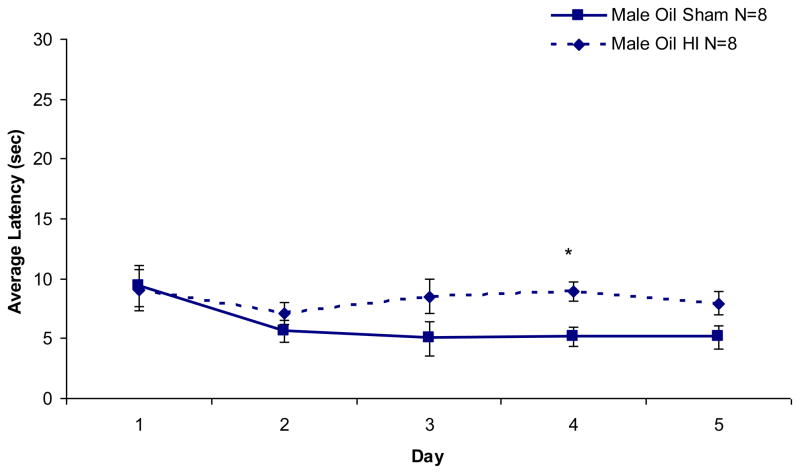
A repeated measures ANOVA performed on latency scores across five days of testing for male animals revealed a significant effect of Treatment, [F (1,14) = 4.39, p < .03 (one-tailed)], indicating HI males were significantly impaired as compared to sham males (see Figure 4). This same analysis performed for oil-treated females revealed a significant Day effect, [F (4,56) = 2.62, p < .05] (data not shown), but no effect of Treatment, indicating that both sham and HI female animals performed better on each consecutive day, but neither group performed better than the other.
Figure 4. Non-Spatial Water Maze.
Male subjects performed better on each consecutive day of testing (p < .05), but HI males were significantly impaired overall as compared to sham males (p < .05, one-tailed). No effects of HI were seen for oil or TP-treated female animals. Average latency (mean seconds ± SEM) across 5 days of testing: male HI 9.23 ± 0.86, male sham 6.92 ± 0.69, female HI 12.29 ± 1.59, female sham 12.47 ± 1.04, TP female HI 17.34 ± 3.45, TP female sham 14.66 ± 2.17.
A repeated measures ANOVA performed on latency scores across five days of testing for TP and oil-treated female animals revealed no significant effects. Likewise, no effect of Treatment was found for TP-treated female animals, though a significant Day effect was revealed, [F (4,56) = 2.60, p < .05] (data not shown), indicating TP females performed better on each consecutive day of testing, but HI and sham animals performed similarly.
4. Anatomy Results
4.1 Brain Weight
To determine Treatment and Hormone effects on brain weight, one-way ANOVA’s were performed for total brain weight within specific groups (male, females, and TP females). Results showed male HI brain weight (2.21g (mean) ± .04 (SEM)) to be significantly lower than that of male shams (2.38g ± .05), [F (1,7) = 6.62, p < .05; Table 1], while no differences were found between brain weights of oil-treated female HI (2.09g ± .05) and female sham animals (2.19g ± .02; see Table 1). Oil-treated female sham brain weight was significantly less than male sham brain weight; however, treatment with TP increased female sham brain weight to nearly equal to that of male shams. Similarly, brain weights were significantly different between TP-treated female HI (2.18g ± .05) and sham female animals (2.30g ± .02), [F (1,36) = 5.04, p < .05], with smaller brains in TP-treated HI animals (Table 1).
