Inactivation of integrin-linked kinase in the stem cells of the hair follicle bulge results in impaired skin regeneration after injury but does not affect hair follicle entry into anagen.
Abstract
Integrin-linked kinase (ILK) is key for normal epidermal morphogenesis, but little is known about its role in hair follicle stem cells and epidermal regeneration. Hair follicle stem cells are important contributors to newly formed epidermis following injury. We inactivated the Ilk gene in the keratin 15–expressing stem cell population of the mouse hair follicle bulge. Loss of ILK expression in these cells resulted in impaired cutaneous wound healing, with substantially decreased wound closure rates. ILK-deficient stem cells produced very few descendants that moved toward the epidermal surface and into the advancing epithelium that covers the wound. Furthermore, those few mutant cells that homed in the regenerated epidermis exhibited a reduced residence time. Paradoxically, ILK-deficient bulge stem cells responded to anagen growth signals and contributed to newly regenerated hair follicles during this phase of hair follicle growth. Thus ILK plays an important modulatory role in the normal contribution of hair follicle stem cell progeny to the regenerating epidermis following injury.
INTRODUCTION
The epidermis is a stratified squamous epithelium, formed by one basal and several suprabasal layers of keratinocytes, and is responsible for the barrier properties of the skin. The basal keratinocyte layer is closest to the dermis and is composed of undifferentiated cells with proliferative capacity, including stem cells and their transit-amplifying progeny. Basal keratinocytes are key for epidermal maintenance and regeneration after injury (Martin, 1997; Singer and Clark, 1999). Keratinocytes at the surface of the epidermis are constantly shed, so epidermal cells must be continually replenished to maintain tissue integrity and homeostasis. This is achieved through the contribution of keratinocyte stem cells.
Keratinocyte stem cells have been identified in different parts of the epidermis, including the interfollicular epidermis (Jones and Watt, 1993), as well as in hair follicles (Cotsarelis et al., 1990) and sebaceous glands (Horsley et al., 2006). Keratinocyte stem cells can give rise to all epidermal lineages in reconstitution experiments, indicating a high level of plasticity (reviewed by Watt and Jensen, 2009). However, the lineage determination of keratinocyte stem cells appears to be strongly influenced by their microenvironment. For example, in the intact epidermis, epidermal keratinocyte stem cells essentially replenish only the interfollicular epidermis, whereas hair follicle stem cells contribute exclusively to the follicles (Ito et al., 2005).
Hair follicles are epidermal appendages formed during embryogenesis. They consist of an upper permanent region (infundibulum), a midarea (isthmus), and a lower region termed the bulb. After birth, the bulb and suprabulbar sections undergo repeated cycles of growth (anagen), regression (catagen), and rest (telogen) that continue throughout the life of the individual (Schneider et al., 2009). The execution of repeated regeneration rounds is sustained by stem cells located in the bulge area around the isthmus, which produce progeny that regenerate the anagen follicle at every cycle (Schneider et al., 2009). Bulge hair follicle stem cells are multipotent and have the capacity to give rise to all epidermal lineages (Blanpain et al., 2004; Morris et al., 2004). A considerable body of work has defined some of the signals involved in the activation of stem and precursor cells to initiate anagen growth, including WNT pathway activation through stabilization of β-catenin, inhibition of bone morphogenetic protein signaling, and stimulation by fibroblast growth factor-7 (Greco et al., 2009).
Given their location, the epidermis and its appendages are highly susceptible to trauma. Epidermal regeneration after wounding is accomplished through the activation of both epidermal and hair follicle stem cells (Ito et al., 2005; Levy et al., 2005, 2007). Injury induces follicular stem cells to proliferate, and their progeny move toward the surface and become committed to an epidermal phenotype, thus contributing to reepithelialization (Ito et al., 2005; Levy et al., 2007). Notably, the pathways involved in modulating the transition of hair follicle to epidermal keratinocytes necessary for wound repair have yet to be identified.
Central for hair follicle morphogenesis and epidermal homeostasis is integrin-linked kinase (ILK) (Lorenz et al., 2007; Nakrieko et al., 2008). ILK is a scaffold protein that localizes to focal adhesions and mediates cell responses induced by the interaction of integrins with the extracellular matrix (reviewed by Wickstrom et al., 2010). Depending on the cell type and context, ILK can modulate the proliferation, viability, migration, and acquisition of cell polarity. Inactivation of the Ilk gene in the epidermis causes abnormal proliferation, defects in hemidesmosomes and in attachment of basal keratinocytes to the basement membrane, as well as alterations in the cytoskeleton and in epidermal architecture (Lorenz et al., 2007; Nakrieko et al., 2008). Notably loss of ILK expression in the embryonic ectoderm prior to initiation of epidermal formation severely impairs hair follicle development (Nakrieko et al., 2008). In contrast, Ilk gene inactivation in keratinocytes, once the epidermis has begun stratification and hair follicle morphogenesis is under way, results in the formation of follicles with abnormal hair shafts, which lose the ability to respond to growth signals as early as the first anagen, eventually leading to alopecia (Lorenz et al., 2007). Although it has become clear that ILK fulfills important functions in keratinocytes, its specific role in hair follicle stem cell biology remains unexplored. To address this issue, we conditionally inactivated the Ilk gene in keratin 15–expressing hair follicle bulge stem cells, and we characterized the consequent alterations in their properties. We now show that, although ILK-deficient bulge stem cells are able to support anagen follicle growth, their ability to contribute to the interfollicular epidermis following injury is compromised, significantly reducing the regenerative properties of the skin.
