Abstract
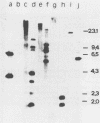
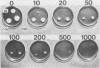
A transformation system for the ringworm-producing dermatophyte Trichophyton mentagrophytes has been developed. The system employs the plasmid pHIS, which contains a bacterial hygromycin B phosphotransferase gene linked to Cochliobolus heterostrophus regulatory sequences (B. G. Turgeon, R. C. Garber, and O. C. Yoder, Mol. Cell. Biol. 7:3297-3305, 1987). This plasmid confers hygromycin B resistance to T. mentagrophytes. The DNA was stably integrated into the fungal genome, and the number and sites of integrations varied among transformants. Transformant clones were capable of infecting guinea pigs. This system opens the way for the molecular genetic analysis of the interaction of T. mentagrophytes with epithelial animal tissues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard H. U., Krämmer G., Röwekamp W. G. Construction of a fusion gene that confers resistance against hygromycin B to mammalian cells in culture. Exp Cell Res. 1985 May;158(1):237–243. doi: 10.1016/0014-4827(85)90446-x. [DOI] [PubMed] [Google Scholar]
- González A., Jiménez A., Vázquez D., Davies J. E., Schindler D. Studies on the mode of action of hygromycin B, an inhibitor of translocation in eukaryotes. Biochim Biophys Acta. 1978 Dec 21;521(2):459–469. doi: 10.1016/0005-2787(78)90287-3. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Kaster K. R., Burgett S. G., Rao R. N., Ingolia T. D. Analysis of a bacterial hygromycin B resistance gene by transcriptional and translational fusions and by DNA sequencing. Nucleic Acids Res. 1983 Oct 11;11(19):6895–6911. doi: 10.1093/nar/11.19.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. M., Barnason A. R., Rogers S. G., Byrne M. C., Fraley R. T., Horsch R. B. Transformation of Arabidopsis thaliana with Agrobacterium tumefaciens. Science. 1986 Oct 24;234(4775):464–466. doi: 10.1126/science.234.4775.464. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Zalacaín M., Jiménez A., Davies J. Molecular cloning and expression in streptomyces lividans of a hygromycin B phosphotransferase gene from Streptomyces hygroscopicus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):6–12. doi: 10.1016/0006-291x(83)91533-4. [DOI] [PubMed] [Google Scholar]
- Ramón D., Carramolino L., Patiño C., Sánchez F., Peñalva M. A. Cloning and characterization of the isopenicillin N synthetase gene mediating the formation of the beta-lactam ring in Aspergillus nidulans. Gene. 1987;57(2-3):171–181. doi: 10.1016/0378-1119(87)90120-x. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Allen N. E., Hobbs J. N., Jr, Alborn W. E., Jr, Kirst H. A., Paschal J. W. Genetic and enzymatic basis of hygromycin B resistance in Escherichia coli. Antimicrob Agents Chemother. 1983 Nov;24(5):689–695. doi: 10.1128/aac.24.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., Yoder O. C. Selectable genes for transformation of the fungal plant pathogen Glomerella cingulata f. sp. phaseoli (Colletotrichum lindemuthianum). Gene. 1987;54(1):73–81. doi: 10.1016/0378-1119(87)90349-0. [DOI] [PubMed] [Google Scholar]
- Santerre R. F., Allen N. E., Hobbs J. N., Jr, Rao R. N., Schmidt R. J. Expression of prokaryotic genes for hygromycin B and G418 resistance as dominant-selection markers in mouse L cells. Gene. 1984 Oct;30(1-3):147–156. doi: 10.1016/0378-1119(84)90115-x. [DOI] [PubMed] [Google Scholar]
- Singh A., Ursic D., Davies J. Phenotypic suppression and misreading Saccharomyces cerevisiae. Nature. 1979 Jan 11;277(5692):146–148. doi: 10.1038/277146a0. [DOI] [PubMed] [Google Scholar]
- Turgeon B. G., Garber R. C., Yoder O. C. Development of a fungal transformation system based on selection of sequences with promoter activity. Mol Cell Biol. 1987 Sep;7(9):3297–3305. doi: 10.1128/mcb.7.9.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernars K., Goosen T., Wennekes L. M., Visser J., Bos C. J., van den Broek H. W., van Gorcom R. F., van den Hondel C. A., Pouwels P. H. Gene amplification in Aspergillus nidulans by transformation with vectors containing the amdS gene. Curr Genet. 1985;9(5):361–368. doi: 10.1007/BF00421606. [DOI] [PubMed] [Google Scholar]
- Zalacain M., González A., Guerrero M. C., Mattaliano R. J., Malpartida F., Jiménez A. Nucleotide sequence of the hygromycin B phosphotransferase gene from Streptomyces hygroscopicus. Nucleic Acids Res. 1986 Feb 25;14(4):1565–1581. doi: 10.1093/nar/14.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]