Abstract
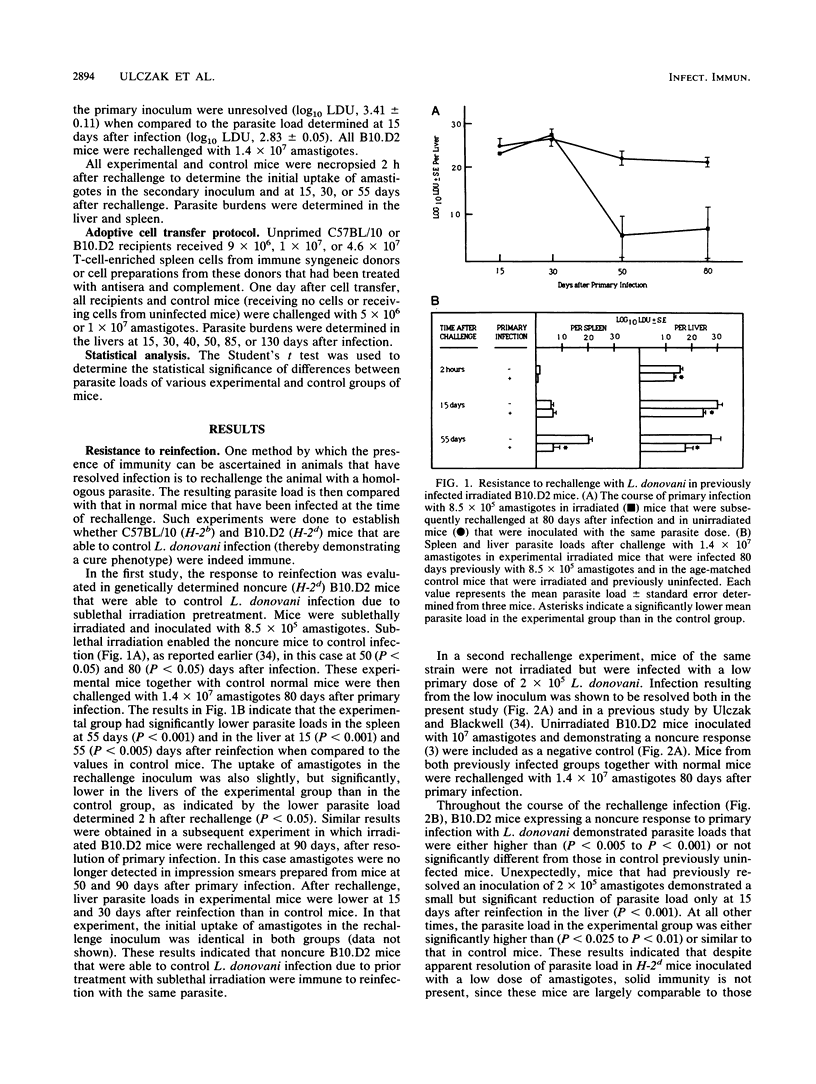
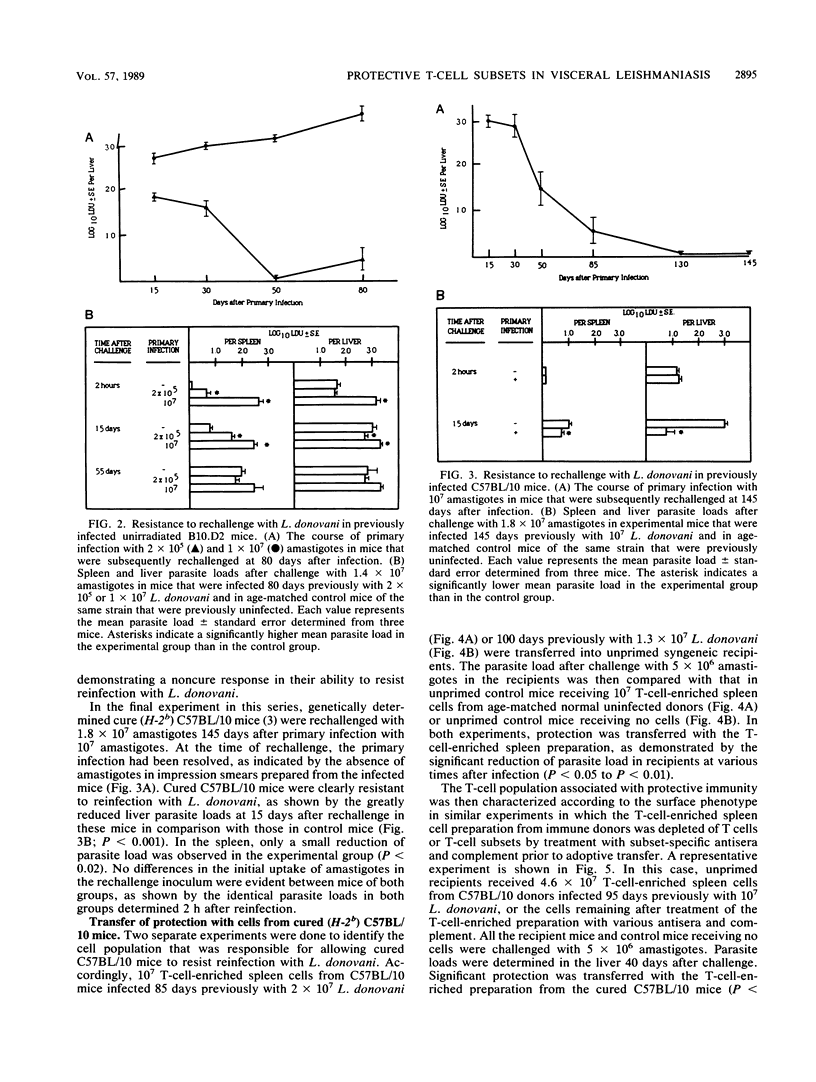
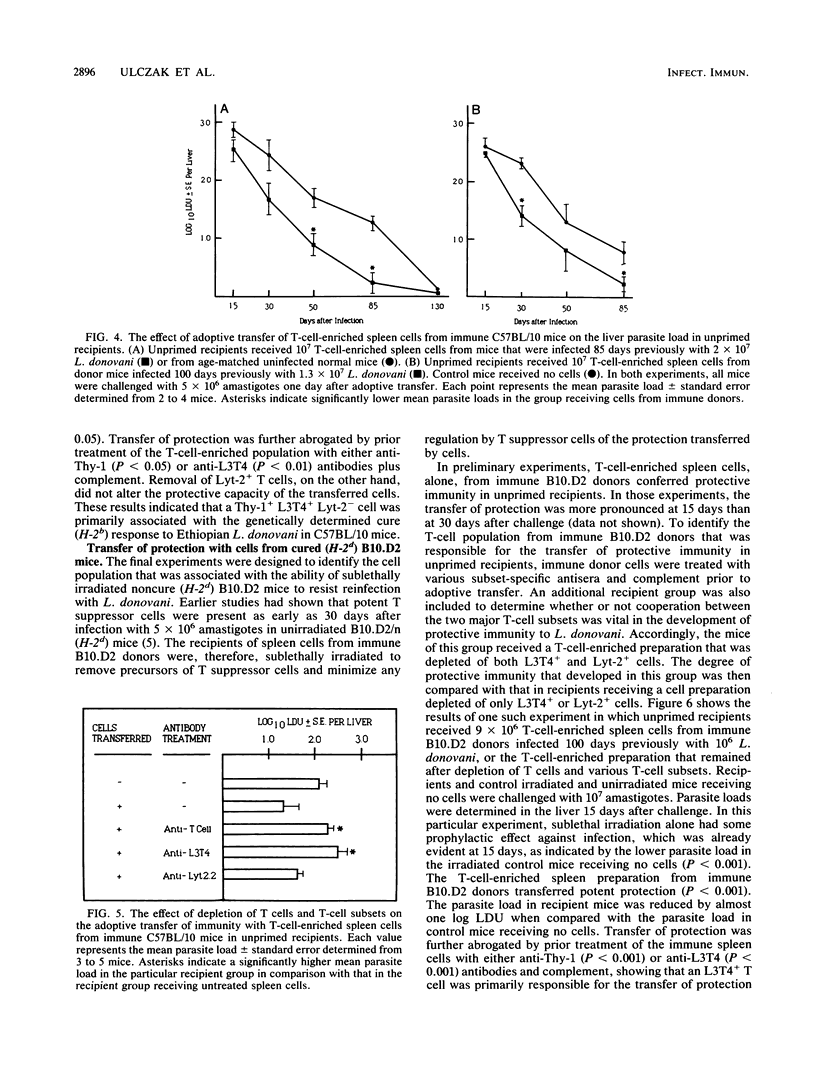
The response to reinfection with Ethiopian Leishmania donovani was evaluated in genetically determined noncure (H-2d) B10.D2 mice that are able to resolve infection due to sublethal irradiation pretreatment after inoculation with a low parasite dose and in C57BL/10 mice that demonstrate the genetically determined cure (H-2b) response to L. donovani. It was found that after resolution of primary infection, C57BL/10 (cure) mice and sublethally irradiated B10.D2 (noncure) mice were resistant to rechallenge with L. donovani. Noncure mice inoculated with a low dose of amastigotes were not, however, solidly immune to reinfection. Adoptive-cell transfer experiments were then done to determine the T-cell subset that was associated with resistance to reinfection, and thus the development of immunity, in sublethally irradiated B10.D2 noncure mice and in C57BL/10 cure mice. T-cell-enriched preparations from spleens of immune donors were treated with subset-specific antibodies and complement prior to adoptive transfer in unprimed recipients. The results of the adoptive transfer experiments provide evidence that the genetically determined cure (H-2b) response in C57BL/10 mice and the cure response in genetically determined noncure (H-2d) B10.D2 mice brought about by sublethal irradiation pretreatment are mediated primarily by an L3T4+ Lyt-2- T cell.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badaro R., Jones T. C., Carvalho E. M., Sampaio D., Reed S. G., Barral A., Teixeira R., Johnson W. D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Behforouz N. C., Amirhakimi G. H., Rezai H. R., Saberi M. S. Immunological findings in kala-azar, Iran. Trop Geogr Med. 1983 Mar;35(1):27–32. [PubMed] [Google Scholar]