Further anatomical analyses of ventricular size (an indirect measure of pathology) were computed within in each group using the difference score (the amount of difference between the left and right ventricle), as measured from serial sections (ImageJ). A univariate ANOVA was computed using the difference score of the ventricular volume for oil-treated male and female animals, and revealed a significant Treatment effect, [F (1,54) = 5.50, p = <.05], with HI animals having larger difference scores. One-way ANOVA’s performed for oil-treated male and females groups independently revealed a significant effect of Treatment for male HI versus sham only, [F (1,27) = 4.23, p = <.05], indicating HI treatment caused an increase in the left-right ventricular volume difference in male animals only. Further simple effects analysis performed for male and female animals independently revealed significant differences between the left and right absolute ventricular volume (right larger than left) for male HI animals (p <.05, one-tailed), indicating induced HI injury lead to a significant expansion of the right ventricle in male animals only (Table 1).
A univariate ANOVA computed using the difference score of ventricular volumes for TP and oil-treated female animals did not reveal a significant Treatment or Hormone effect.
5. Discussion
Perinatal hypoxic-ischemic injury, and term-birth trauma resulting in HI, represent leading causes of brain damage in the neonatal population (Boylan et al., 2000; Volpe, 2001; for review see Fatemi et al., 2009 and Scafidi et al., 2009). Further, clinical data reveal that early events leading to brain damage (Gualtieri and Hicks, 1985; Donders and Hoffman, 2002; Lauterbach et al., 2008; Raz et al., 2010) and multiple brain-based disorders (e.g., learning and language disabilities; Tioseco et al., 2006) occur at higher incidence for males as compared to females. Sex differences in androgen levels represent one principal difference characterizing the male/female neonatal brain, with neonatal ovaries largely quiescent in the perinatal human female, but testicular androgens at near-pubertal levels in males (Knickmeyer and Baron-Cohen, 2006; McCarthy, 2008). This difference may account, at least in part, for sex differences seen in behavioral and neuropathological outcome following early HI injury.
In humans, the testes develop around gestational week (GW) 6, and detectable plasma levels of testosterone (from the testes and adrenals) are circulating in males by GW 8 (Knickmeyer and Baron-Cohen, 2006; Hines, 2008). Fetal testosterone production is highest from GW 10–20, with a subsequent postnatal surge during the first week of life (Knickmeyer and Baron-Cohen, 2006). Levels of plasma androgens in males remain high for the first year, and peak during the 3rd–4th month at plasma levels similar to the second stage of puberty (200–300ng/dL; Knickmeyer and Baron-Cohen, 2006). Research has shown that testosterone exerts intra-neuronal masculinizing effects on brain development via the P-450 enzyme, aromatase [see McCarthy, 2008 for review], with the intra-neuronal conversion of testosterone to 17-β estradiol allowing the estradiol synthesized within individual neurons to bind to estrogen receptors within the brain of both humans and rodents (MacLusky et al., 1985; see McCarthy, 1994 for review; Fitch and Denenberg, 1998).
In rats, elevated levels of testosterone are seen in males starting at E18 and lasting through P5 (Weisz and Ward, 1980). These differential levels of plasma testosterone may modulate sex differences in early response to neuronal disruption as measured by behavioral outcome. These effects may be mediated by organizational changes to the neural substrate rather than interaction with circulating hormone at the time of injury, since our injections ended on P5 and HI surgery occurred on P7. Evidence of such effects includes data showing that male rats and mice with neuronal disruptions including microgyria and ectopia, were found to be impaired on fast, but not slow, auditory discrimination tasks, even though females with identical injuries were able to perform both tasks successfully (Fitch et al., 1997; Peiffer et al., 2002, 2004). Anatomical analysis also revealed more small and fewer large neurons in the medial geniculate nuclei (MGN) of male rats with microgyria as compared to shams, while in female animals MGN neuronal size distribution did not differ (Herman et al., 1997). Interestingly, androgenizing female rat pups via P1–P5 injections of TP, followed by induction of microgyria, led to a shift in MGN neuronal size distribution similar to that seen in male microgyric rats. Conversely, the brain histology of vehicle-treated females undergoing microgyric surgery was found to be identical to sham females (Rosen et al., 1999). On tasks of rapid auditory processing, male BXSB/MpJ mice (Peiffer et al., 2002) and rats (Peiffer et al., 2004) with neonatal cortical malformations (ectopia and microgyria) were found to be significantly impaired as compared to female littermates with equivalent injury. Evidence of sex differences in response to brain injury has also been obtained in non-rodent species. In a classic study by Clark and Goldman-Rakic (1989), female rhesus monkeys with prefrontal cortical lesions were unimpaired on reversal learning, while male monkeys and females receiving testosterone (at differing ages) were significantly impaired following lesion when compared to shams. These collective findings suggest that the actions of perinatal gonadal steroids may mediate some of the sex differences seen following early injury to the cortex — a suggestion consistent with evidence that testosterone may enhance neuronal excitotoxicity in vitro (Yang et al., 2002).
