Abstract
Dietary compounds can influence the risk of cancer and other diseases through diverse mechanisms which include the activation or inhibition of macroautophagy. Macroautophagy is a catabolic process for the lysosomal degradation and recycling of cytoplasmic constituents which has been implicated in several pathologies, including cancer and neurodegeneration. In some instances, macroautophagy acts to suppress tumor formation and neural degeneration. Thus, it may be feasible to design diets, supplements or therapeutics that can alter the level of macroautophagy within cells to prevent or treat disease. While critical questions still need to be answered before we can safely and effectively implement such a strategy, we provide here a review of the literature regarding dietary constituents that have a demonstrated macroautophagy-modulating function.
Keywords: macroautophagy, autophagy, diet, nutrition, natural compounds, LC3, cancer, prevention
Nutrition in Health and Disease
It is generally recognized that nutrition has a significant role in health. For example, it has been estimated that diet accounts for about 30 percent of cancers in Western countries1 and dietary modification has been proposed as a means of reducing cancer risk.2 Several epidemiological studies have also shown a correlation between dietary habits and the development of neurodegenerative diseases.3 Many common foods contain ingredients that promote health and fight disease. These compounds can act by various mechanisms such as scavenging free radicals, maintaining DNA stability, increasing apoptosis, or decreasing cell proliferation.4
Many natural compounds, including spices and vitamins, are currently being used or assessed for disease prevention and treatment. Approximately half of the drugs used in the clinic today come from natural sources.5 These drugs include many of the most common anticancer therapeutics such as the taxanes, derived from plants of the genus Taxus (yews), and daunomycin-related agents, produced by fungi of the genus Streptomyces.6 Along with their many other mechanisms of action, we are now realizing that many of these natural compounds also affect macroautophagy.7–10
Autophagy
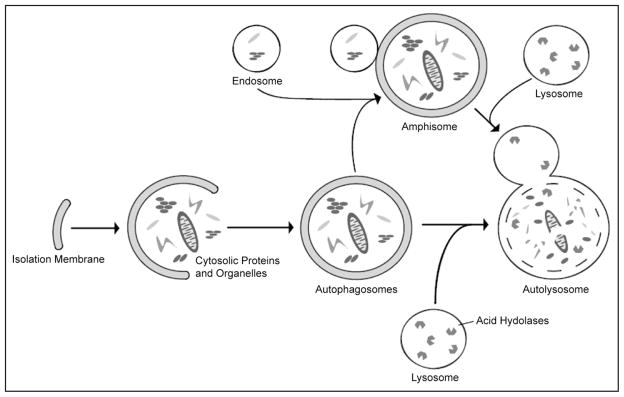
Autophagy, literally self-eating, is a general term for lysosomal degradation of cytosolic components such as long-lived proteins and organelles. There are three main forms of autophagy: macroautophagy, microautophagy and chaperone-mediated autophagy. The focus of this review is macroautophagy, a process that occurs when a double membrane of unknown origin, called an isolation membrane, forms and encompasses a portion of cytoplasm (Fig. 1). Closure of the double membrane forms an autophagosome which can fuse with an endosome to form an amphisome. Fusion of an autophagosome or amphisome with a lysosome forms an autolysosome where the hydrolytic enzymes from the lysosome degrade the components which can then be released back into the cytoplasm for use in bioen-ergetic and biosynthetic processes.11–13
Figure 1.
Diagram of macroautophagy. Macroautophagy occurs when a double membrane structure, called an isolation membrane, forms and encompasses a portion of cytoplasm. Completion of the double membrane forms an autophagosome which is able to fuse with an endosome to form an amphisome. Fusion of an autophagosome or amphisome with a lysosome forms an autolysosome where the acid hydrolases from the lysosome break down the inner membrane and cytoplasmic contents which are released back to the cytoplasm for use by the cell.
Macroautophagy, hereafter referred to as autophagy, occurs at basal levels in many cells to turn over long-lived cytosolic proteins and organelles, however it is upregulated in response to cell stresses such as starvation,14,15 radiation16 and an increase in reactive oxygen species.17 There is much controversy over the role of autophagy in cells under such stresses, with evidence supporting functions in both cell survival and cell death. For several recent reviews on this topic, see refs. 11 and 18–20.
Autophagy and Life Span
Almost all organisms experience a decrease in autophagy with age.21 Accordingly, studies in worms and flies have shown that blocking autophagy decreases life span, while overexpressing autophagy genes can increase life span.22,23 Calorie restriction (CR), which involves a reduction in dietary calorie intake, was shown to be the most effective method for increasing life span in many species, such as yeast, Drosophila and mammals.24,25 In addition to affecting life span, CR was also shown to delay the onset or reduce incidence of many age-related diseases such as cancer and neurodegeneration.25 While it is unclear how CR produces these benefits, much research has been done to determine potential mechanisms of action.
CR has been shown to activate sirtuins,25 which are also known as silent information regulator (SIRT) proteins. Sirtuins are nicotinamide adenine dinucleotide (NAD)-dependent deacetylases that regulate expression of several genes. For a review of sirtuins see refs. 26 and 27. SIRT1 is one of the main mammalian sirtuins that is upregulated in response to CR.26,28 Recent studies have shown that SIRT1 acts as a positive regulator of starvation-induced autophagy. One possible explanation for these observations is that SIRT1 increases the acetylation, and thus expression, of many autophagy-related genes.28 While the upregulation of sirtuins may have a direct role in the life-span extension induced by CR, autophagy may be another mechanism by which CR increases life span and maintains healthy cells.29
Autophagy in Health and Disease
Functions of autophagy include the degradation of protein aggregates that are too large for proteosomal degradation, removal of pathogens within the cell, and the removal of damaged proteins and organelles that may produce DNA-damaging reactive oxygen species.11 Loss of autophagy could therefore lead to neurodegen-erative disorders, infectious disease, or possibly genomic instability resulting in cancer. Autophagy has also been shown to play a role in other diseases including liver, muscle and heart disease.11
In the process of neurodegeneration, autophagy has been shown to play a protective role. Some neurodegenerative disorders, such as Parkinson disease and Huntington disease, are caused by an accumulation of mutant proteins that aggregate and interfere with cell trafficking and the normal function of cytoplasmic proteins. Typically these aggregates are too large to be dealt with by the proteasome; cell model studies have shown that they are instead cleared by autophagy.30,31 Mouse models have demonstrated that loss of autophagy leads to an accumulation of mutant protein aggregates as well as dispersed cytosolic mutant proteins which may otherwise form aggregates. Both are defects that resemble features observed in several neurodegenerative diseases and thus suggest that autophagy has a neuroprotective function.31–33
In cancer, autophagy appears to have dual roles dependent on a number of factors including stage of disease, cell type and cell stimulus. Prior to cancer development, autophagy can act as a tumor suppressor by removing oxidative stresses and damaged organelles which could otherwise have DNA-damaging effects. Conversely, in established tumors, autophagy can promote tumor survival by allowing cells to adapt to the harsh conditions of the tumor microenvironment and can protect cells from undergoing apoptosis in response to anticancer therapies. Alternatively, autophagy appears to promote cell death in response to some anticancer therapies. The role of autophagy in cancer is discussed extensively in several recent reviews.11,31,34–36
Monitoring Autophagy
There are several methods, thoroughly described in reference 37, used to monitor autophagy. The methods relevant to this review are described briefly here, along with some of their limitations. One common approach is the use of acidotropic stains such as monod-ansylcadaverine (MDC),38 LysoTracker and acridine orange16 which label acidic compartments. These stains label autolysosomes but also other acidic compartments within cells, such as lysosomes and endosomes and thus should be substantiated by other assays that are specific to autophagy.37 Electron microscopy can also be used to monitor autophagy. The resolution of electron micrographs allows the identification of many autophagic structures, with the double-membrane autophagosome being the most easily recognized.39
Microtubule-associated protein 1 light chain 3β, or LC3, the mammalian homologue of yeast Atg8, is an autophagy protein that can be used to monitor autophagy. LC3 exists in two forms within cells. LC3-I, the cytosolic form, is predominant under normal conditions but is converted into LC3-II during autophagy by the addition of phospatidylethanolamine (PE) which allows for inclusion in the autophagosome membrane. The LC3-II form is located on both the inner and outer surfaces of the autophagosome membrane. The conversion of LC3-I to LC3-II can be visualized with a western blot and comparison of the amounts of LC3-II to appropriate controls (such as actin) can give an indication of relative levels of autophagy between samples. Relative changes in LC3-II are tissue and cell-type specific40 so it is very important to have both positive and negative controls to confirm that the assay is behaving as expected.37 Fusion of a fluorescent marker, such as green fluorescent protein (GFP), to the N-terminus of LC3 (or yeast Atg8) allows one to assess the subcellular localization of LC3. Under normal conditions LC3 appears diffuse in the cytoplasm, while under autophagy-inducing conditions, LC3 appears as punctate structures (puncta) which represent autophagosomes.41 The localization of LC3 in cells can also be performed using anti-LC3 antibodies by immunohistochemistry42 or immunofluorescence.43
A caveat of the above-mentioned methods is that they are steady state measurements. Increases in the number of autophagosomes or autolysosomes may be due to an increase in autophagy occurring in the cell but it could also be caused by a blockage at a later stage in the process. It is necessary to use other methods to measure autophagic flux (i.e., lysosomal delivery and degradation) to determine how exactly autophagy is being affected.37
There are several methods that have been employed to monitor autophagic flux. One such method is the sequestration assay. This is typically conducted by injecting cytosolic [3H]-raffinose into cells and measuring the transfer of this cargo into the insoluble cell fraction, which includes autophagic compartments.44 Another method to analyze autophagic flux is by monitoring protein degradation using radiolabelled proteins and measuring the release of radioactive amino acids into the cytosol.37 A method used to analyze autophagic flux in yeast is the Ape1 (aminopeptidase 1) maturation assay which involves conversion of the Ape1 precursor to the mature protein within the vacuole (the yeast equivalent of the lysosome).45–48 Additional strategies to measure autophagic flux, including monitoring the turnover of LC3-II or the clearance of autophagic substrates, are described in reference 37.