- Blackwell J. M., Roberts M. B. Immunomodulation of murine visceral leishmaniasis by administration of monoclonal anti-Ia antibodies: differential effects of anti-I-A vs. anti-I-E antibodies. Eur J Immunol. 1987 Nov;17(11):1669–1672. doi: 10.1002/eji.1830171125. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Ulczak O. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: demonstration and characterization of suppressor T cells in noncure mice. Infect Immun. 1984 Apr;44(1):97–102. doi: 10.1128/iai.44.1.97-102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- CAHILL K. M. LEISHMANIASIS IN THE SUDAN REPUBLIC. XXI. INFECTION IN AMERICAN PERSONNEL. Am J Trop Med Hyg. 1964 Nov;13:794–799. doi: 10.4269/ajtmh.1964.13.794. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Teixeira R. S., Johnson W. D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- ERICKSON R. P., TACHIBANA D. K., HERZENBERG L. A., ROSENBERG L. T. A SINGLE GENE CONTROLLING HEMOLYTIC COMPLEMENT AND A SERUM ANTIGEN IN THE MOUSE. J Immunol. 1964 Apr;92:611–615. [PubMed] [Google Scholar]
- Haldar J. P., Ghose S., Saha K. C., Ghose A. C. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun. 1983 Nov;42(2):702–707. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner Levy L., Mendes E. Impaired cell-mediated immunity in patients with kala-azar. Allergol Immunopathol (Madr) 1981 Mar-Apr;9(2):109–112. [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Roberts M. B., Blackwell J. M. Analysing the immune response to L. donovani infection. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):762–768. doi: 10.1016/s0769-2625(87)80034-x. [DOI] [PubMed] [Google Scholar]
- Mathew R. C., Boros D. L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986 Dec;54(3):820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath M. J., Murray H. W., Cohn Z. A. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988 Jun 1;167(6):1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Murray H. W., Carriero S. M., Donelly D. M. Presence of a macrophage-mediated suppressor cell mechanism during cell-mediated immune response in experimental visceral leishmaniasis. Infect Immun. 1986 Nov;54(2):487–493. doi: 10.1128/iai.54.2.487-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Visceral leishmaniasis in congenic mice of susceptible and resistant phenotypes: immunosuppression by adherent spleen cells. Infect Immun. 1985 Oct;50(1):160–168. doi: 10.1128/iai.50.1.160-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampiglione S., Manson-Bahr P. E., Giungi F., Giunti G., Parenti A., Canestri Trotti G. Studies on Mediterranean leishmaniasis. 2. Asymptomatic cases of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1974;68(6):447–453. doi: 10.1016/0035-9203(74)90067-4. [DOI] [PubMed] [Google Scholar]