5.1 Conclusions
Based on clinical data, coupled with prior data gathered from our lab indicating poorer performance by HI male rats as compared to HI female rats on tasks of auditory processing (preliminary studies; Hill et al., 2007), the current study sought to assess a potentially modulating role for perinatal testosterone relative to long-term behavioral response to hypoxic ischemic injury. We found: 1) evidence of more deleterious behavioral effects of early HI injury for male as compared to female subjects, replicating earlier reports (preliminary studies; Hill et al., 2007); 2) evidence of increased pathology as a result of HI injury in males relative to similarly treated female animals; and 3) new evidence that early exposure to high levels of testosterone propionate prior to HI injury causes females to exhibit both an increase in neuropathological damage as measured by reduced brain weight, and also larger behavioral deficits (characteristic of males) on an acoustic processing task.
Results from tests of RAP ability in the current study show that male HI animals were significantly worse at detecting silent gaps in broadband white noise as compared to sham counterparts, while both female HI and sham animals were able to perform this task equivalently. Interestingly, treatment with testosterone propionate prior to induced injury led HI females to show significantly impaired performance on the Silent Gap task compared to oil-treated counterparts. Indeed, this impaired performance by TP-treated HI females was equivalent to that of oil-treated HI males. Maze testing results also show a deficit in performance by HI males as compared to sham males for both MWM and the NSM, while female HI and sham animals performed the tasks equally well. However, treatment with TP produced no significant effects on water maze tasks in female HI animals (though a p = .2 suggests that TP effect size may be smaller than baseline sex differences in response to HI and might therefore require a larger N than was used). Anatomical analysis revealed decreased brain weight in both male HI and TP-treated female HI animals as compared to their sham counterparts, but no brain weight differences between oil-treated female HI and sham animals. Finally, only male HI animals showed increases in ventricular volume relative to sham counterparts. These cumulative findings show that TP-treated HI females had significant deficits in some aspects of behavioral performance (with failure to mimic TP-induced differences as seen for male HI animals on water maze tasks). Moreover, the neuropathological indices of brain weight and ventricular volume indicated effects mid-way between intact males and females, with significant effects on brain weight, but not ventricular size. Although it is evident that TP treatment had a detrimental effect on HI outcome for female animals, recent work exploring mechanisms of the apoptotic cascade suggest hormone levels may not be the only factor underlying sex differences in response to injury (see Lang and McCullough, 2008 and Renolleau et al., 2008 for review). Finally, because the developing brain responds to brain injury (or genetic disruption) through wide-spread, but subtle, alterations to the neurodevelopmental cascade, these disruptions are often more difficult to quantify as compared to more overt damage such as that caused by adult stroke. However, studies are underway to examine potential neural correlates to the behavioral deficits described here, such as changes in medial geniculate nucleus cell size (Herman et al., 1997).