RESULTS
Conditional inactivation of ILK in keratinocyte stem cells of the hair follicle bulge
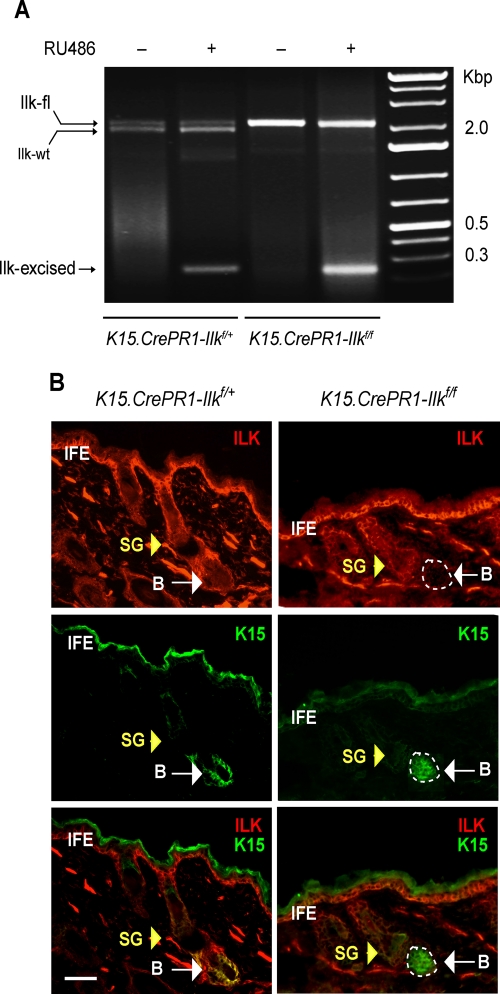
Keratin 15 (K15) is present in keratinocyte stem cells of the hair follicle bulge, as well as in their progeny found in the hair germ (Fuchs, 2009). To determine the role of ILK in hair follicle bulge cell functions, we generated mice in which we could conditionally inactivate the Ilk gene specifically in the K15-expressing cell population. These mice, hereafter termed K15.CrePR1-Ilkf/f, have floxed Ilk alleles and express Cre recombinase fused to a modified progesterone receptor, which is activated by RU486, under the control of the K15 promoter (Morris et al., 2004). The K15-CrePR1 transgene has been demonstrated to specifically target hair follicle stem cells and is not expressed in other keratinocyte types, including those in the sebaceous glands, the inner and outer root sheath of the hair follicle, and the adult interfollicular epidermis (Liu et al., 2003; Ito et al., 2005; Kim et al., 2009). We first treated the mice with topical RU486 or vehicle for 5 or 10 d and analyzed by PCR genomic DNA from dorsal skin. We readily detected genomic DNA amplicons corresponding to the excised, floxed allele in animals treated with RU486 but not in vehicle-treated skin, indicating that the K15-Cre transgene is tightly regulated in our system and that noninduced Cre activity is negligible, if any (Figure 1A). Because the skin contains both K15-expressing bulge cells and interfollicular keratinocytes and fibroblasts that do not express K15, we also detected an amplicon corresponding to the floxed, unexcised Ilk allele, which is expected in the DNA of other skin cells (Figure 1A). We next assessed by immunofluorescence microscopy ILK protein expression in the K15-expressing hair follicle bulge stem cell population. Treatment of K15.CrePR1-Ilkf/f dorsal skin with RU486 was accompanied by a loss of ILK immunofluorescence in the K15-expressing cell compartment (Figure 1B), indicating the effectiveness of the drug regimen used and its specificity to target the desired bulge cell population. In contrast, ILK immunofluorescence was detected in all K15-positive cells in control K15.CrePR1-Ilkf/+ skin, in the presence or absence of RU486 treatment (Figure 1B and unpublished data). Furthermore, we observed that RU486 treatment of K15.CrePR1-Ilkf/f dorsal skin resulted in the loss of ILK expression in CD34-expressing hair follicle cells (Supplemental Figure 1). CD34 is an established marker of hair follicle bulge stem cells. Thus, in our model, ILK protein decreases in hair follicle bulge stem cells to undetectable levels after a 5-d RU486 treatment without causing detectable alterations in the interfollicular epidermis, as evidenced by normal expression of keratin 14 and involucrin in basal and suprabasal interfollicular keratinocytes, respectively (Supplemental Figure 2).
FIGURE 1:
Conditional Ilk gene inactivation in stem cells of the hair follicle bulge. (A) Dorsal skin of P50 K15.CrePR1-Ilkf/+ or K15.CrePR1-Ilkf/f mice was treated daily with topical RU486 (+) or vehicle (–) for 5 d. Genomic DNA from treated skin was isolated and genotyped. Amplicons corresponding to the floxed (2.1 Kb), wild-type (wt, 1.9 Kb), and Cre-excised (230 base pairs) Ilk alleles are indicated. (B) Dorsal skin of P50 K15.CrePR1-Ilkf/+ or K15.CrePR1-Ilkf/f mice was treated daily with topical RU486 for 5 d. The skin was harvested 5 d later, and tissue sections were processesed for immunofluorescence microscopy, using rabbit anti-ILK and mouse anti-K15 antibodies, as indicated. Yellow and white arrows show the sebaceous gland (SG) and the hair follicle bulge (B), respectively. IFE, interfollicular epidermis. The apparent K15 staining in the IFE is due to antibody trapping by keratin filaments overlaying the cornified layer. Bar, 50 μm.
Role of ILK in anagen-induced hair follicle stem cell activation and differentiation
During postnatal life, bulge stem cells divide, generating progeny that contribute to all cell lineages of the cycling hair follicle (Morris et al., 2004). At birth, hair follicles are not fully developed but are in an active growth (anagen) phase. Following anagen, the catagen phase ensues, which is characterized by extensive apoptosis of matrix cells and involution of the hair follicle. Catagen is followed by a resting telogen phase (Cotsarelis, 2006), and these three phases continue throughout the life of the individual. To examine the possibility that ILK is required for follicular fate selection during hair cycling, we bred K15.CrePR1-Ilkf/f mice with a Rosa26 reporter strain that expresses yellow fluorescent protein (YFP), which allowed us to conduct lineage analyses. In the resulting mice (hereafter termed K15.CrePR1-YFP-Ilkf/f mice), Cre-mediated excision in the K15-expressing population produces ILK-deficient cells that express YFP. We also generated control mice with only one floxed Ilk allele, in which YFP-positive hair follicle stem cells and their progeny still express ILK from the wild-type locus (hereafter termed K15.CrePR1-YFP-Ilkf/+mice). We then treated P50 mice with RU486 (during the second telogen) and analyzed their hair follicles 20 d later, during the third anagen. This time course was selected to separate any potential acute effects of RU486 on hair follicle growth from changes due to Ilk gene inactivation.
