Abstract
San-Huang-Xie-Xin-Tang (SHXT), composed of Coptidis rhizoma, Scutellariae radix and Rhei rhizoma, is a traditional Chinese herbal medicine used to treat gastritis, gastric bleeding and peptic ulcers. This study investigated the neuroprotective effects of SHXT on microglia-mediated neurotoxicity using co-cultured lipopolysaccharide (LPS)-activated microglia-like BV-2 cells with neuroblastoma SH-SY5Y cells. Effects of SHXT on 6-hydroxydopamine (6-OHDA)-induced neurotoxicity were also examined in SH-SY5Y cells. Results indicated SHXT inhibited LPS-induced inflammation of BV-2 cells by downregulation of iNOS, NO, COX-2, PGE2, gp91phox, iROS, TNF-α, IL-1β, inhibition of IκBα degradation and upregulation of HO-1. In addition, SHXT increased cell viability and down regulated nNOS, COX-2 and gp91phox of SH-SY5Y cells co-cultured with LPS activated BV-2 cells. SHXT treatment increased cell viability and mitochondria membrane potential (MMP), decreased expression of nNOS, COX-2, gp91phox and iROS, and inhibited IκBα degradation in 6-OHDA-treated SH-SY5Y cells. SHXT also attenuated LPS activated BV-2 cells- and 6-OHDA-induced cell death in differentiated SH-SY5Y cells with db-cAMP. Furthermore, SHXT-inhibited nuclear translocation of p65 subunit of NF-κB in LPS treated BV-2 cells and 6-OHDA treated SH-SY5Y cells. In conclusion, SHXT showed protection from activated microglia- and 6-OHDA-induced neurotoxicity by attenuating inflammation and oxidative stress.
1. Introduction
Inflammation appears to be a complicating factor in many age-related neurodegenerative diseases, such as Parkinson's disease (PD) [1], Alzheimer's disease (AD) [2] and multiple sclerosis [3]. Microglia are thought to mediate the innate defense system in the central nervous system [4]. Activated microglia enhances pro-inflammatory and neurotoxic responses and neuronal injury [5, 6]. Microglia-mediated neurotoxicity was induced through release of pro-inflammatory cytokines such as tumor-necrosis factor alpha (TNF)-α, interleukin 1β (IL-1β), nitric oxide (NO) and prostaglandin E2 (PGE2), this yields enhanced reactive oxygen species (ROS) [7] and reactive nitric oxide species (RNS) [8, 9]. The deleterious consequences of excessive oxidation and pathophysiological role of ROS have been intensively studied in Alzheimer's disease. Studies indicated that anti-inflammatory or anti-oxidative stress drugs exert neuroprotective effects [10–13]. Therefore, anti-inflammatory agents and antioxidants are considered a promising approach to slowing the progression and limiting the extent of neuronal cell loss in these disorders.
Many herbal medicines and dietary supplements are sold as aids to improve memory, treat neurodegenerative diseases or create favorable effects on the central nervous system. Increasing evidence has suggested that traditional Chinese medicines or herbal extracts possess neuroprotective benefits through their distinct and multiple mechanisms, including anti-inflammation and anti-oxidation [14–19]. San-Huang-Xie-Xin-Tang (SHXT), a traditional Chinese medicinal formula containing Coptidis rhizome (Coptis chinesis Franch), Scutellariae radix (Scutellaria baicalensis Georgi) and Rhei rhizome (Rheum officinale Baill), has been used to treat gastritis, gastric bleeding and peptic ulcers [20]. The preventive effects of SHXT on lipopolysaccharide (LPS) and Helicobacter pylori-induced inflammatory responses have been confirmed by our previous studies [21–23]. Studies indicate that several components in SHXT show inhibitory effects on neurotoxicity. Baicalein and baicalin, the major flavonoids in Scutellaria baicalensis, can reduce beta amyloid protein-induced neurotoxicity [24]. Baicalein [14, 18] and wogonin [19] attenuate inflammation-mediated neurodegeneration [14, 18, 19]. Baicalin attenuates oxygen-glucose deprivation-induced injury in neurons [25]. Emodin, an anthraquinone derivative extracted from Rhei rhizome, decreases glutamate-induced neurotoxicity [26].