The effects of administration of testosterone propionate to female rats in the current study — combined with comparable data such as Rosen (1999) and Clark and Goldman-Rakic (1989) — provide evidence for a detrimental effect of this predominantly male steroid hormone modulating early brain injury. Though mechanisms underlying these effects require further assessment, with a goal that the basis for “protection” in females might be better understood (and potentially adapted to males via neuroprotective treatment), it is evident that testosterone acts in some manner to exacerbate response to early hypoxic-ischemic injury in rats as measured by behavioral and neuropathological outcome. Because testosterone propionate is aromatizable to estrogen, the current study cannot determine whether these TP effects occur through androgenic or estrogenic mechanisms. This will be an issue for future studies, which must also account for species differences in aromatization as results are extrapolated to human clinical conditions (McCarthy, 2008). The current work holds important implications for future research on gender differences in response to early brain hemorrhages/HI injury, including the possible implementation of gender-specific neuroprotectants.
Acknowledgments
Thank you to the laboratory of Glenn D. Rosen at Beth Israel Deaconess Medical Center for histological preparation of brain tissue. This research was funded by NIH Grant HD049792.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benasich AA. Impaired processing of brief, rapidly presented auditory cues in infants with a family history of autoimmune disorder. Dev Neuropsychol. 2002;22(1):351–372. doi: 10.1207/S15326942dn2201_2. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136(1):31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Thomas JJ, Choudhury N, Leppanen PH. The importance of rapid auditory processing abilities to early language development: Evidence from converging methodologies. Dev Psychobiol. 2002;40(3):278–92. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44(3):396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan GB, Young K, Panerai RB, Rennie JM, Evans DH. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res. 2000;48:12–17. doi: 10.1203/00006450-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Casiro OG, Moddemann DM, Stanwick RS, Panikkar-Thiessen VK, Cowan H, Cheang MS. Language development of very low birth weight infants and fullterm controls at 12 months of age. Early Hum Dev. 1990;24(1):65–77. doi: 10.1016/0378-3782(90)90007-6. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Leppanen PH, Leever HJ, Benasich AA. Infant information processing and family history of specific language impairment: converging evidence for RAP deficits from two paradigms. Dev Sci. 2007;10(2):213–36. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Goldman-Rakic PS. Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behav Neurosci. 1989;103(6):1287–1295. doi: 10.1037//0735-7044.103.6.1287. [DOI] [PubMed] [Google Scholar]
- Donders J, Hoffman NM. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16(4):491–499. doi: 10.1037//0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- Diaz DR, Fleming DE, Rhees RW. The hormone-sensitive early postnatal periods for sexual differentiation of feminine behavior and luteinizing hormone secretion in male and female rats. Dev Brain Res. 1995;86:227–232. doi: 10.1016/0165-3806(95)00029-d. [DOI] [PubMed] [Google Scholar]
- Downie AL, Jakobson LS, Frisk V, Ushycky I. Auditory temporal processing deficits in children with periventricular brain injury. Brain Lang. 2002;80(2):208–225. doi: 10.1006/brln.2001.2594. [DOI] [PubMed] [Google Scholar]
- Dudink J, Lequin M, can Pul C, Buijs J, Conneman N, van Goudoever J, Govaert P. Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatr Radiol. 2007;37:1216–1223. doi: 10.1007/s00247-007-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi A, Wilson MA, Johnston MV. Hypoxic-Ischemic encephalopathy in the term infant. Clin Perinatol. 2009;36:835–858. doi: 10.1016/j.clp.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman RB, Breedlove SM. The androgenic induction of spinal sexual dimorphism is independent of supraspinal afferents. Dev Brain Res. 1985;23:255–258. doi: 10.1016/0165-3806(85)90047-1. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Brown CP, Tallal R, Rosen GD. Effects of sex and MK-801 on auditory-processing deficits associated with developmental microgyric lesions in rats. Behav Neurosci. 1997;111(2):404–412. doi: 10.1037//0735-7044.111.2.404. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Denenberg VH. A role for ovarian hormones in sexual differentiation of the brain. Behav Brain Sci. 1998;21:311–352. doi: 10.1017/s0140525x98001216. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Tallal P. Neural mechanisms of language-based learning impairments: insights from human populations and animal models. Behav Cog Neurosci Rev. 2003;2(3):155–178. doi: 10.1177/1534582303258736. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 2008;76(1–2):1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri T, Hicks RE. An immunoreactive theory of selective male affliction. Beh Brain Sci. 1985;8:427–441. [Google Scholar]
- Herman AE, Galaburda AM, Fitch RH, Carter AR, Rosen GD. Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cereb Cortex. 1997;7(5):453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- Hill CA, Threlkeld SW, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits associated with neonatal hypoxia-ischemia in rats. Society for Neuroscience; San Deigo, CA: Abstract. [Google Scholar]
- Hines M. Early androgen influences on human neural and behavioural development. Early Hum Dev. 2008;84:805–807. doi: 10.1016/j.earlhumdev.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CD, Valer JC, Shryne JE, Gorski RA. The influence of gonadectomy, androgen exposure, or a gonadal graft in the neonatal rat on the volume of the sexually dimorphic nucleus of the preoptic area. J Neurosci. 1981;1(10):1142–1147. doi: 10.1523/JNEUROSCI.01-10-01142.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. Int J Dev Neurosci. 2002;20:339–347. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences. Early Hum Dev. 2006;82:755–760. doi: 10.1016/j.earlhumdev.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6(33) doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm infants: the influence of sex and severity of respiratory distress on cognitive recovery. J Neuropsychiatry Clin Neurosci. 2008;20(4):409–418. [PubMed] [Google Scholar]
- MacLusky NJ, Philip A, Hurlburt C, Naftolin F. Estrogen formation in the developing rat brain: sex differences in aromatase activity during early post-natal life. Pyschoneuroendocrino. 1985;10(3):355–361. doi: 10.1016/0306-4530(85)90013-7. [DOI] [PubMed] [Google Scholar]
- Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005;90:F380–F387. doi: 10.1136/adc.2004.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Molecular aspects of sexual differentiation of the rodent brain. Psychoneuroendocrinology. 1994;19(5–7):415–427. doi: 10.1016/0306-4530(94)90029-9. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–134. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean C, Ferriero D. Mechanisms of hypoxic-ischemic injury in the term infant. Semin Perinatol. 2002;28:425–432. doi: 10.1053/j.semperi.2004.10.005. [DOI] [PubMed] [Google Scholar]
- McClure MM, Peiffer AM, Rosen GD, Fitch RH. Auditory processing deficits in rats with neonatal hypoxic-ischemic injury. Int J Dev Neurosci. 2005a;23(4):351–62. doi: 10.1016/j.ijdevneu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Auditory processing deficits in unilaterally and bilaterally injured hypoxic-ischemic rats. NeuroReport. 2005b;16(12):1309–1312. doi: 10.1097/01.wnr.0000175613.16183.6c. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Fitch RH. The effects of erythropoietin on auditory processing following neonatal hypoxic-ischemic injury. Brain Res. 2006a;1087:190–195. doi: 10.1016/j.brainres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Rapid auditory processing and learning deficits in rats with P1 versus P7 neonatal hypoxic-ischemic injury. Behav Brain Res. 2006b;172(1):114–21. doi: 10.1016/j.bbr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Fitch RH. Auditory processing and learning/memory following erythropoietin administration in neonatally hypoxic-ischemic injured rats. Brain Res. 2007;1132(1):203–209. doi: 10.1016/j.brainres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Mouton R The Johns Hopkins University Press. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. Baltimore: 2002. The Cavalieri point-counting method; pp. 97–101. [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Chitnis X, Williams SC, Murray RM. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131:205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Dunleavy CK, Frenkel M, Gabel LA, LoTurco JJ, Rosen GD, Fitch RH. Impaired detection of variable duration embedded tones in ectopic NZB/BINJ mice. Neuroreport. 2001;12(13):2875–2879. doi: 10.1097/00001756-200109170-00024. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in ectopic BXSB/MpJ mice. Neuroreport. 2002;13(17):2277–2280. doi: 10.1097/00001756-200212030-00021. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in microgyric rats. Brain Res Dev Brain Res. 2004;148(1):53–57. doi: 10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Raz S, Lauterbach MD, Hopkins TL, Glogowski BK, Porter CL, Riggs WW, Sander CJ. A female advantage in cognitive recovery from early cerebral insult. Dev Psychol. 1995;31(6):958–966. [Google Scholar]
- Raz S, Debastos AK, Newman JB, Batton D. Extreme prematurity and neuropsychological outcome in the preschool years. J Int Neuropsychol Soc. 2010;16(1):169–179. doi: 10.1017/S1355617709991147. [DOI] [PubMed] [Google Scholar]
- Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14(1):46–52. doi: 10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Bran Res Dev Brain Res. 1990;52(1–2):17–23. doi: 10.1016/0165-3806(90)90217-m. [DOI] [PubMed] [Google Scholar]
- Robertson C, Finer N. Term infants with hypoxic-ischemic encephalopathy: outcome at 3.5 years. Dev Med Child Neurol. 1985;27:473–484. doi: 10.1111/j.1469-8749.1985.tb04571.x. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Herman AE, Galaburda AM. Sex differences in the effects of early neocortical injury on neuronal size distribution of the medial geniculate nucleus in the rat are mediated by perinatal gonadal steroids. Cereb Cortex. 1999;9:27–34. doi: 10.1093/cercor/9.1.27. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry. 2003;44(8):1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Scafidi J, Fagel DM, Ment LR, Vaccarino FM. Modeling premature brain injury and recovery. Int J Devl Neuroscience. 2009;27:863–871. doi: 10.1016/j.ijdevneu.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie LT, van der Knapp MS, Oosting J, de Vries LS, Lafeber HN, Valk J. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics. 2000;31:128–136. doi: 10.1055/s-2000-7496. [DOI] [PubMed] [Google Scholar]
- Steinman KJ, Gorno-Tempini ML, Glidden DV, Kramer JH, Miller SP, Barkovich AJ, Ferriero DM. Neonatal watershed brain injury on MRI correlates with verbal IQ at four years. Pediatrics. 2009;123(3):1025–1030. doi: 10.1542/peds.2008-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Itoh M, Oka A. A history of our understanding of cerebral vascular development and pathogenesis of perinatal brain damage over the past 30 years. Semin Pediatr Neurol. 2009;16:226–236. doi: 10.1016/j.spen.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, McClure MM, Rosen GD, Fitch RH. Developmental timeframes for induction of microgyria and rapid auditory processing deficits in the rat. Brain Res. 2006;1109(1):22–31. doi: 10.1016/j.brainres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, McClure MM, Bai J, Wang Y, LoTurco JJ, Rosen GD, Fitch RH. Developmental disruptions and behavioral impairments in rats following in utero RNAi of Dyx1c1. Brain Res Bull. 2007;71(5):508–514. doi: 10.1016/j.brainresbull.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Hill CA, Clearly CE, Truong DT, Rosen GD, Fitch RH. Developmental learning impairments in a rodent model of nodular heterotopia. J Neurodevelop Disord. 2009;1:237–250. doi: 10.1007/s11689-009-9026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tioseco JA, Aly H, Essers J, Patel K, El-Mohandes AAE. Male sex and intraventricular hemorrhage. Pediatr Crit Care Med. 2006;7(1):40–44. doi: 10.1097/01.pcc.0000192341.67078.61. [DOI] [PubMed] [Google Scholar]
- van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. 2007;166:645–654. doi: 10.1007/s00431-007-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Hypoxic-ischemic encephalopathy and intracranial hemorrhage. In: Volpe JJ, editor. Neurology of the Newborn. Saunders; Philadelphia: 2001. pp. 217–496. [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106(1):306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Jordan CL, Breedlove SM. Sexual differentiation of the brain. Encyclo Neurosci. 2009:745–750. [Google Scholar]