At anagen, hair follicle stem cells are activated to divide, and their progeny migrate out of the bulge, forming SOX9-expressing outer root sheath cells (Vidal et al., 2005). Some outer root sheath keratinocytes later give rise to LEF1-expressing matrix cells. The latter are highly proliferative transit-amplifying cells, which eventually become quiescent and differentiate into the cell lineages of the hair shaft and the inner root sheath (Schneider et al., 2009). We examined RU486-treated K15.CrePR1-YFP-Ilkf/f mice and observed the presence of targeted, YFP-positive cells that also expressed SOX9 (Figure 2A). Similarly, YFP-positive cells in these mice contributed to the LEF1-expressing matrix population (Figure 2A), indicating that ILK is not essential for differentiation of stem cells into either outer root sheath or matrix keratinocytes. To determine if ILK is essential to maintain the proliferative capacity of matrix cells, we also assessed the expression of Ki67. Expression of this marker in ILK-deficient matrix cells was indistinguishable from that in ILK-expressing cells, indicating that proliferation of postnatal matrix keratinocytes during anagen is not dependent on ILK (Figure 2B). Finally, we evaluated the role of ILK in susceptibility to apoptosis in these anagen hair follicles and found very few terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive apoptotic cells in either K15.CrePR1-YFP-Ilkf/f or K15.CrePR1-YFP-Ilkf/+ animals, irrespective of whether they received RU486 or vehicle (Figure 2B and unpublished data). In keeping with the preceding observations, the pelage of K15.CrePR1-Ilkf/f mice treated at P20 (first telogen) with RU486 grew in a manner undistinguishable from that of K15.CrePR1-Ilkf/+ animals during the following two hair cycles (Supplemental Figure 3), suggesting that inactivation of Ilk in keratinocyte bulge stem cells does not appreciably alter hair growth and is not essential for cell survival within this time frame.
FIGURE 2:
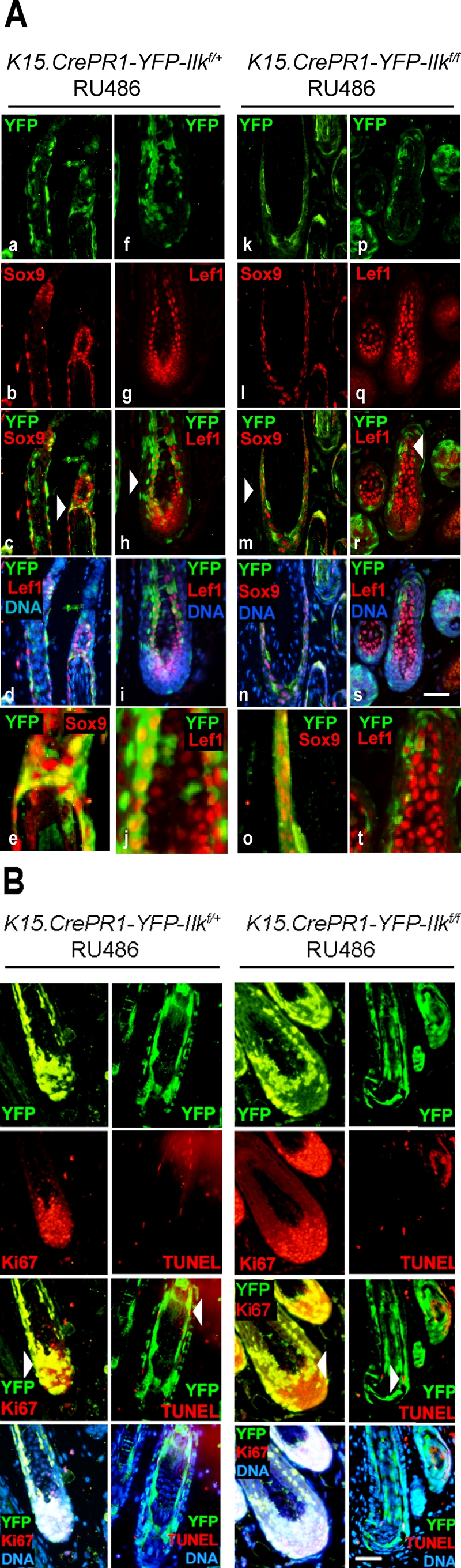
Differentiation of ILK-deficient hair follicle stem cells into various hair follicle cell lineages. (A) The dorsal skin of P50 K15.CrePR1-Ilkf/+ or K15.CrePR1-Ilkf/f mice (during the second telogen) was treated daily with topical RU486 for 5 d, harvested during the following anagen phase (P70), and processed for immunofluorescence microscopy using antibodies against SOX9 or LEF1, as indicated. Anti–green fluorescent protein (anti-GFP) antibodies were used to detect YFP. Micrographs in panels e, j, o, and t represent higher-magnification images of the areas indicated by the arrows in panels c, h, m, and r, respectively. Bar, 100 μm (for all panels except e, j, o, and t). (B) The tissues described in (A) were also analyzed for Ki67-associated immunoreactivity. Ki67-expressing cells are indicated by arrows. Apoptotic cells, shown by arrowheads, were identified using TUNEL staining. Nuclear DNA was visualized with Hoescht 33342. Bar, 100 μm.