Major Dietary Components and Autophagy
Nutrient availability is the best characterized regulator of autophagy. As such it is not surprising that the major dietary components, protein, carbohydrates and fats, are able to influence the levels of autophagy.
Amino acids, the breakdown products of proteins, are well known to be regulators of autophagy. Amino acid starvation induces autophagy while the presence of some amino acids is sufficient to inhibit autophagy. Specifically, leucine, tyrosine and phenylalanine are able to suppress autophagy in various cell types.49,50 The suppression of autophagy in the presence of amino acids occurs by signaling through mTOR complex 1.51,52 Amino acids cause an increase in intracellular calcium which leads to enhanced binding of calmodulin to hVps34 and activation of mTOR complex 1, a suppressor of autophagy.52 This hVps34 containing complex is distinct from the Beclin 1/hVps34 complex which is required for autophagosome formation. Thus hVps34 appears to be able to regulate two distinct autophagy responses via different complexes.52 As both plasma and muscle amino acid levels change in response to dietary modification,53 it may be possible to induce or inhibit autophagy by eating or not eating particular proteins.
Carbohydrates and lipids have not been shown to have a direct effect on autophagy, however they may be able to regulate autophagy indirectly through their effects on insulin levels. Carbohydrates are broken down and presented to cells as glucose, which is the main energy source for the body.54 The main role of glucose is to regulate endocrine pathways such as the insulin pathway.55 The presence of glucose induces insulin secretion which leads to the activation of mTOR and the suppression of autophagy. An increase in glucose uptake by cells can lead to increases in both ATP and H2O2 and render the insulin receptor constitutively active, thus constantly suppressing autophagy.54 In addition, an increase in glucose uptake also results in an increase in glycolysis. The result of this is an increase in NADH and a decrease in NAD, which causes a decrease in sirtuin activity and may also play a role in suppressing autophagy.54
High glucose availability causes a decrease in the life span of C. elegans.54 This is not surprising as lack of autophagy has been associated with shortened life span, and high levels of glucose suppress autophagy.22,23
Insulin resistance induced by a high-fat diet was shown recently to increase autophagic vacuole formation in the β-cells of nondiabetic C57BL/6 mice.56 Serum levels of free fatty acids are increased in insulin-resistant states.56 Fatty acids can interact with the GPR40 receptor which leads to an increase in intracellular calcium ions, which is thought to increase insulin secretion.57 As discussed above, insulin leads to activation of mTOR and suppresses autophagy. Therefore, one might expect that in the case of insulin resistance, mTOR fails to be activated and thus autophagy can occur.
Dietary Compounds which Affect Autophagy
Given the associations between autophagy and human disease, as well as the impact of nutrition on disease occurrence, we review here the literature regarding specific dietary compounds that affect autophagy. Where relevant, we have included information regarding the investigation of these compounds as chemopreventative agents or therapeutic agents for cancer and other diseases. The compounds described below have been shown to affect autophagy based on changes in the levels of autophagic and/or acidic structures or measurements of autophagic flux. The latter has not been measured in the majority of the cited literature so it is currently unknown, in those cases, whether the compound actually induces or inhibits the autophagic process. The following information is summarized in Table 1.
Table 1.
Summary of dietary compounds that affect autophagy
| Dietary Compound | Major Dietary Source | Effect on Autophagic & Acidic Structures | Effect on Autophagic Flux | Concentration | Cell Line and Type | Method of Autophagy Analysis | +/− Apoptosis | Reference |
|---|---|---|---|---|---|---|---|---|
| Amentoflavone | Gingko and some other plants | ND | Inhibit | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 |
| Apigenin | Celery and parsley | ND | Inhibit | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 |
| Benzyl isothiocyanate | Cruciferous vegetables | Increase autophagosomes | ND | 2.5μM | In vitro: MDA-MB-231 breast cancer cells | EM | + | 67 |
| Bromovanin | Derivative of vanillin from vanilla beans | Increase, autophagosomes | ND | 40μM | In vitro: HepG2 liver cancer cells | EM | + | 69 |
| Caffeine | Coffee, tea, cocoa, and soft drinks | Increase autophagosomes | Induce | 10mM | Yeast strains: Saccharomyces cervisiae and Zygosaccharomyces bailii | GFP-ATG8 localization Ape I maturation assay | ND | 78 |
| Concanavalin A | Jack bean seeds | Increase autophagosomes and acidic structures | ND | 40μg/ml | In vitro: ML-1 hepatoma cells | GFP-LC3 localization EM L03 conversion Acridine orange staining |
- | 79 |
| Increase autophagosomes and acidic structures | ND | 40μg/ml | In vitro: Huh-7, HepG2 and CT-26 hepatoma cells | LC3 conversion Acridine orange staining |
- | 79 | ||
| Increase autophagosomes | ND | 40mg/kg | In vivo: Mouse hepatocytes | LC3 IF | ND | 80 | ||
| Curcumin | Tumeric | Increase autophagosomes and acidic structures | ND | 40μM | In vitro: U87-MG and U373-MG malignant glioma cells | AVO quantification GFP-LC3 localization LC3 western blotting |
- | 84 |
| Increase autophagosomes | ND | 100mg/kg | In vivo: U87-MG malignant glioma cells | LC3 IHC LC3 conversion |
- | 84 | ||
| Fenugreek | Wheat and maize flour (in Egypt) | Increase autophagosomes | ND | 20mg/kg | In vivo: DMBA induced breast tumours in female Wistar rats | EM | ND | 92 |
| Fisetin | Several fruits and vegetables | ND | Inhibit | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 |
| Genistein | Soy | Increase autophagosomes | ND | 50μM, 100μM | In vitro: A2780 ovarian cancer cells | GFP-LC3 localization | + | 95 |
| ND | Inhibit | 300μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 | ||
| ND | No effect | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 | ||
| Hesperetin | Citrus fruits and peppermint | ND | Inhibit | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 |
| Hop-derived prenylflavanones | Beer | Increase vacuoles | ND | 200μM | In vitro: PC-3 and DU145 prostate cancer cells | Vacuole accumulation | - | 103 |
| Lithium | Grains, vegetables and some drinking water | Increase autophagosomes | Induce | 10mM | In vitro: COS-7 African green monkey kidney cells PC12 rat pheochromocytoma cells | LC3 IF LC3 conversion Autophagic substrate clearance |
ND | 105 |
| Luteolin (aglycone) | Perilla seeds | ND | Inhibit | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 |
| Luteolin (glycosylated) | Celery, green pepper, and chamomile tea | ND | Inhibit | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 |
| MK615 | Japanese apricot | Increase autophagosomes | ND | 300μg/ml | In vitro: SW480, COLO and WiDr colon cancer cells | EM LC3 IF |
+ | 109 |
| Increase autophagosomes | ND | 300μg/ml | In vitro: MDA-MB-468 and MCF-7 breast cancer cells | EM | + | 108 | ||
| Quercetin | Apple skins and red onions | ND | Inhibit | 100μM | In vitro: Rat hepatocytes | Sequestration of [3H]-raffinose | ND | 59 |
| No effect | ND | 20μM | In vitro: Cuco-2 colorectal cancer cells | MDC staining GFP-LC3 localization | + | 111 | ||
