Abstract
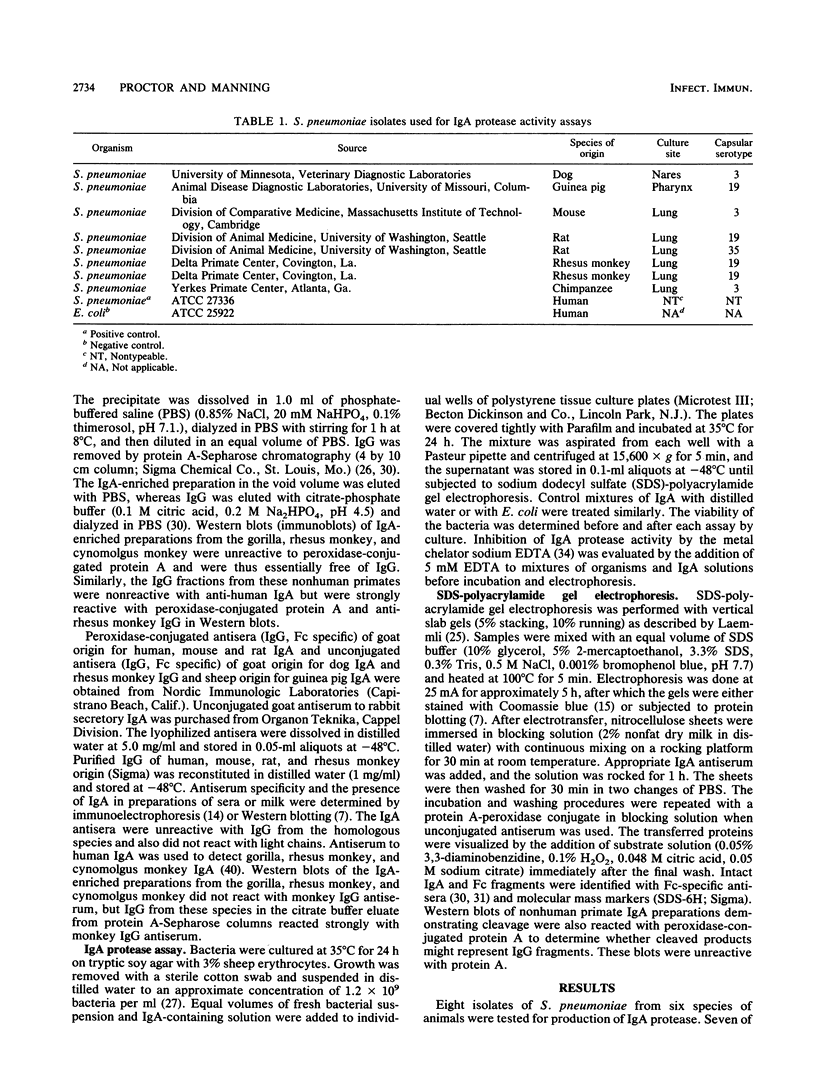
Human isolates of Streptococcus pneumoniae tested by traditional immunochemical methods produce a protease that cleaves human immunoglobulin A1 (IgA1) into Fab and Fc fragments. The protease may be an important virulence factor, but studies of its pathogenetic significance have been hampered by lack of a suitable animal model. Since S. pneumoniae is a respiratory pathogen for several species of animals, we sought to determine whether isolates of this organism from animals with pneumococcal infection, including fatal diplococcal pneumonia, produced an IgA protease. Isolates from six animal species including the mouse, rat, dog, guinea pig, rhesus monkey, and chimpanzee were tested for protease activity against IgA preparations from the mouse, rat, dog, guinea pig, rabbit, rhesus and cynomolgus monkeys, gorilla, and human. Cleavage of IgA was demonstrated by the appearance of Fc fragments in Western blots (immunoblots) treated with specific antisera. All these isolates except that from the guinea pig produced a protease that cleaved IgA of human, rhesus monkey, and gorilla origin. Cleavage was inhibited by 5 mM EDTA. IgA cleavage from the other species could not be demonstrated. Although S. pneumoniae can colonize the respiratory tracts of several animal species, it is a significant pathogen principally of humans and some other primates. Our data suggest that some species of nonhuman primates including the rhesus monkey could be suitable for experimental studies on the significance of IgA protease in the pathogenesis of pneumococcal disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7260–7269. [PubMed] [Google Scholar]
- Baer H. Diplococcus pneumoniae type 16 in laboratory rats. Can J Comp Med Vet Sci. 1967 Sep;31(9):216–218. [PMC free article] [PubMed] [Google Scholar]
- Baer H., Preiser A. Type 3 diplococcus pneumonia in laboratory rats. Can J Comp Med. 1969 Apr;33(2):113–117. [PMC free article] [PubMed] [Google Scholar]
- Benson C. E., Sweeney C. R. Isolation of Streptococcus pneumoniae type 3 from equine species. J Clin Microbiol. 1984 Dec;20(6):1028–1030. doi: 10.1128/jcm.20.6.1028-1030.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert P. L. Human Scarlatinal Streptococci in Monkeys. J Bacteriol. 1940 Jun;39(6):727–738. doi: 10.1128/jb.39.6.727-738.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker J., Mulks M., Moxon E. R., Plaut A. G., Wright A. Physical and genetic analysis of DNA regions encoding the immunoglobulin A proteases of different specificities produced by Haemophilus influenzae. Infect Immun. 1985 Feb;47(2):370–374. doi: 10.1128/iai.47.2.370-374.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Fallon M. T., Reinhard M. K., Gray B. M., Davis T. W., Lindsey J. R. Inapparent Streptococcus pneumoniae type 35 infections in commercial rats and mice. Lab Anim Sci. 1988 Apr;38(2):129–132. [PubMed] [Google Scholar]