Spreading and viability of ILK-deficient keratinocyte stem cells
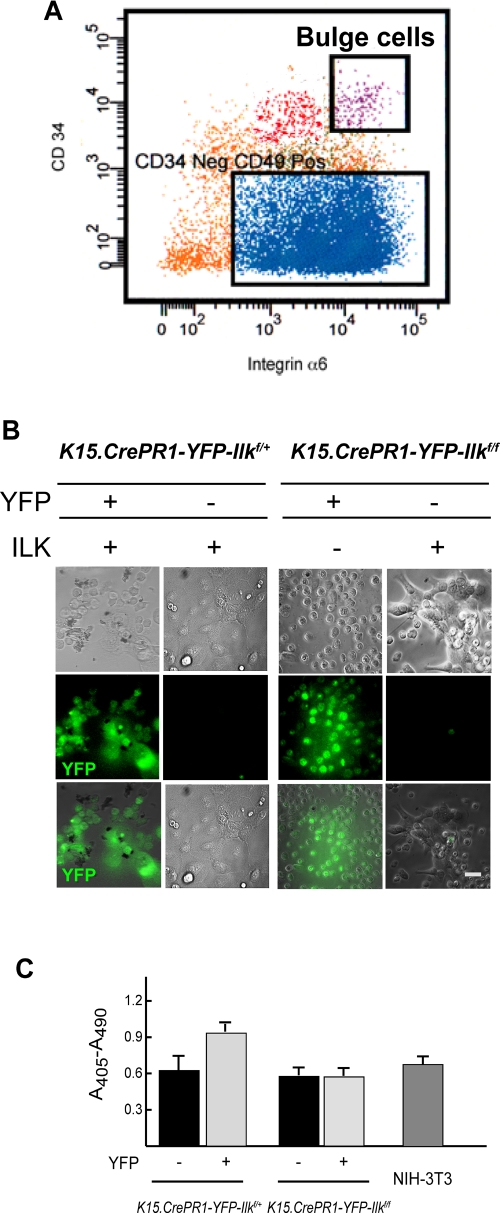
To gain a better understanding of the consequences of ILK loss on bulge keratinocytes, we used fluorescence-activated cell sorting (FACS) to isolate and culture these cells. Keratinocytes from RU486-treated K15.CrePR1-YFP-Ilkf/f or K15.CrePR1-YFP-Ilkf/+ mice were sorted based on the joint high-level expression of the stem cell marker CD34 and of integrin α6 (Trempus et al., 2003; Blanpain et al., 2004) (Figure 3A). We further fractionated these cells to separate YFP-expressing cells from the fraction of YFP-negative cells present. We found that CD34HIGH/integrin α6HIGH stem cells constituted ∼1% of the total keratinocyte population analyzed and, within this fraction, ∼50% of stem cells from RU486-treated animals were YFP-positive (Supplemental Figure 4). This would suggest that about half of the hair follicle stem cell population was targeted by the drug treatments. Extending the daily RU486 administration to 10 d did not significantly change these proportions (unpublished data). Sorted cells were plated onto laminin 332 matrix- and collagen-coated surfaces, and their ability to attach and spread was scored. ILK-deficient stem cells were able to attach, albeit with somewhat reduced efficiency. However, they did not show appreciable spreading until after 72 h following plating (Figure 3B and unpublished data). In contrast, YFP-negative K15.CrePR1-Ilkf/f keratinocytes showed attachment and spreading comparable to those observed in ILK-expressing cells isolated from K15.CrePR1-YFP-Ilkf/+ animals. All these defects mimic those present in ILK-deficient keratinocytes isolated from newborn epidermis (Nakrieko et al., 2008) and are consistent with the notion that ILK is necessary for normal bulge keratinocyte interaction with the extracellular matrix, at least in culture.
FIGURE 3:
Adhesion, spreading, and viability of ILK-deficient hair follicle bulge cells. The dorsal skin of P50 K15.CrePR1-YFP-Ilkf/+ or K15.CrePR1-YFP-Ilkf/f mice was treated daily with topical RU486 for 5 d. Five days after the last treatment, the skins were harvested and keratinocytes were isolated by trypsin digestion. (A) The keratinocyte suspension was labeled with antibodies against CD34 and integrin α6, as described in Materials and Methods. Viable stem cells from the hair follicle bulge (“Bulge”) were purified by FACS based on high CD34 and integrin α6 fluorescence intensity. These cells were further separated into YFP-positive and YFP-negative populations. (B) Phase contrast and direct fluorescence micrographs of the YFP-positive and YFP-negative bulge keratinocytes isolated in (A) (1 × 104 cells), seeded onto collagen- and laminin 332 matrix-coated dishes, and cultured for 72 h. Bar, 25 μm. (C) YFP-positive or YFP-negative hair follicle bulge keratinocytes of the indicated genotypes were isolated as described in (A), and duplicate samples (1 × 104 cells each) were cultured on collagen- and laminin 332 matrix-coated dishes for 96 h. The cells were lysed and cytoplasmic mono- and oligonucleosomes were quantified using a colorimetric enzyme-linked immunosorbent assay method. Cytoplasmic oligonucleosomes in a culture containing 1 × 104 exponentially proliferating NIH-3T3 cells (≥ 95% viable) are shown for comparison. The results are expressed as the mean absorbance at 405 nm (reference wavelength 490 nm) plus standard error of the mean (SEM) (n = 3).
FIGURE 4:
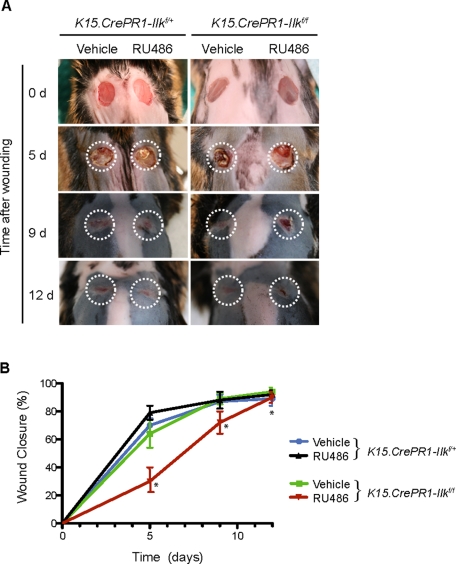
Impaired epidermal regeneration after injury following Ilk gene inactivation in hair follicle stem cells. (A) The right and left halves of dorsal skin of P50 K15.CrePR1-Ilkf/+ or K15.CrePR1-Ilkf/f mice (during the second telogen) were treated daily with topical RU486 or vehicle, respectively, for 5 d. Five days after the last treatment, the mice received two full-thickness wounds with a 6-mm biopsy punch. Wound appearance at the indicated days (d) postwounding is shown, and the dashed lines represent the wound surface at the time of injury (0 d). (B) Rates of wound closure in K15.CrePR1-Ilkf/+ or K15.CrePR1-Ilkf/f mice treated with RU486 or vehicle. Remaining wound surface was measured at the indicated times postwounding and plotted as the percent of total closure, set at 100%. The results are expressed as the mean ± SD (n = 8). Asterisks indicate p < 0.05 (ANOVA), relative to vehicle-treated K15.CrePR1-Ilkf/+ skin at the corresponding time postinjury.