| Increase autophagosomes and acidic structures | ND | 20μM | In vitro: Ras transformed Caco-2 colorectal cancer cells | MDC staining GFP-LC3 localization | + | 111 | ||
| Resveratrol | Grapes, nuts, and red wine | Increase autophagosomes and acidic structures | ND | 50μM | In vitro: A2780 ovarian cancer cells | EM MDC staining |
- | 115 |
| Increase autophagosomes | ND | 50μM, 100μM | In vitro: A2780 ovarian cancer cells | GFP-LC3 localization | - | 116 | ||
| Increase autophagosomes | ND | 100μM |
In vitro: H460 lung cancer cells Heel A and Heel B endometrial cancer cells HSY salivary gland carcinuma cells MCF-7 breast cancer cells |
GFP-LC3 localization | ND | 117 | ||
| Increase autophagosomes | Induce | 64μM | In vitro: MCF-7 breast cancer cells | GFP-LC3 localization LC3 conversion Degradation of long-lived proteins |
+ | 118 | ||
| Sulforaphane | Cruciferous vegetables | Increase autophagosomes and acidic structures | ND | 40μM | In vitro: PC-3 and LNCaP prostate cancer cells | EM Acridine orange LC3 IF |
+ | 124 |
| Increase autophagosomes | ND | 40μM | In vitro: PC-3 prostate cancer cells | GFP-LC3 localization LC3 conversion |
+ | 124 | ||
| Tocotrienols | Subclass of vitamin E; Palm oil and rice bran | Increase autophagosomes and acidic structures | ND | 20μM | In vitro: Pancreatic stellate cells | MDC LC3 conversion |
+ | 126 |
| Triterpenoid Saponins (Group B) | Intact legumes and soy foods | Increase autophagosomes and acidic structures | ND | 100ppm | In vitro: HCT-15 colon adenocarcinoma | EM MDC LC3-I and LC3-II concentrations |
ND | 128 |
| Vitamin C (aseorbate) | Fruit and vegetables | Increase autophagosomes and acidic structures | ND | 500μM | In vitro: H1299 non-small cell lung cancer cells | Acridine orange GFP-LC3 localization |
+ | 131 |
| Vitamin D (and EB 1089) | Fatty fish and fortified dairy products | Increase autophagosomes and acidic structures | ND | 100nM | In vitro: MCF-7 breast cancer cells | MDC staining Lysotracker staining dsRed-LC3 localization EM |
+ | 135 |
| Increase autophagosomes | Induce | 100nM | In vitro: MCF-7 breast cancer cells | GFP-LC3 localization EM Degradation of long-lived proteins |
ND | 136 | ||
| Vitamin K2 (menaquinone) | Fermented foods | Increase autophagosomes and autolysosomes | Induce | 10μM | Irt vivo: HL-60 leukemia cells | Acridine orange LC3 conversion LC3 localization LC3-II turnover |
+ | 140 |
Abbreviations: EM, electron microscopy; IF, immunofluorescence; IHC, immunohistochemistry; ND, not done.
Note: Apoptosis annotations refer specifically to information contained in listed references. See text for additional information.
Amentoflavone
Amentoflavone is a secondary metabolite found in many plants with medicinal properties, including Ginkgo biloba.58 In vitro studies in rat hepatocytes showed that 100 μM amen-toflavone inhibits autophagy. The authors used sequestration of [3H]-raffinose as a measure of autophagy and found a greater than five-fold decrease in autophagy upon treatment with amentoflavone compared to untreated controls.59
Later studies on amentoflavone found the compound also has anti-inflammatory and antioxidative properties. In addition, amen-toflavone is able to inhibit phospholipase C1 and cAMP-dependent phosphodiesterase, iNOS expression, NFκB activation and IκB degradation.60
Apigenin
Apigenin is a plant secondary metabolite and phytoestrogen found in celery, olives, hot peppers, parsley, oregano, rosemary and thyme.61 In vitro studies in rat hepatocytes showed that 100 μM apigenin resulted in autophagy inhibition. Sequestration of [3H]-raffinose revealed a greater than five-fold decrease in autophagy upon treatment with apigenin compared to untreated controls.59
Apigenin was shown to increase SIRT1 activity in a yeast model62 and another study suggested that SIRT1 is a positive regulator of autophagy.28 However, the latter study was conducted in mammalian cells so it may be that these potentially contrasting findings are due to cell type or species differences. Apigenin has many other molecular targets, including mitochondrial F0F1-ATPase.63 In addition, apigenin induces cell cycle arrest in epidermal, fibroblast and human colon cancer cell lines, and inhibits growth in colon cancer cells and skin cancer mouse models, suggesting it may be useful as an anticancer therapy.64
Benzyl isothiocyanate
Benzyl isothiocyanate is a compound found in cruciferous vegetables which has chemoprotective properties.65,66 Treatment of MDA-MB-231 breast cancer cells with 2.5 to 10 μM benzyl isothiocyanate resulted in cell cycle arrest, apoptosis, and an increase in the number of autophagic structures as determined by electron microscopy.67 The authors also found that treatment with 2.5 μM benzyl isothiocyanate led to a decrease in the amount of Bcl-2 protein and an increases in the generation of reactive oxygen species,67 both of which have been associated with an increase in autophagy.17,68
Bromovanin
Bromovanin is a derivative of vanillin, the agent responsible for the aroma and flavor of vanilla. In vitro studies in the HepG2 liver cancer cell line showed that treatment with 40 M bromovanin led to an accumulation of autophagic structures. The authors used electron microscopy to quantify autophagic structures and showed that bromovanin treatment led to an increase of organelle-containing vacuoles, presumably autophagosomes.69 Additional analyses showed that bromovanin also induced apoptosis and double-stranded DNA breaks in some cell lines, decreased the activity of the DNA-dependent protein kinase catalytic subunit, promoted the degradation of c-Myc, a proto-oncogene, and led to an increase in reactive oxygen species,69 which has been shown to be required for autophagy.17
Caffeine
Caffeine occurs naturally in coffee, tea and cocoa, and is added to soft-drinks and cola as a flavoring component.70 A study performed in food spoilage yeasts Zygosaccharomyces bailii and Saccharomyces cervisiae found that autophagy is induced upon treatment with 10 mM caffeine.71 The authors used the Ape1 maturation assay as well as GFP-Atg8 localization to measure autophagy flux and induction.
It is unknown whether caffeine also induces autophagy in humans, however studies have shown that caffeine can inhibit many phosphotidylinositol kinase-related kinases in vivo, with preference for mTOR, a negative regulator of autophagy.72 In addition, caffeine has been shown to have many functions such as inducing apoptosis, antagonizing adenosine receptors, and interfering with several regulatory proteins such as p53.73,74
Several studies have shown the ability of caffeine to improve learning and memory in humans.75–77 One study showed that caffeine was able to reverse the cognitive impairments of Parkinson disease, a neurodegenerative disorder involving cytoplasmic inclusion bodies and aggregates, in 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyrindine (MPTP) treated rats, a model for early stage Parkinson disease.78 As autophagy has been shown to be involved in the clearance of these aggregates in cell models,31 it is possible that caffeine exhibits neurological effects by inducing autophagy.
It has been estimated that human consumption of caffeine is around 1–2 mg/kg per day with one cup of coffee resulting in a peak plasma concentration of 1–10 μM, much lower than the tested concentrations. Interestingly, regular coffee consumption has been linked to decreased mortality.72
Calcium
See Vitamin D below.