- Pampiglione S., Manson-Bahr P. E., La Placa M., Borgatti M. A., Musumeci S. Studies in Mediterranean leishmaniasis. 3. The leishmanin skin test in kala-azar. Trans R Soc Trop Med Hyg. 1975;69(1):60–68. doi: 10.1016/0035-9203(75)90012-7. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Farrell J., Soulsby E. L. Immunological responses of L. donovani infection in mice and significance of T cell in resistance to experimental leishmaniasis. Clin Exp Immunol. 1980 Jun;40(3):508–514. [PMC free article] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semprevivo L. H., DeTolla L. J., Passmore H. C., Palczuk N. C. Spectral model of leishmaniasis in congenic strains of mice. J Parasitol. 1981 Feb;67(1):8–14. [PubMed] [Google Scholar]
- Skov C. B., Twohy D. W. Cellular immunity to Leishmania donovani. I. The effect of T cell depletion on resistance to L. donovani in mice. J Immunol. 1974 Dec;113(6):2004–2011. [PubMed] [Google Scholar]
- Skov C. B., Twohy D. W. Cellular immunity to Leishmania donovani. II. Evidence for synergy between thymocytes and lymph node cells in reconstitution of acquired resistance to L. donovani in mice. J Immunol. 1974 Dec;113(6):2012–2019. [PubMed] [Google Scholar]
- Stern J. J., Oca M. J., Rubin B. Y., Anderson S. L., Murray H. W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988 Jun 1;140(11):3971–3977. [PubMed] [Google Scholar]
- Ulczak O. M., Blackwell J. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: the effects of parasite dose, cyclophosphamide and sublethal irradiation. Parasite Immunol. 1983 Sep;5(5):449–463. doi: 10.1111/j.1365-3024.1983.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Weinbaum F. I., Herrod H. R. Characterization of in vitro proliferative responses of human lymphocytes to leishmanial antigens. J Infect Dis. 1979 Aug;140(2):215–221. doi: 10.1093/infdis/140.2.215. [DOI] [PubMed] [Google Scholar]