Although the loss of ILK in stem cells did not decrease their viability in situ, we also investigated the possibility that alterations in the microenvironment could generate conditions upon culture of ILK-deficient stem cells that might uncover increased susceptibility to apoptosis. To this end, we measured the formation of oligonucleosomes, indicative of apoptosis, in these cultures. As observed in vivo, no significant changes in cell viability were present in ILK-deficient cells relative to normal keratinocytes under these conditions (Figure 3C).
ILK is essential for normal interfollicular epidermal regeneration
The role of bulge keratinocyte stem cells in intact epidermis is mainly confined to maintaining all follicular cell lineages during the hair cycle. However, after cutaneous injury, these cells are also recruited into a distinct cell fate, differentiating into interfollicular keratinocytes and contributing ∼30% of cells to the newly formed epithelium (Ito et al., 2005; Levy et al., 2005). Given that keratinocytes cultured onto laminin 332–coated surfaces can be induced to model some responses to injury (Harper et al., 2005), and based on the observed defects in cultured ILK-deficient bulge stem cells, we reasoned that ILK expression in follicular stem cells might be required for normal epidermal repair. To address this possibility, we treated the right half of the dorsal skin of 50-d-old K15.CrePR1-Ilkf/f or K15.CrePR1-Ilkf/+ mice with RU486 as before. The left half of the dorsal skin was treated with vehicle. This experimental design allowed us to measure changes in the rates of reepithelialization specifically due to Ilk gene inactivation within individual animals, because both wounds were exposed to identical systemic responses to injury. Five days after the last drug treatment, 6-mm full-thickness excisional wounds were produced on the animals, and reepithelialization was examined at timed intervals postwounding. Regeneration of vehicle-treated skin in K15.CrePR1-Ilkf/f mice was undistinguishable from that of K15.CrePR1-Ilkf/+ animals. Specifically, ∼80 and 90% of the wound in most animals had been reepithelialized as early as 5 and 12 d, respectively, following injury (Figure 4). In stark contrast, regeneration in the epidermis containing ILK-deficient bulge stem cells was significantly delayed. This impairment was most pronounced 5 d after injury, when only ∼26% of the wound had been reepithelialized. The delay was maintained all throughout the healing process, although the relative differences between ILK-expressing and ILK-deficient animals decreased with time and all mice eventually healed. Similar delays in reepithelialization were observed in K15.CrePR1-YFP-Ilkf/f relative to K15.CrePR1-YFP-Ilkf/+ mice (unpublished data).
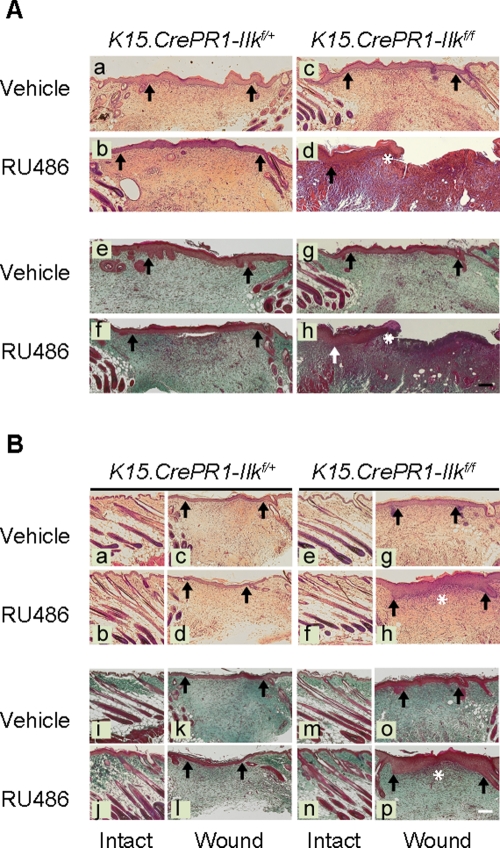
Histological examination of the regenerating epidermis showed that the keratinocyte migrating edge had advanced significantly less in epidermis containing ILK-deficient bulge stem cells, both 5 and 9 d after wounding (Figure 5A and unpublished data). Abundant cell infiltration under the wounded area persisted 9 d after injury exclusively in RU486-treated K15.CrePR1-Ilkf/f skin (Figure 5A), indicative of a delayed course of tissue regeneration. This effect is unlikely to be related to RU486-induced inflammation, because it was not observed in drug-treated ILK-expressing skin. Trichrome staining indicated that collagen deposition was also delayed in RU486-treated K15.CrePR1-Ilkf/f skin 9 and 12 d following wounding compared with controls (Figure 5, A and B). Collectively, these observations are consistent with the notion that ILK expression in bulge keratinocyte stem cells and/or their progeny is essential for normal epidermal regeneration and cutaneous wound healing.
FIGURE 5:
Delayed reepithelialization in wounds from mice with ILK-deficient hair follicle stem cells. The right and left halves of dorsal skin of P50 K15.CrePR1-Ilkf/+ or K15.CrePR1-Ilkf/f mice (during the second telogen) were treated daily with topical RU486 or vehicle, respectively, for 5 d. Five days after the last treatment, the mice received two full-thickness wounds with a 6-mm biopsy punch. (A) Tissues were harvested 9 d postwounding and processed for histological analysis. Sections were stained with hematoxylin and eosin (micrographs a–d) or with trichrome stain (micrographs e–h). Arrows indicate wound margins, and the asterisks in panels d and h indicate the epithelium migrating edge. (B) Tissues were harvested 12 d postwounding and processed for histological analysis. Sections were stained with hematoxylin and eosin (micrographs a–h) or with trichrome stain (micrographs i–p). The sections correspond to either the reepithelialized area at the center of the wound or intact epidermis adjacent to the wound, shown for comparison. Arrows indicate wound edges at t = 0, and the asterisks indicate the edge of the migrating tongue in the newly formed epithelium. Bar, 500 μm.