Concanavalin A
Concanavalin A (Con A) is a mannose-specific lectin found in Jack bean seeds. In vitro studies showed that 40 μg/ml Con A was able to induce nonapoptotic cell death in ML-1 hepatoma cells. Further studies showed that Con A leads to the accumulation of autophagic and acidic structures as detected by GFP-LC3 localization, LC3 conversion, electron microscopy and acridine orange staining. Similar results were also seen in Huh-7, HepG2 and CT-26 hepatoma cells. The authors found that Con A is also cytotoxic to hepatoma cells in vivo and less cytotoxic to normal hepatocytes, suggesting that Con A may be useful as an antihepatoma therapeutic.79,80
Curcumin
Curcumin, the active ingredient in turmeric, is the compound that gives curry its yellow colouring and distinctive taste.81 As a nontoxic and pharmacologically active compound, curcumin has been suggested as a potential anticancer drug.82 In vitro studies in U87-MG and U373-MG glioma cells showed that treatment with 40 μM curcumin resulted in an increase in the number of acidic and autophagic structures as detected by quantification of acidic vesicular organelles (AVO’s), GFP-LC3 localization and LC3 conversion. Curcumin also induced cell death which was inhibited by treatment with the nonspecific autophagy inhibitor 3-MA,83 suggesting that autophagy may help to promote cell death in curcumin treated glioma cells.84 Further in vivo studies found that administration of 100 mg/kg curcumin was able to inhibit growth of subcutaneous U87-MG tumors and increase the number of autophagosomes as determined by immunohistochemistry of LC3 and analysis of LC3 conversion.84 Curcumin was found to induce autophagy by downregulating the Akt/mTOR/p70S6K pathway and activating the ERK1/2 pathway.84,85
Curcumin has been used in traditional Indian medicine for centuries; in 2007, there were already 13 clinical trials underway employing curcumin. Several studies have shown curcumin to have potential for treatment of several cancers as well as Alzheimer disease.81
The average daily intake of curcumin in France, where curry is frequently consumed, is about 1 mg/kg86 with up to 100 mg/day being nontoxic.87 The bioavailability of ingested curcumin is low88 with the gastrointestinal tract exposed to the largest concentration of unmetabolized curcumin.89 Studies have shown that the bioavailability of curcumin can be enhanced with ingestion of food additives such as piperine (a constituent of pepper)90 which are commonly consumed in Asian diets.89
Fenugreek
Fenugreek is a legume grown widely in India, Egypt and Middle Eastern countries. It is often used as whole seed and is processed into flour and added to wheat products as a protein supplement.91 Studies conducted in Wistar rats showed that administration of 20 mg/kg Fenugreek seed extract daily for seven days was protective against 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer. Electron microscopy showed that breast tissues that were first treated with Fenugreek had extensive vacuolization compared to breast tissue not treated with Fenugreek. Several vacuoles contained cytoplasmic material and organelles, suggesting an autophagic origin.92 The active ingredient in Fenugreek seeds that is responsible for this effect is unknown.
Fisetin
Fisetin is a plant secondary metabolite found in many fruits and vegetables such as tomatoes, onions, grapes and apples.61 In vitro studies in rat hepatocytes showed that 100 μM fisetin was able to inhibit autophagy by more than five-fold compared to untreated controls. The authors used sequestration of [3H]-raffinose to measure autophagic flux.59
Fisetin was shown to increase SIRT1 activity in a yeast model62 and, as noted above, activation of SIRT1 was associated with an increase in autophagic structures.28 The apparently discrepant findings may be explained by the different model systems employed. A study in HL-60 leukemia cells showed that fisetin is also able to induce apoptosis.93 Other studies have demonstrated the ability of fisetin to reduce cell proliferation of colon cancer cells without cytotoxic effects,94 suggesting that it may be useful for prevention of colon cancer.
Genistein
Genistein is a plant secondary metabolite found in soy products. Having many mechanisms of action, such as apoptosis induction, cell cycle arrest and inhibition of angiogenesis, genistein has been found to be both chemopreventative and chemotherapeutic in several cancers.95,96 In vitro studies in A2780 ovarian cancer cells found that both 50 and 100 μM genistein increased the number of autophagosomes, as determined by GFP-LC3 localization. Treatment of A2780 cells with genistein was associated with a decrease in phosphorylated Akt.95 Accordingly, a study in yeast showed that genistein slightly activates SIRT1,62 which has been shown to be associated with an increase in autophagic structures.28 Conversely, a study in rat hepatocytes found that 100 μM genistein had no autophagy-related effect, while 300 μM genistein moderately inhibited autophagy. In this study the investigators used sequestration of [3H]-raffinose as a measure of autophagic flux.59 The opposing findings of the studies cited above may be attributed to several factors such as different methods used to measure autophagy and the use of different cell types, such as cancerous cells versus normal cells.
Genistein also inhibits mitochondrial proton F0F1-ATPase/ATP synthase with an IC50 of 55 μM.63 The blockage of ATP synthesis by genistein may be one way that the compound signals the cell to undergo autophagy. Consistent with this idea, the ATP synthase inhibitor oligomycin A was shown to induce autophagy in the IPLB-LdFB insect cell line.97 A decrease in ATP concentrations leads to an increase in the AMP/ATP ratio and activation of AMP-activated protein kinase (AMPK) by binding of AMP. AMPK activates tuberous sclerosis complex 2 (TSC2) which suppresses mTOR and thus activates autophagy.98 It has been suggested that AMPK may also activate autophagy via other mechanisms which have yet to be tested.99
Several studies have shown genistein to have poor bioavailability. A review of several studies showed that maximum plasma concentrations range from 0.4–25.4 μM,100 lower than the tested concentrations mentioned above.
Hesperetin
Hesperedin is a plant secondary metabolite found in peppermint and citrus fruits.61 Hesperedin is deglycosylated by bacteria in the intestine to form hesperetin, which is the biologically active form of the compound.101 In vitro studies in rat hepatocytes showed that 100 μM hesperetin has moderate inhibitory effects on autophagy. The authors used sequestration of [3H]-raffinose as a measure of autophagy and found almost a two and a half-fold decrease in autophagy upon treatment with hesperetin.59
Hesperetin has also been found to inhibit cell proliferation and induce cell cycle arrest and apoptosis in MCF-7 breast cancer cells, and thus has been suggested as a potential anticancer agent.101 However, the bioavailability of hesperetin is quite low with maximum plasma levels reaching only 1.3–2.2 μM after intake of 130–220 mg hesperetin.102
Hop-derived prenylflavanones
Hop, an essential ingredient for making beer, contains prenylchalcones that are isomerized into prenylflavanones. In vitro studies showed that 200 μM hop-derived prenylflavanones induced cell death in PC-3 and DU145 prostate cancer cells which was not characteristic of apoptosis, but was characterized by an accumulation of vacuoles suggesting an autophagy-associated mechanism.103
Lithium
Lithium is an alkali metal found in grains, vegetables, meat and drinking water in a number of regions.104 In vitro studies in COS-7 African green monkey kidney cells showed that 10 mM lithium increases the number of autophagosomes as visualized by LC3 immunofluorescence and LC3 conversion. The authors found this autophagy effect was independent of mTOR signaling but rather occurred through the inhibition of inositol monophosphatase.105 These findings may explain the ability of lithium to protect against Huntington disease, a neurodegenerative disorder involving protein aggregation. The authors also found that autophagy, as induced by lithium administration, was able to facilitate clearance of mutant huntingtin as well as mutant α-synuclein which is found in some forms of Parkinson disease.105
The estimated daily intake of lithium for a 70 kg American adult is 650–3100 μg with serum levels of 7–28 μg/L corresponding to intakes of 385–1540 μg.104
Luteolin
Luteolin is a flavone that occurs in various forms in nature. Luteolin aglycone is found in perilla seeds, while glycosylated luteolin is found in celery, green pepper and chamomile tea.106 In vitro studies in rat hepatocytes showed that 100 μM luteolin agly-cone and glycosylated luteolin were able to inhibit autophagy almost seven-fold and three-fold, respectively. The authors used sequestration of [3H]-raffinose as a measure of autophagic flux.59 In apparent contrast, luteolin was shown to increase SIRT1 activity in a yeast model.62
Luteolin has been found to have antitumorigenic properties in several human cancer cell lines and there is also in vivo evidence that it can improve the activity of paclitaxel therapy for oral squamous cell carcinoma.107 It is possible that luteolin exerts this synergistic effect by inhibiting autophagy; in some instances autophagy provides a way for cells to avoid or delay apoptosis. Thus, reducing autophagy may enhance anticancer therapy in these cases.31
MK615
MK615 is an extract of compounds from the Japanese apricot. In vitro studies on MDA-MB-468 and MCF-7 breast cancer cells showed that 300 μg/ml MK615 is able to inhibit cell proliferation and induce apoptosis accompanied by an accumulation of vacuoles.108 Further studies in SW480, COLO and WiDr colon cancer cells showed similar antiproliferative and apoptosis inducing effects and characterized the cytoplasmic vacuoles as autophagic structures using GFP-LC3 localization.109
Quercetin
Quercetin is a plant secondary metabolite that accounts for the pigment found in many fruits and vegetables, such as apple skins and red onions.110 In vitro studies in rat hepatocytes showed that 100 μM quercetin was able to inhibit autophagy greater than four-fold as determined by the sequestration of [3H]-raffinose.59 Another study found that 20 μM quercetin had no effect on autophagy in Caco-2 colon cancer cells but was able to increase the number of autophagosomes and acidic structures in Caco-2 cells transformed with oncogenic Ras. These studies were conducted using GFP-LC3 localization and MDC staining, respectively.111 The opposing findings of the studies cited above may be attributed to several factors such as different methods used to measure autophagy and the use of different cell types.