- Fox J. G., Wikse S. E. Bacterial meningoencephalitis in rhesus monkeys: clinical and pathological features. Lab Anim Sci. 1971 Aug;21(4):558–563. [PubMed] [Google Scholar]
- Henderson J. D., Jr, Webster W. S., Bullock B. C., Lehner N. D., Clarkson T. B. Naturally occurring lesions seen at necropsy in eight woolly monkeys (Lagothrix sp.). Lab Anim Care. 1970 Dec;20(6):1087–1097. [PubMed] [Google Scholar]
- Hendley J. O., Sande M. A., Stewart P. M., Gwaltney J. M., Jr Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J Infect Dis. 1975 Jul;132(1):55–61. doi: 10.1093/infdis/132.1.55. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Schrohenloher R. E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide N., Muramatsu T. Endo-beta-N-acetylglucosaminidase acting on carbohydrate moieties of glycoproteins. Purification and properties of the enzyme from Diplococcus pneumoniae. J Biol Chem. 1974 Aug 10;249(15):4897–4904. [PubMed] [Google Scholar]
- Kornfeld S. J., Plaut A. G. Secretory immunity and the bacterial IgA proteases. Rev Infect Dis. 1981 May-Jun;3(3):521–534. doi: 10.1093/clinids/3.3.521. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Applications of immobilized protein A in immunochemical techniques. J Immunol Methods. 1982 Dec 30;55(3):277–296. doi: 10.1016/0022-1759(82)90088-6. [DOI] [PubMed] [Google Scholar]
- MIRICK G. S., RICHTER C. P., SCHAUB I. G., FRANKLIN R., MacCLEARY R., SCHIPPER G., SPITZNAGEL J. An epizootic due to pneumococcus type II in laboratory rats. Am J Hyg. 1950 Jul;52(1):48–53. doi: 10.1093/oxfordjournals.aje.a119408. [DOI] [PubMed] [Google Scholar]
- Male C. J. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect Immun. 1979 Oct;26(1):254–261. doi: 10.1128/iai.26.1.254-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. N. Chromatographic fractionation of rhesus monkey (Macaca mulatta) IgG subclasses using deae cellulose and protein A-sepharose. J Immunol Methods. 1982;50(3):319–329. doi: 10.1016/0022-1759(82)90170-3. [DOI] [PubMed] [Google Scholar]
- Mehta S. K., Plaut A. G., Calvanico N. J., Tomasi T. B., Jr Human immunoglobulin A: production of an Fc fragment by an enteric microbial proteolytic enzyme. J Immunol. 1973 Oct;111(4):1274–1276. [PubMed] [Google Scholar]
- Mestecky J., Kulhavy R., Kraus F. W. Studies on human secretory immunoglobulin A. II. Subunit structure. J Immunol. 1972 Mar;108(3):738–747. [PubMed] [Google Scholar]
- Mulks M. H., Kornfeld S. J., Plaut A. G. Specific proteolysis of human IgA by Streptococcus pneumoniae and Haemophilus influenzae. J Infect Dis. 1980 Apr;141(4):450–456. doi: 10.1093/infdis/141.4.450. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Grey H. M. Structure and function of immunoglobulin A. Prog Allergy. 1972;16:81–213. [PubMed] [Google Scholar]
- Weisbroth S. H., Freimer E. H. Laboratory rats from commercial breeders as carriers of pathogenic pneumococci. Lab Anim Care. 1969 Aug;19(4):473–478. [PubMed] [Google Scholar]