ILK is required for normal contribution of bulge stem cell progeny to the interfollicular epidermis
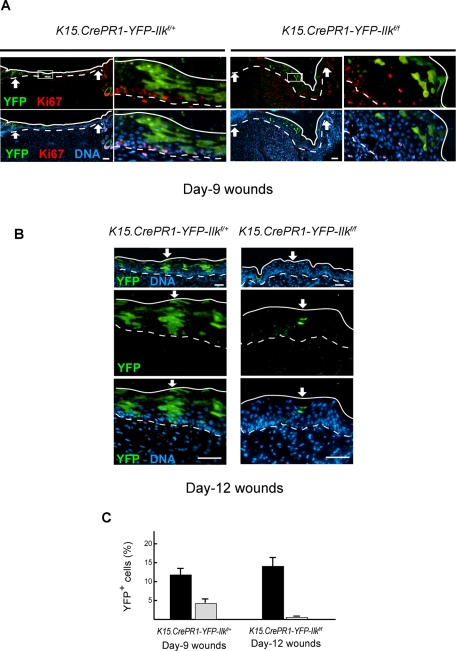
The delays in epidermal repair described earlier might result from several factors, including reduced activation of ILK-deficient hair follicle stem cells in response to injury and/or from defects in their progeny once they reach the newly formed epidermis. To address these issues, we treated with RU486 and wounded K15.CrePR1-YFP-Ilkf/f and K15.CrePR1-YFP-Ilkf/+ mice as before. We first conducted tracing studies at timed intervals after injury. We observed YFP-positive basal keratinocytes derived from bulge stem cells in the newly formed epithelium of K15.CrePR1-YFP-Ilkf/+ mice as early as 1 d after wounding. In 9- and 12-d wounds, YFP-expressing epidermal keratinocytes (basal plus suprabasal) were readily detected (Figure 6) and constituted ∼12 and 14%, respectively, of the basal plus suprabasal cell population in the newly formed epidermis, in agreement with the reported contribution of bulge-derived progeny to epidermis regenerated following excisional injury (Ito et al., 2005). In stark contrast, the contribution of ILK-deficient bulge stem cells to the new epidermis was substantially lower (Figure 6). Specifically, YFP-expressing cells constituted only ∼5% of the basal plus suprabasal cell population in the regenerated epithelium of RU486-treated K15.CrePR-YFP1-Ilkf/f animals 9 d after wounding. Furthermore, the fraction of ILK-deficient cells that contributed to reepithelialization decreased to only ∼0.3% in 12-d wounds. Thus, although ILK-deficient bulge cells are capable of generating interfollicular progeny, the abundance and characteristics of the latter are abnormal.
FIGURE 6:
Reduced contribution of ILK-deficient cells to the regenerated epidermis. The dorsal skin of P50 K15.CrePR1-YFP-Ilkf/+ or K15.CrePR1-YFP-Ilkf/f mice was treated daily with topical RU486 for 5 d. Five days after the last treatment, the mice received a full-thickness excisional wound with a 6-mm biopsy punch. (A) Tissues were harvested 9 d postwounding and processed for immunofluorescence microscopy using antibodies against Ki67. YFP was detected using anti-GFP antibodies. Arrows indicate wound margins. Continuous and dashed lines indicate, respectively, the cornified layer and the dermal–epidermal junction. The box in the panels on the left represents regions shown at higher magnification in the adjacent panels at right. (B) Tissues were harvested 12 d postwounding and processed for immunofluorescence microscopy using anti-GFP antibodies to visualize YFP. The arrow in the micrographs indicates areas shown below at higher magnification. Solid and dashed lines represent the outer surface of the epidermis and the dermal–epidermal junction, respectively. Nuclear DNA was visualized with Hoescht 33342. Bars, 100 μm. (C) The number of YFP-positive cells in the reepithelialized epidermis between the wound margins of mice with the indicated genotype was scored. The results are expressed as the percentage of YFP-expressing cells (basal plus suprabasal) relative to total cell number (basal plus suprabasal). Total cell numbers were assessed by counting the Hoescht 33342–stained cell nuclei. The data are shown as the mean plus SEM (n = 8).
ILK is involved in clonal expansion and organization of interfollicular keratinocytes during epidermal regeneration
Trauma induces the cells of the hair follicle to divide and migrate outward, to contribute to the regenerating epidermis (Ito et al., 2005; Levy et al., 2005). These cells and their descendants can be visualized as discrete columns that span all epidermal layers. Indeed we clearly observed columns of YFP-positive cells in regenerated epidermis of ILK-expressing K15.CrePR1-YFP-Ilkf/+ mice 9 and 12 d postwounding, and they contributed to both basal and suprabasal layers (Figure 6). On the contrary, those few YFP-positive, ILK-deficient keratinocytes present in 9-d wounds from K15.CrePR1-Ilkf/f animals appeared randomly organized, rather than distributed into vertical columns, and were frequently found within the suprabasal layers (Figure 6A). By 12 d postwounding, no ILK-deficient cells were detected in the basal layer (Figure 6B).