In Caco-2 colorectal cancer cells with oncogenic Ras, quercetin was able to induce Ras degradation, and thus has the potential to be used for colorectal cancer prevention.111 Quercetin has other characteristics which make it attractive as a chemopreventative agent, such as antioxidative activity, the ability to inhibit enzymes that activate carcinogens, and the ability to modify receptor interactions and signaling pathways.112
Quercetin also has effects on SIRT1 activation,62 F0F1-ATPase and several other ATPases,63 cell proliferation, survival and differentiation.111 As such, it has been suggested as a potential anticancer agent and initial studies in animal models and humans have shown that quercetin is able to inhibit tumor growth.111
Quercetin has quite low bioavailability with maximum plasma concentrations of 0.3–7.5 μM, lower than the tested concentrations, after administration of 80–100 mg dietary quercetin.102
Resveratrol
Resveratrol is a natural phytoalexin found in grape skin, nuts and red wine. Resveratrol has been studied for many years and has been shown to have antioxidant, anti-inflammatory and anticancer properties,113 and several molecular targets have been identified, including p53 and many kinases.27,114 Several recent studies have focused on determining the molecular activity of resveratrol that accounts for its anticancer function. In vitro studies in A2780 ovarian cancer cells showed that 50 μM resveratrol induces cell cycle arrest and nonapoptotic cell death. Further analysis confirmed that resveratrol induces the accumulation of autophagic and acidic structures as determined by electron microscopy and MDC staining.115,116 It was subsequently determined that the induction of autophagy by resveratrol occurs through downregulation of phospho-Akt and mTOR.116
The autophagy-regulating function of resveratrol was analyzed in several other cell lines with the same effect. GFP-LC3 localization in H460 lung cancer cells, Hec1A and Hec1B endometrial cancer cells, HSY salivary gland carcinoma cells and MCF-7 breast cancer cells also showed an increase in autophagic structures after treatment with 100 μM resveratrol.117
Further studies in MCF-7 breast cancer cells showed that 64 μM resveratrol increases autophagic flux and induces autophagy-associated cell death as determined by MDC staining, GFP-LC3 localization and assays measuring the degradation of long-lived proteins. The authors further determined that this induction of autophagy occurs via a noncanonical Beclin 1-independent pathway that is insensitive to PI3K inhibition.118
Resveratrol also inhibits mitochondrial proton F0F1-ATPase/ATP synthase at concentrations between 0.7–70 μM.63 The blockage of ATP synthesis by resveratrol may be one way that the compound signals the cell to undergo autophagy. Consistent with such a mechanism, the ATP synthase inhibitor oligomycin A was shown to induce autophagy in the IPLB-LdFB insect cell line.97 As described above, reduced ATP levels lead to activation of AMPK, suppression of mTOR, and subsequent activation of autophagy.98
The ability of resveratrol to induce autophagy is particularly interesting in light of the finding that resveratrol is able to prevent the harmful effects of a diet high in calories. Several studies have shown that CR is able to extend the life span of many model organisms, and at least some of this effect has been attributed to the activation of SIRT1 and the induction of autophagy under such conditions.11,119 Studies in mice have shown that consumption of resveratrol is able to rescue mice from the harmful effects of a high calorie diet and improve overall health and survival.120 As resveratrol has been shown to induce autophagy and activate SIRT1, both of which have been associated with the health benefits of CR, it is possible that these are the mechanisms by which resveratrol also increases life span.114,121
Although resveratrol is rapidly absorbed after oral administration, it has low bioavailability, with plasma levels below 5 ng/ml.114 One reason for this is that resveratrol is quickly metabolized. It is very possible that some of the biological benefits we see from resveratrol administration are due to the activity of its metabolites but this has not yet been determined.
Sulphoraphane
Sulphoraphane is an isothiocyanate found in cruciferous vegetables and some other plants. Sulphoraphane has several anticancer functions such as protecting cells from genotoxic damage, and inducing cell cycle arrest and apoptosis.122 As such, it has been suggested both as a cancer chemopreventative agent as well as a potential cancer therapeutic.123 In vitro work in PC-3 prostate cancer cells showed that sulphoraphane can also affect autophagy. Cells treated with 40 μM sulphoraphane exhibited an increase in autophagic and acidic structures as visualized by electron microscopy, LC3 immunofluorescence, LC3 conversion, GFP-LC3 localization, and acridine orange. This effect on autophagy was associated with decreases in both cytochrome c release and apoptosis, also induced by sulphoraphane, which were reversed when autophagy was blocked. These findings suggest that autophagy has a protective effect in sulphoraphane treated PC-3 cells. Similar effects were also seen in sulphoraphane treated LNCaP prostate cancer cells.124
Sulphoraphane also leads to a decrease in Bcl-2 expression.122 As Bcl-2 has been shown to suppress autophagy by binding Beclin1,68 it is possible that reducing available Bcl-2 is one way in which sulphoraphane activates autophagy.
Tocotrienols
Tocotrienols are a naturally occurring subclass of vitamin E compounds that are found in palm oil and rice bran and are well-known as antioxidants.125 When treated with 20 μM tocotrienols, cells that can lead to pancreatic fibrosis, called pancreatic stellate cells, showed an increase in acidic and autophagic structures as determined by MDC staining and LC3 conversion, respectively.126
Tocotrienols have been found to have both anticancer and neuroprotective effects (reviewed in ref. 127) While yet uninvestigated, these health-promoting characteristics of tocotrienols may be somewhat attributable to their ability to induce autophagy.
Triterpenoid saponins (Group B)
Soyasaponins are a class of trit-erpenoids encompassing three groups of sapogenols, A, B and E. The B-group is the predominant class in the human diet, found in intact legumes and soy products.128 In addition to inhibiting cell cycle progression, proliferation and phosphokinase C acitivty, B-group triterpenoid saponins are able to affect autophagy. An in vitro study in HCT-15 colon adenocarcinoma cells showed that treatment with 100 p.p.m. B-group soyasaponins resulted in an increase in acidic structures as determined by MDC staining, and an increase in autophagic structures as determined by electron microscopy or LC3-I and LC3-II concentrations.128 Treatment with triterpenoid saponins resulted in inactivation of Akt and activation of the ERK1/2 pathway. Soyasaponins have low bioavailabilty, appearing to pass undigested through the small intestine after consumption of soy.128
Soy products contain both B-group triterpenoid saponins and genistein (mentioned previously), both of which have anticancer properties. Diets high in soy products, such as diets of many Asian countries, have been associated with decreased levels of breast and prostate cancer,96 and several in vitro, and animal studies have supported this notion.129
Vitamin C
Vitamin C, also known as ascorbate, is found in many fruits and vegetables, especially citrus fruits.130 In addition to inducing apoptosis, 500 μM vitamin C increases the number of autophagosomes and acidic structures in H1299 nonsmall cell lung cancer cells as determined by GFP-LC3 localization and acridine orange staining, respectively.131 This finding is consistent with data showing that some patients with neurodegeneration have low serum levels of vitamin C132 which may translate to low levels of autophagy, a process implicated in neurodegeneration.
It has been estimated that eating at least five fruits and vegetables a day will result in an intake of 210–280 mg of vitamin C. At these doses, vitamin C has about 100 percent bioavailability, however these calculations were done based on a fasted state and the true bioavailability when administered as part of a normal diet is unknown.133
Vitamin D
Vitamin D is a prohormone that is found in fatty fish and fortified dairy products and is produced naturally in the skin (as vitamin D3) upon exposure to ultraviolet light.134 It has long been known that vitamin D has health benefits. High intake of both calcium and vitamin D is associated with a decreased risk of colon, breast, prostate and ovarian cancer.134
EB1089 (seocalcitol) is a vitamin D analogue that is being developed for chemotherapeutic use. In vitro studies in MCF-7 breast cancer cells showed an increase in autophagosomes and acidic structures after treatment with 100 nM EB1089. Autophagy was measured using electron microscopy, MDC and Lysotracker staining and dsRed-LC3 localization.135 Another study showed that 100 nM vitamin D3 (the active form of vitamin D) as well as 100 nM EB1089 induced autophagy in MCF-7 breast cancer cells as determined by GFP-LC3 localization, electron microscopy and an assay for the degradation of long-lived proteins. The authors found that the induction of autophagy by both vitamin D compounds was dependent on their ability to increase intracellular calcium levels. The release of calcium was shown subsequently to induce autophagy through a signaling cascade involving mTOR.136 Specifically, intracellular calcium enhances the binding of calcium/calmodulin to hVps34 which induces kinase activity and activates mTOR complex 1.52
The average daily intake of vitamin D is between 7.33 and 8.12 μg for American adults.137 The levels of vitamin D achieved in serum are not easy to determine as there are several factors that affect the absorbance of vitamin D including the starting plasma concentration and the individual’s body weight.138
Vitamin E
See Tocotrienols.