LPS is commonly used to investigate the impact of inflammation on neuronal death, and studies indicate that microglia are necessary for LPS-induced neurotoxicity [27, 28]. LPS is not toxic to neurons, but LPS stimulates glial cells to produce factors that are toxic to neurons [28–30]. On the other hand, the auto-oxidation of the neurotransmitter dopamine to 6-hydroxydopamine (6-OHDA) generates ROS and reactive quinones subsequently inducing cell death [31, 32]. Therefore, we investigated the effects of SHXT on LPS activated microglia-induced neurotoxicity by using the invitro model of co-culture microglia-like BV-2 cells with the neuronal-like neuroblastoma SH-SY5Y cell. We also examined the effects of SHXT on 6-OHDA-induced neurotoxicity in dopaminergic neuroblastoma SH-SY5Y cells.
2. Materials and Methods
2.1. Reagents
The voucher specimen and method for extraction and analysis of SHXT were described previously [22]. Briefly, a blended mixture of Coptidis rhizome, root of Scutellariae radix and rhizome of Rhei officinale Baill was prepared in a ratio of 1 : 1 : 2, respectively. The content of each component in SHXT is shown as follows (μg/mL): baicalin 1153.07 ± 56.36, baicalein 82.81 ± 3.74, emodin 11.15 ± 1.22, wogonin 19.55 ± 0.83, rhein 126.12 ± 3.84, berberine 62.14 ± 4.27, coptisine 6.15 ± 0.34, palmatine 25.11 ± 3.78, sennoside A 128.02 ± 13.56 and sennoside B 95.90 ± 3.59. Dimethyl sulfoxide (DMSO), lipopolysaccharide (LPS, L8274) from Escherichia coli (O26:B6), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), 2′,7′-dichloro-dihydrofluorescein diacetate (H2DCF-DA), 6-hydroxydopamine (6-OHDA), dibutyryl cyclic AMP (db-cAMP), mouse antibody against iNOS and β-actin were purchased from Sigma Aldrich (USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin, amphotericin B and streptomycin were purchased from GIBCO/BRL Life Technologies (USA). Mouse antibody against gp91phox and all materials for SDS-polyacrylamide gel electrophoresis were purchased from Bio-Rad (USA). Goat antibody against COX-2 and HO-1, mouse antibody against IκBα, rabbit antibody against nNOS and all horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (USA). Enhanced chemiluminescence agent was purchased from PerkinElmer Life and Analytical Sciences (USA). TNF-α, IL-1β and PGE2 enzyme immunoassay kits and NE-PER Nuclear and Cytoplasmic Extraction Reagents were purchased from Pierce Endogen (USA). TransFactor Family Colorimetric Kit NF-κB was purchased from BD Bioscience, Clontech (USA). All other chemicals were purchased from Sigma Chemical Company (USA).
2.2. Microglial BV-2 Cell Culture and Activated Microglia with LPS
Mouse microglial cell line BV-2 was cultured in DMEM containing 10% (v/v) heat-inactivated FBS, 4 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin and 0.25 mg/mL amphotericin B at 37°C in a humidified incubator under 5% CO2 and 95% air. For the purpose of experiment, BV-2 cells were plated in 6-, 24- or 96-well sterile plates (1 × 105 cells/mL). Then, cells were stimulated with LPS alone (100 ng/mL) or LPS with different concentrations of SHXT.
2.3. Human SH-SY5Y Neuroblastoma Cell Culture
The human neuroblastoma cell line SH-SY5Y (ATCC CRL-2266) was cultured in a medium consisting of a 1 : 1 mixture of DMEM and Ham's F-12 medium containing 10% heat-inactivated FBS, 4 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin and 0.25 mg/mL amphotericin B at 37°C in a humidified incubator under 5% CO2 and 95% air. In order to investigate the neuroprotective effect of SHXT on differentiated neuronal cell, SH-SY5Y cells were treated with 1 mM db-cAMP, a permeable analog of cAMP, to induce neuronal differentiation. SH-SY5Y cells (1 × 105 cells/mL) were plated in 96-well sterile plates and db-cAMP was dissolved in distilled water and added to each well at a concentration of 1 mM in DMEM containing 10% FBS. The cells were then incubated for 4 days at 37°C, with the medium refreshed daily. Then, cells were treated with 6-OHDA alone (100 μM) or 6-OHDA with different concentrations of SHXT for 24 h.