The poor contribution of ILK-deficient stem cell progeny to the regenerated epidermis could result from decreased cell proliferation and/or survival. We first analyzed Ki67 expression in newly formed skin from K15.CrePR1-YFP-Ilkf/+ and K15.CrePR1-Ilkf/f mice. In day 9 wounds, Ki67 immunoreactivity was confined to basal cells and was observed in ∼30% of YFP-negative keratinocytes from either mouse strain (Figure 6A and unpublished data). YFP-positive keratinocytes from K15.CrePR1-YFP-Ilkf/+ mice were similarly labeled with Ki67. In stark contrast, we detected Ki67 expression only in ∼5% of YFP-positive, ILK-deficient basal keratinocytes in day 9 wounds and did not detect any ILK-deficient cells that were Ki67-positive in reepithelialized tissue in day 12 wounds (Figure 6A), consistent with the notion that epidermal cells derived from ILK-deficient bulge keratinocytes in the newly formed epidermis exhibit reduced proliferation potential. Similar to our observations in the cycling hair follicle, the proportion of apoptotic cells in the ILK-deficient epidermal population (<3%) was undistinguishable from that in ILK-expressing keratinocytes (unpublished data), indicating that impairment in regeneration in RU486-treated K15.CrePR1-Ilkf/f skin is unlikely to arise from decreased viability. Together our data indicate that ILK is essential to mobilize hair follicle stem cells out of their niche to contribute to epidermal recapitulation after damage. Moreover, ILK also appears to be involved in maintaining the bulge-derived stem cell progeny in the newly regenerated epidermis.
DISCUSSION
We examined the role that ILK plays in hair follicle stem cells and their progeny through conditional inactivation of the Ilk gene in the K15-expressing bulge stem cell population. When we treated K15.CrePR1-YFP-Ilkf/f dorsal skin with RU486 during the first (P20) or second (P50) telogen and examined follicle entry into the following anagen phase, hair follicles in which Ilk had been targeted grew and generated hair shafts indistinguishable from those in ILK-expressing mice. ILK-deficient bulge stem cells were capable of giving rise to SOX9-expressing outer root sheath cells, as well as LEF1-expressing matrix keratinocytes, indicating that ILK is not required for cell fate selection of at least a subset of follicular lineages. Whether ILK-deficient cells contribute to all hair follicle lineages exactly to the same extent as ILK-expressing cells over the short and long term remains to be determined. It has been shown that inactivation of Ilk in the embryonic epidermis impairs matrix cell proliferation and hair follicle development (Lorenz et al., 2007; Nakrieko et al., 2008). In contrast, ILK-deficient bulge stem cell progeny contribute to the proliferating transit-amplifying matrix population during postnatal anagen growth, suggesting that keratinocyte proliferation and survival in response to anagen induction is not dependent on ILK-mediated processes. Thus, although ILK is essential for hair follicle morphogenesis, it appears to be dispensable for at least some aspects of postnatal hair follicle cycling. All these observations provide evidence of novel roles for ILK in modulating signaling pathways that distinguish embryonic versus postnatal hair follicle growth.
ILK is required for the normal contribution of hair follicle stem cells and their progeny to the regenerating epidermis. The intact epidermis is maintained through differentiation and self-renewal of interfollicular keratinocyte stem and/or progenitor cells, with minimal, if any, contribution from the hair follicle (Ito et al., 2005). However, trauma activates bulge stem cells, inducing them to proliferate, to migrate outward, and to acquire an interfollicular epidermis phenotype (Ito et al., 2005; Levy et al., 2007). The contribution of hair follicle stem cells to newly formed epidermis is very important, and its absence results in substantial and significant delays in regeneration after injury (Langton et al., 2008). We now show that ILK is essential for hair follicle stem cell contribution to reepithelialization, which places this protein as a key factor in the regenerative pathways of the epidermis.
Following injury during telogen, ILK-deficient bulge stem cells are able to respond to activation and proliferation signals, as evidenced by the presence of their descendants in the growing hair follicles adjacent to the wounded regions. However, the capacity of ILK-deficient cells to travel toward the surface and participate in reepithelialization is severely compromised, possibly at various levels. First, very few ILK-deficient bulge stem cell progeny were mobilized from the follicles adjacent to the wound. Specifically, in RU486-treated K15.CrePR1-YFP-Ilkf/+ mice, we readily detected numerous YFP-positive cells derived from the hair follicle bulge, which had moved toward the surface, populated the infundibulum, and advanced to the edge of the wound, as early as 1 d after injury (unpublished data). In contrast, we found only a few ILK-deficient cells in the upper regions of the hair follicle, and they were not detected prior to about 5 d after wounding. This suggests that ILK is essential for the migration of hair follicle stem cell progeny out of the bulge toward the regenerating epidermis. Furthermore, ILK-expressing cells derived from bulge stem cells went on to form abundant, well-organized YFP-expressing columns in the regenerated epidermis by 9 d postwounding. These cells were able to divide and became a larger fraction of the reepithelialized areas by 12 d following injury. In stark contrast, 9 d after wounding, those few ILK-deficient cells that contributed to the newly formed epidermis were rarely found in the basal layer, they were unable to expand and organize in vertical columns, and their abundance had substantially decreased 12 d after wounding. These observations suggest that ILK may also contribute to basal keratinocyte attachment to the underlying extracellular matrix, which may have important implications in the execution of a normal differentiation program. Finally, it is also possible that ILK-deficient stem cell progeny in the anagen hair follicle may also exhibit impaired ability to contribute to the newly formed epithelium.
The presence of transit-amplifying matrix cells in RU486-treated K15.CrePR1-YFP-Ilkf/f follicles would suggest that ILK is dispensable for the short-term proliferation of bulge stem cells and their immediate descendants in vivo. Whether ILK-deficient cells can support hair follicle growth over multiple anagen cycles is an important area for future studies.
ILK-deficient bulge hair follicle cells cultured on a laminin 332 matrix have adhesion, spreading, and migration defects reminiscent of those observed in ILK-deficient epidermal keratinocytes (Nakrieko et al., 2008). Altered interactions between these cells and their surrounding extracellular matrix in vivo may contribute to their inability to leave the follicular regions adjacent to wounds and to move toward areas of active reepithelialization. It has been demonstrated that ILK-deficient basal epidermal keratinocytes exhibit abnormalities in hemidesmosomes and do not attach properly to the underlying basement membrane (Lorenz et al., 2007; Nakrieko et al., 2008). It is conceivable that similar defects exist in those few epidermal keratinocytes derived from ILK-deficient hair follicle bulge stem cells during reepithelialization, which might contribute to their inability to remain anchored to the basement membrane.