Vitamin K2
Vitamin K2, or menaquinone, is found in fermented foods such as fermented soybeans.139 In vitro studies using HL-60 leukemia cells showed that 10 μM vitamin K2 leads to an induction of autophagy, as determined by acridine orange staining, LC3 localization, LC3 conversion, and LC3-II turnover. This autophagic response was especially high in cells that were deficient for apoptosis.140
The average intake of vitamin K in North American adults is 59–86 μg/day with bioavailability of 4–20%.141
Unanswered Questions
As we increasingly focus our attention on disease prevention strategies employing dietary modification, it is critical to develop a better understanding of health-promoting dietary constituents and their modes of action. Such food components may act, at least in part, by altering the cellular process of autophagy. While there is an exponentially growing body of literature regarding autophagy,142 there is limited information to date on how autophagy is affected by diet. It has long been realized that molecules such as amino acids can regulate autophagy.143 In this review, we reveal many connections between diverse dietary components and autophagy regulation (Table 1), though the specifics are still largely unknown. It also remains to be tested whether many of the autophagy-modulating dietary components result in flux through the entire autophagic pathway (i.e., lysosomal degradation) as most of the studies performed only steady state analyses. As researchers are becoming increasingly aware of the importance of measuring autophagic flux, it is likely that this information will become available for many of the described compounds.
There are also many outstanding questions regarding the effect of autophagy on human health. It is generally accepted that autophagy is protective against neurodegeneration, however the role of autophagy in cancer is still unclear. In some circumstances autophagy acts as a tumor suppressor, while in others it promotes tumor survival. It is important to determine when autophagy is beneficial and when it is harmful before we can effectively manipulate the process to improve human health.
In addition, there are still many unanswered questions with regards to autophagy-regulating dietary agents and how they can affect autophagy in humans. Many of the studies cited were conducted in vitro, which is extremely different from what occurs in the human body, and raises several questions: (1) How, when and where are these compounds metabolized? Would they be available in an active form for long enough and in high enough concentrations to exhibit an effect in vivo? Are their metabolites also biologically active? (2) How do the tested concentrations compare to the amount of these compounds consumed by the average person? How much would need to be consumed to achieve bioactive doses in the body? And are these concentrations safe? (3) Where will these compounds be available to induce an effect? Would they only exert a response in the organs that they directly contact, such as the gastrointestinal tract? Or would they be able to enter the bloodstream and reach other organs? (4) Would it be necessary to store and prepare foods in a certain way to gain the most autophagy-related benefit from them? (5) Do these compounds interfere or interact with pharmaceutical drugs? (6) How are these compounds regulating autophagy? Are they signaling through the classical mammalian target of rapamycin (mTOR) pathway? Are they acting through novel signaling pathways? (7) What are the effects of these compounds in other organs and tissues? We may find that it is sometimes favorable to use a compound to inhibit autophagy to achieve a health benefit, but what effect will that compound have on other parts of the body such as the brain? Do these compounds cross the blood-brain barrier? And if so, is it possible that a compound which inhibits autophagy would speed up neurodegeneration?
Furthermore, it is necessary to consider that each compound is only one of several in any given food. It is important to know how all the components would affect autophagy and other biological processes. For example, if proposed for the treatment or prevention of cancer (e.g., breast cancer), it would be essential to know the effects of the given dietary compound on hormone levels. It is also possible that various dietary components may interact or may affect individuals differently depending on their genetic background.
Concluding Remarks
Clearly there are important associations between diet and health, with many possible mechanisms of action. This review focuses on the links between dietary constituents and autophagy, both of which have known associations with cancer and neurodegeneration. It was suggested previously that fasting can promote good health and extend longevity through the induction of autophagy,11,119 but perhaps a more appealing approach would be to change what we eat to achieve the same effect. Similarly, perhaps we should consider altering our diets depending on our health status, eating to promote autophagy at some times but to inhibit autophagy at others. There are still many questions that need to be answered before we can effectively and safely manipulate our diet to prevent or treat disease, but with further research this may be achieved. Curried tofu and a glass of red wine, anyone?
Acknowledgments
This work was supported by CIHR grant MOP-78882 to SMG. We thank J. Lum, S. Ng and I. Tai for critical reading of the manuscript and for helpful suggestions and comments.
Abbreviations
- AMPK
AMP-activated protein kinase
- Ape1
aminopeptidase 1
- AVO
acidic vesicular organelles
- CR
calorie restriction
- Con A
concanavalin A
- Cvt
cytoplasm to vacuole targeting
- DMBA
7,12-dimethylbenz[a]anthracene
- GFP
green fluorescent protein
- GPR40
G-protein coupled receptor 40
- LC3
microtubule-associated protein 1 light chain 3
- MDC
monodansylcadaverine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine
- mTOR
mammalian target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- PE
phospatidylethanolamine
- PI3K
phosphoinositide 3-kinase
- TSC 2
tuberous sclerosis complex 2
References
- 1.Key TJ, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC. Diet, nutrition and the prevention of cancer. Public Health Nutrition. 2004;7:187–200. doi: 10.1079/phn2003588. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948–65. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 3.Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenera-tive processes. Neurobiol Aging. 2002;23:719–35. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Shahidi F. Functional foods: Their role in health promotion and disease prevention. J Food Sci. 2004;69:146–9. [Google Scholar]
- 5.Paterson I, Anderson EA. The renaissance of natrual products as drug candidates. Science. 2005;310:451–3. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- 6.da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol. 2001;1:364–9. doi: 10.1016/s1471-4892(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 7.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 8.Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85–90. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- 9.Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14:500–10. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- 10.Singletary K, Milner J. Diet, autophagy and cancer: A review. Cancer Epidemiol Biomarkers Prev. 2008;17:1596–610. doi: 10.1158/1055-9965.EPI-07-2917. [DOI] [PubMed] [Google Scholar]
- 11.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z, Klionsky DJ. Autophagosome formation: Core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 13.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: Cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 14.Mortimore GE, Schworer CM. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature. 1977;270:174–6. doi: 10.1038/270174a0. [DOI] [PubMed] [Google Scholar]
- 15.Filkins JP. Lysosomes and hepatic regression during fasting. Am J Phys. 1970;219:923–7. doi: 10.1152/ajplegacy.1970.219.4.923. [DOI] [PubMed] [Google Scholar]
- 16.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–44. [PubMed] [Google Scholar]
- 17.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang C, Avery L. To be or not to be, the level of autophagy is the question: Dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4:82–4. doi: 10.4161/auto.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Codogno P, Meijer AJ. Autophagy and signaling: Their role in cell survival and cell death. Cell Death Differ. 2005;12:1509–18. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 20.Baehrecke EH. Autophagy: Dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–10. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 21.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: The importance of maintaining “clean” cells. Autophagy. 2005;1:131–40. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 22.Hars E, Qi H, Ryazanov A, Jin S, Cai L, Hu C, Liu LF. Autophagy regulates ageing in C. elegans. Autophagy. 2007;3:93–5. doi: 10.4161/auto.3636. [DOI] [PubMed] [Google Scholar]
- 23.Simonsen A, Cumming R, Brech A, Isakson P, Schubert D, Finley K. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult drosophila. Autophagy. 2007;4:176–84. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 24.Piper MDW, Bartke A. Diet and aging. Cell Metab. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Dilova I, Easlon E, Lin SJ. Calorie restriction and the nutrient sensing signaling pathways. Cell Mol Life Sci. 2007;64:752–67. doi: 10.1007/s00018-007-6381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanfi Y, Peshti V, Gozlan YM, Rathaus M, Gil R, Cohen HY. Regulation of SIRT1 protein levels by nutrient availability. FEBS Letters. 2008;582:2417–23. doi: 10.1016/j.febslet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Haigis MC, Guarente LP. Mammalian sirtuins-emerging roles in physiology, aging and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 28.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. PNAS. 2008;105:3374–9. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomedicine and Pharmacotherapy. 2003;57:203–8. doi: 10.1016/s0753-3322(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: When the cleaning crew goes on strike. The Lancet Neurology. 2007;6:352–61. doi: 10.1016/S1474-4422(07)70076-5. [DOI] [PubMed] [Google Scholar]
- 31.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–12. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 32.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 33.Komatsu M, Waguri S, Chiba T, Murata S, Iwata JI, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurode-generation in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 34.Levine B. Cell biology: Autophagy and cancer. Nature. 2007;446:745–7. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 35.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyer-Hansen M, Jaattela M. Autophagy: An emerging target for cancer therapy. Autophagy. 2008;4:574–80. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- 37.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3–14. [PubMed] [Google Scholar]
- 39.Eskelinen EL. Fine structure of the autophagosome. Methods Mol Biol. 2008;445:11–28. doi: 10.1007/978-1-59745-157-4_2. [DOI] [PubMed] [Google Scholar]
- 40.Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von Figura K, Mizushima N, Saftig P, Uchiyama Y. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (batten disease) Am J Pathol. 2005;167:1713–28. doi: 10.1016/S0002-9440(10)61253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinet W, De Meyer GRY, Andries L, Herman AG, Kockx MM. In situ detection of starvation-induced autophagy. J Histochem Cytochem. 2006;54:85–96. doi: 10.1369/jhc.5A6743.2005. [DOI] [PubMed] [Google Scholar]
- 43.Aoki H, Kondo Y, Aldape K, Yamamoto A, Iwado E, Yokoyama T, Hollingsworth EF, Kobayashi R, Hess K, Shinojima N, Shingu T, Tamada Y, Zhang L, Conrad C, Bogler O, Mills G, Sawaya R, Kondo S. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy. 2008;4:467–75. doi: 10.4161/auto.5668. [DOI] [PubMed] [Google Scholar]
- 44.Seglen PO, Gordon PB, Tolleshaug H, Høyvik H. Use of [3H]raffinose as a specific probe of autophagic sequestration. Exp Cell Res. 1986;162:273–7. doi: 10.1016/0014-4827(86)90446-5. [DOI] [PubMed] [Google Scholar]
- 45.Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3:181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- 46.Hutchins MU, Klionsky DJ. Vacuolar localization of oligomeric alpha—mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in saccharomyces cerevisiae. J Biol Chem. 2001;276:20491–8. doi: 10.1074/jbc.M101150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Mizushima N, Klionsky DJ. Protein turnover via autophagy: Implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 49.Mordier S, Deval C, Bechet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem. 2000;275:29900–6. doi: 10.1074/jbc.M003633200. [DOI] [PubMed] [Google Scholar]
- 50.Kanazawa T, Taneike I, Akaishi R, Yoshizawa F, Furuya N, Fujimura S, Kadowaki M. Amino acids and insulin control autophagic proteolysis through different signaling pathways in relation to mTOR in isolated rat hepatocytes. J Biol Chem. 2004;279:8452–9. doi: 10.1074/jbc.M306337200. [DOI] [PubMed] [Google Scholar]
- 51.Blommaart EFC, Luiken JJFP, Blommaart PJE, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–6. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 52.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–65. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergstrom J, Furst P, Vinnars E. Effect of a test meal, without and with protein, on muscle and plasma free amino acids. Clin Sci. 1990;79:331–7. doi: 10.1042/cs0790331. [DOI] [PubMed] [Google Scholar]
- 54.Kassi E, Papavassiliou AG. Could glucose be a proaging factor? J Cell Mol Med. 1008;12:1194–8. doi: 10.1111/j.1582-4934.2008.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Proud CG, Hundal HS, Taylor PM. Nutrient sensing in animal cells and integration of nutrient and endocrine signalling pathways. Topics in Current Genetics. 2004;7:25–64. [Google Scholar]
- 56.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–32. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–6. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 58.Pan X, Tan N, Zeng G, Zhang Y, Jia R. Amentoflavone and its derivatives as novel natural inhibitors of human cathepsin B. Bioorg Med Chem. 2005;13:5819–25. doi: 10.1016/j.bmc.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 59.Gordon PB, Holen I, Seglen PO. Protection by naringin and some other flavonoids of hepatocytic autophagy and endocytosis against inhibition by okadaic acid. J Biol Chem. 1995;270:5830–8. doi: 10.1074/jbc.270.11.5830. [DOI] [PubMed] [Google Scholar]
- 60.Woo ER, Lee JY, Cho IJ, Kim SG, Kang KW. Amentoflavone inhibits the induction of nitric oxide synthase by inhibiting NFκB activation in macrophages. Pharmacol Res. 2005;51:539–46. doi: 10.1016/j.phrs.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–88. [Google Scholar]
- 62.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend saccharomyces cerevisiae life span. Nature. 2003 doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 63.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Brit J Pharmacol. 2000;130:1115–23. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang W, Heiderman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinogen. 2000;28:102–10. [PubMed] [Google Scholar]
- 65.Nakamura Y, Kawakami M, Yoshihiro A, Miyoshi N, Ohigashi H, Kawai K, Osawa T, Uchida K. Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. J Biol Chem. 2002;277:8492–9. doi: 10.1074/jbc.M109760200. [DOI] [PubMed] [Google Scholar]
- 66.Kassie F, Pool-Zobel B, Parzefall W, Knasmuller S. Genotoxic effects of benzyl isothiocyanate, a natural chemopreventive agent. Mutagenesis. 1999;14:595–603. doi: 10.1093/mutage/14.6.595. [DOI] [PubMed] [Google Scholar]
- 67.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by bax and bak. Mol Cancer Ther. 2006;5:2931–45. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 68.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Yan Y, Zhang B, Wang L, Xie Y, Peng T, Bai B, Zhou P. Induction of apoptosis and autophagic cell death by the vanillin derivative 6-bromine-5-hydroxy-4-methoxybenz-aldehyde is accompanied by the cleavage of DNA-PKcs and rapid destruction of c-myc oncoprotein in HepG2 cells. Cancer Lett. 2007;252:280–9. doi: 10.1016/j.canlet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 71.Winter G, Hazan R, Bakalinsky AT, Abeliovich H. Caffeine induces macroautophagy and confers a cytocidal effect on food spoilage yeast in combination with benzoic acid. Autophagy. 2008;4:28–36. doi: 10.4161/auto.5127. [DOI] [PubMed] [Google Scholar]
- 72.Wanke V, Cameroni E, Uotila A, Piccolis M, Urban J, Loewith R, De Virgilio C. Caffeine extends yeast life span by targeting TORC1. Mol Microbi. 2008;69:277–85. doi: 10.1111/j.1365-2958.2008.06292.x. [DOI] [PubMed] [Google Scholar]
- 73.Bode AM, Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007;247:26–39. doi: 10.1016/j.canlet.2006.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- 75.Lieberman HR, Wurtman RJ, Emde GG, Roberts C, Coviella ILG. The effects of low doses of caffeine on human performance and mood. Psychopharmacology. 1987;92:308–12. doi: 10.1007/BF00210835. [DOI] [PubMed] [Google Scholar]
- 76.Jarvis MJ. Does caffeine intake enhance absolute levels of cognitive performance? Psychopharmacology. 1993;110:45–52. doi: 10.1007/BF02246949. [DOI] [PubMed] [Google Scholar]
- 77.Warburton DM. Effects of caffeine on cognition and mood without caffeine abstinence. Psychopharmacology. 1995;119:66–70. doi: 10.1007/BF02246055. [DOI] [PubMed] [Google Scholar]
- 78.Gevaerd MS, Takahashi RN, Silveira R, Da Cunha C. Caffeine reverses the memory disruption induced by intra-nigral MPTP-injection in rats. Brain Res Bull. 2001;55:101–6. doi: 10.1016/s0361-9230(01)00501-9. [DOI] [PubMed] [Google Scholar]
- 79.Chang CP, Yang MC, Liu HS, Lin YS, Lei HY. Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology. 2007;45:286–96. doi: 10.1002/hep.21509. [DOI] [PubMed] [Google Scholar]
- 80.Lei HY, Chang CP. Induction of autophagy by concanavalin A and its application in anti-tumor therapy. Autophagy. 2007;3:402–4. doi: 10.4161/auto.4280. [DOI] [PubMed] [Google Scholar]