In summary, we have shown that ILK expression in the hair follicle stem cells is necessary for proper epidermal regeneration after injury, constituting a potential therapeutic target for healing abnormalities and aging-related epidermal disorders.
MATERIALS AND METHODS
Generation of K15.CrePR1-Ilkf/f mice
Hemizygous K15.CrePR1 transgenic mice (Morris et al., 2004) (B6;SJL-Tg(Krt1–15-cre/PGR)22Cot/J; The Jackson Laboratory, Bar Harbor, ME) were bred with Ilkf/f mice (Terpstra et al., 2003) to produce progeny hemizygous for the K15.CrePR1 transgene and either heterozygous (K15.CrePR1-Ilkf/+) or homozygous (K15.CrePR1-Ilkf/f) for the floxed Ilk alleles. The K15.CrePR1-Ilkf/f mice were also bred into a ROSA26-enhanced YFP background (mice obtained from The Jackson Laboratory) to produce K15.CrePR1YFP-Ilkf/+ and K15.CrePR1YFP-Ilkf/f animals. Mice were genotyped for Ilk and Cre as described (Nakrieko et al., 2008), and for the Rosa-YFP loci with 5′-GGAGCGGGAGAAATGGATATG-3′, 5′-AAGACCGCGAAGAGTTTGTC-3′, and 5′AAAGTCGCTCTGAGTTGTTAT-3′ primers, which yield 600– and 320–base pair amplicons corresponding, respectively, to the wild-type and the mutant (YFP-encoding) alleles. CrePR1 was activated in dorsal skin of age-matched female mice following depilation by applications of 200 mg Neutrogena skin cream (Johnson and Johnson, New Brunswick, NJ) containing 1% (wt/wt) RU486 (Tokyo Chemical Industry, Portland, OR). The drug was first dissolved in 1 ml ethanol, mixed with the cream, and allowed to stand until all excess ethanol had evaporated. Drug treatments were conducted daily for 5 d. Control animals were treated with vehicle only. All animal procedures and maintenance were conducted in accordance with animal care guidelines from the University of Western Ontario.
Excisional wounds
Age-matched (50–55-d-old) female K15.CrePR1-Ilkf/+ and K15.CrePR1-Ilkf/f mice, or K15.CrePR1YFP-Ilkf/+ and K15.CrePR1YFP-Ilkf/f mice, were treated daily with RU486 or vehicle for 5 d. In these experiments, the right half of the skin was shaved and treated with RU486, whereas the left half was treated with vehicle. A central portion of 7–9-mm width was left untouched to better distinguish vehicle-treated areas from drug-treated areas. The treatments with RU486 were conducted on animals housed individually. During treatment, the animals were housed in cages with appropriate filters to prevent RU486 spreading through dust or aerosols. The mice were treated individually in a laminar flow hood and housed this way until 24 h after the last RU486 treatment. One day after the last treatment, the mice were anesthetized by isoflurane inhalation. Two full-thickness excisional wounds through the dorsal skin and on each side of the spine were made just under the scapula with a 6-mm-diameter circular biopsy punch (Miltex, Plainsboro, NJ). The wound surface was measured and tissues were then harvested for further analyses 5, 9, or 12 d postinjury. Wound closure rates were determined, and differences were assessed by analysis of variance (ANOVA) with post hoc Bonferroni correction. All wounding analyses were conducted using eight animals per group, examining at least eight epidermal sections per wound, which contained reepithelialized areas as well as adjacent nonwounded skin. To estimate the contribution of targeted cells to newly formed epidermis, wounded and adjacent normal tissues were harvested from K15.CrePR1YFP-Ilkf/+ and K15.CrePR1YFP-Ilkf/f mice. Tissue sections were processed for immunofluorescence microscopy to detect YFP as described in the Supplemental Material. YFP-positive cells in the reepithelialized areas between the wound margins were counted, and their abundance was expressed as the percentage of total cells counted in that same area of newly formed epidermis. The cells counted included both basal and suprabasal cells. These analyses were conducted using at least five epidermal sections per wound and eight animals per group.
Supplementary Material
Acknowledgments
We thank K. Chadwick for assistance with FACS experiments, S. A. Charlesworth for help with histology, and A. MacGillivary for excellent technical assistance. We are grateful to D. Hess and G. DiGuglielmo for helpful comments on the manuscript. This work was supported with grants to L.D. from the Canadian Institutes of Health Research, the Canadian Cancer Society, and the Cancer Research Society.
Abbreviations used:
- ANOVA
analysis of variance
- FACS
fluorescence-activated cell sorting
- GFP
green fluorescent protein
- ILK
integrin-linked kinase
- K15
keratin 15
- P
postnatal day
- SEM
standard error of the mean
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- YFP
yellow fluorescent protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-01-0035) on May 18, 2011.
REFERENCES
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EG, Alvarez SM, Carter WG. Wounding activates p38 map kinase and activation transcription factor 3 in leading keratinocytes. J Cell Sci. 2005;118:3471–3485. doi: 10.1242/jcs.02475. [DOI] [PubMed] [Google Scholar]
- Horsley V, O'Oarroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair, but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Kataoka K, Rao D, Kiguchi K, Cotsarelis G, Digiovanni J. Targeted disruption of Stat3 reveals a major role for follicular stem cells in skin tumor initiation. Cancer Res. 2009;69:7587–7594. doi: 10.1158/0008-5472.CAN-09-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128:1311–1318. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct cell populations regenerate the follicle and the interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumalley M, Fassler R. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol. 2007;177:501–513. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotech. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Nakrieko KA, Welch I, Dupuis H, Bryce DM, Pajak A, St-Arnaud RS, Dedhar S, D'souza SJA, Dagnino L. Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation opf integrin-linked kinase in the epidermis. Mol Biol Cell. 2008;19:1462–1473. doi: 10.1091/mbc.E07-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase. EMBO J. 2010;29:281–291. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.