- 81.Singh S. From exotic spice to modern drug? Cell. 2007;130:765–8. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 82.Salvioli S, Sikora E, Cooper EL, Franceschi C. Curcumin in cell death processes: A challenge for CAM of age-related pathologies. eCAM. 2007;4:181–90. doi: 10.1093/ecam/nem043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–68. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 85.Shinojima N, Yokoyama T, Kondo Y, Konda S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–7. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 86.Verger P, Chambolle M, Babayou P, Le Breton S, Volatier JL. Estimation fo the distribution of the maximum theoretical intake for ten additives in france. Food Addit Contam. 1998;15:1867–76. doi: 10.1080/02652039809374707. [DOI] [PubMed] [Google Scholar]
- 87.Commandeur JN, Vermeulen NP. Cytotoxicity and cytoprotective activities of natural compounds. Xenobiotica. 1996;26:667–80. doi: 10.3109/00498259609046741. [DOI] [PubMed] [Google Scholar]
- 88.Ammon HP, Wahl MA. Pharmacology of curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 89.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin—cellular and molecular mechanisms of action. Crit Rev Food Sci. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 90.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 91.El Nasri NA, El Tinay AH. Functional properties of fenugreek (trigonella foenum graecum) protein concentrate. Food Chem. 2007;103:582–9. [Google Scholar]
- 92.Amin A, Alkaabi A, Al-Falasi S, Daoud SA. Chemopreventive activities of trigonella foenum graecum (fenugreek) against breast cancer. Cell Biol Int. 2005;29:687–94. doi: 10.1016/j.cellbi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Lee W, Shen S, Lin H, Hou W, Yang L, Chen Y. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochem Pharmacol. 2002;63:225–36. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]
- 94.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–42. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 95.Gossner G, Choi M, Tan L, Fogoros S, Griffith KA, Kuenker M, Liu JR. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol. 2007;105:23–30. doi: 10.1016/j.ygyno.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744–57. doi: 10.1081/cnv-120023773. [DOI] [PubMed] [Google Scholar]
- 97.Tettamanti G, Malagoli D, Marchesini E, Congiu T, de Eguileor M, Ottaviani E. Oligomycin A induces autophagy in the IPLB-LdFB insect cell line. Cell Tissue Res. 2006;326:179–86. doi: 10.1007/s00441-006-0206-4. [DOI] [PubMed] [Google Scholar]
- 98.Inoki K, Zhu T, Guan K. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 99.Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–7. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- 100.D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43:348–61. [PubMed] [Google Scholar]
- 101.Choi EJ. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: Involvement of CDK4 and p21. Nutr Cancer. 2007;59:115–9. doi: 10.1080/01635580701419030. [DOI] [PubMed] [Google Scholar]
- 102.Manach C, Scalbert A, Morangd C, Remesy C, Jimenez L. Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 103.Demulle L, Vanden Berghe T, De Keukeleire D, Vandenabeele P. Treatment of PC-3 and DU145 prostate cancer cells by prenylflavonoids from hop (humulus lupulus L) induces a caspase-independent form of cell death. Phytother Res. 2007;22:197–203. doi: 10.1002/ptr.2286. [DOI] [PubMed] [Google Scholar]
- 104.Schrauzer GN. Lithium: Occurrence, dietary intakes, nutritional essentiality. J Am Coll Nutr. 2002;21:14–21. doi: 10.1080/07315724.2002.10719188. [DOI] [PubMed] [Google Scholar]
- 105.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–11. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998;438:220–4. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 107.Yang S, Yang W, Chang H, Chu S, Hsieh Y. Luteolin induces apoptosis in oral squamous cancer cells. J Dent Res. 2008;87:401–6. doi: 10.1177/154405910808700413. [DOI] [PubMed] [Google Scholar]
- 108.Nakagawa A, Sawada T, Okada T, Ohsawa T, Adachi M, Kubota K. New antineoplastic agent, MK615, from UME (a variety of) japanese apricot inhibits growth of breast cancer cells in vitro. Breast J. 2007;13:44–9. doi: 10.1111/j.1524-4741.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 109.Mori S, Sawada T, Okada T, Ohsawa T, Adachi M, Keiichi K. New anti-proliferative agent, MK615, from japanese apricot “prunus mume” induces striking autophagy in colong cancer cell in vitro. World J Gastroenterol. 2007;13:6512–7. doi: 10.3748/wjg.v13.i48.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hollman PCH, van Trijp JMP, Buysman MNCPvd, Gaag MS, Mengelers MJB, de Vries JHM, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–6. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 111.Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A. Quercetin mediates preferential degradation of oncogenic ras and causes autophagy in ha-RAS-transformed human colon cells. Carcinogenesis. 2007;28:1021–31. doi: 10.1093/carcin/bgl232. [DOI] [PubMed] [Google Scholar]
- 112.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. doi: 10.1016/j.canlet.2008.03.046. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 113.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–73. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 114.Pirola L, Frojdo S. Resveratrol: One molecule, many targets. IUBMB Life. 2008;60:323–32. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 115.Opipari AW, Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 116.Kueck A, Opipari AW, Jr, Griffith KA, Tan L, Choi M, Huang J, Wahl H, Liu JR. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol Oncol. 2007;107:450–7. doi: 10.1016/j.ygyno.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 117.Ohshiro K, Rayala SK, El-Naggar AK, Kumar R. Delivery of cytoplasmic proteins to autophagosomes. Autophagy. 2008;4:104–6. doi: 10.4161/auto.5223. [DOI] [PubMed] [Google Scholar]
- 118.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–29. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 119.Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57:203–8. doi: 10.1016/s0753-3322(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 120.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Pecker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holme AL, Pervaiz S. Resveratrol in cell fate decisions. J Bioenerg Biomembr. 2007;39:59–63. doi: 10.1007/s10863-006-9053-y. [DOI] [PubMed] [Google Scholar]
- 122.Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J. 2002;364:301–7. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–35. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 125.Theriault A, Chao JT, Wang QI, Gapor A, Adeli K. Tocotrienol: A review of its therapeutic potential. Clin Biochem. 1999;32:309–19. doi: 10.1016/s0009-9120(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 126.Rickmann M, Vaquero EC, Malagelada JR, Molero X. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology. 2007;132:2518–32. doi: 10.1053/j.gastro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 127.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol Apsects Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ellington AA, Berhow M, Singletary KW. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis. 2005;26:159–67. doi: 10.1093/carcin/bgh297. [DOI] [PubMed] [Google Scholar]
- 129.Fournier D, Erdman J, Jr, Gordon G. Soy, its components, and cancer prevention: A review of the in vitro, animal, and human data. Cancer Epidemiol Biomarkers Prev. 1998;7:1055–65. [PubMed] [Google Scholar]
- 130.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 131.Ohtani S, Iwamaru A, Deng W, Ueda K, Wu G, Jayachandran G, Kondo S, Atkinson EN, Minna JD, Roth JA, Ji L. Tumor suppressor 101F6 and ascorbate synergistically and selectively inhibit non-small cell lung cancer growth by caspase-independent apoptosis and autophagy. Cancer Res. 2007;67:6293–303. doi: 10.1158/0008-5472.CAN-06-3884. [DOI] [PubMed] [Google Scholar]
- 132.Foy CJ, Passmore AP, Vahidassr MD, Young IS, Lawson JT. Plasma chain-breaking antioxidants in alzheimer’s disease, vascular dementia and parkinson’s disease. QJM. 1999;92:39–45. doi: 10.1093/qjmed/92.1.39. [DOI] [PubMed] [Google Scholar]
- 133.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 134.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- 136.Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jäättelä M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 137.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: A global perspective of current status. J Nutr. 2005;135:310–6. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 138.Heaney RP. Vitamin D: How much do we need, and how much is too much? Osteroporos Int. 2000;11:553–5. doi: 10.1007/s001980070074. [DOI] [PubMed] [Google Scholar]
- 139.Shearer MJ, Bach A, Kohlmeier M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J Nutr. 1996;126:1181–6. doi: 10.1093/jn/126.suppl_4.1181S. [DOI] [PubMed] [Google Scholar]
- 140.Yokoyama R, Miyazawa K, Naito M, Toyotake J, Tauchi T, Itoh M, Yuo A, Hayashi Y, Georgescu MM, Kondo Y, Kondo S, Ohyashiki K. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy. 2008;4:1–12. doi: 10.4161/auto.5941. [DOI] [PubMed] [Google Scholar]
- 141.Singh H, Duerksen DR. Vitamin K and nutrition support. Nutr Clin Pract. 2003;18:359–65. doi: 10.1177/0115426503018005359. [DOI] [PubMed] [Google Scholar]
- 142.Klionsky DJ. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–7. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 143.Kadowaki M, Karim MR, Carpi A, Miotto G. Nutrient control of macroautophagy in mammalian cells. Mol Aspects Med. 2006;27:426–43. doi: 10.1016/j.mam.2006.08.010. [DOI] [PubMed] [Google Scholar]