2.4. Co-Culture of SH-SY5Y Cells with Microglial BV-2 Cells
The effects of SHXT on microglia-mediated and inflammation-associated neuronal damage were determined using LPS stimulated BV-2 cells as a model of activated microglia [11]. Non-differentiated or differentiated SH-SY5Y cells were plated in 6- or 24-well plates (1 × 105 cells/mL) and BV-2 cells were seeded onto cell culture inserts (pore size of 0.2 μm; NUNCA/S, Roskilde, Denmark). Then the inserts were placed in the wells where SH-SY5Y cells were growing in DMEM containing 10% (v/v) heat-inactivated FBS, 4 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin and 0.25 mg/mL amphotericin B at 37°C in a humidified incubator under 5% CO2 and 95% air. The BV-2 and SH-SY5Y co-cultured cells were separated by filters present in the insert. In co-culture conditions, BV-2 cells were then stimulated with LPS (100 ng/mL) or LPS + SHXT for 24 h.
2.5. Cell Viability Assay
Cell viability was measured by a quantitative colorimetric assay with MTT, showing mitochondrial activity of living cells. In mono-culture, BV-2 or SH-SY5Y cells in 96-well plates were incubated with 25–200 μg/mL of SHXT for 24 h. In 6-OHDA-induced neurotoxicity, non-differentiated or differentiated SH-SY5Y cells were treated with or without 6-OHDA (100 μM) or 6-OHDA + SHXT for 24 h. Under co-culture conditions, BV-2 cells were treated with or without LPS (100 ng/mL) or LPS + SHXT for 24 h. After incubation, inserts containing BV-2 cells were removed. Non-differentiated or differentiated SH-SY5Y cells were incubated with MTT at a final concentration of 0.5 mg/mL for 3 h at 37°C. The reaction was terminated by addition of 200 μL of DMSO. The amount of MTT formazon product was then determined by measuring the absorbance at 560 nm using a microplate reader.
2.6. Nitrite Measurement
Production of nitric oxide (NO) was determined by measuring the level of accumulated nitrite, a metabolite of NO in the culture supernatant using Griess reagent (1% sulfanilamide and 0.1% N-(1-naphthyl)ethylenediamide in 5% phosphoric acid). After 24 h of treatment with LPS (100 ng/mL) alone or LPS + SHXT, the culture supernatants were collected and mixed with an equal volume of Griess reagent and incubated at room temperature for 10 min. The absorbance was measured at 540 nm (OD540).
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
The productions of IL-1β, PGE2 and TNF-α in supernatant were detected using ELISA kits from Endogen (USA) according to the manufacturer's instructions. IL-1β and PGE2 in the supernatant were determined after 24 h of LPS treatment. TNF-α was determined after 2 h of LPS treatment.
2.8. NF-κB DNA-Binding Activity
The DNA-binding activities of transcription factors in cells were determined by enzyme-linked DNA-protein interaction assay (ELDIA), according to a previously described method [21, 33]. Nuclear protein extracts were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagent as described previously [34]. DNA-p65 NF-κB-binding activity was measured with a BD Mercury TransFactor kit (BD Bioscience, Clontech), which detects DNA binding by specific transcription factors, according to the manufacturer's instructions [35].
2.9. Western Blot Analysis
After treatment with indicated agents, cells were collected and lysed to determine expression of nNOS, iNOS, COX-2, gp91phox, IκBα and HO-1. The lysates were centrifuged at 15 000 × g for 30 min at 4°C. The supernatant was then collected for SDS-polyacrylamide gel electrophoresis. Protein concentration was determined with the Bio-Rad protein assay kit following the manufacturer's guide. Cell membranes were obtained by ultracentrifugation of the supernatant at 26 000 × g for 1 h at 4°C. Equal amounts of protein (20 μg per lane) were separated on a 10% polyacrylamide gel and transferred to polyvinylidene difluoride membranes (PerkinElmer Life and Analytical Sciences). Non-specific binding was blocked with TBST (50 mM Tris- HCl, pH 7.6, 150 mM NaCl, 0.1% Tween 20) containing 5% non-fat milk for 1 h at room temperature. The membranes were then each incubated overnight at 4°C with one of the following specific primary antibodies: mouse anti-iNOS (1 : 1000), mouse anti-gp91phox (1 : 1000), mouse anti-β-actin (1 : 20 000), rabbit anti-nNOS (1 : 1000), mouse anti-IκBα (1 : 500), rabbit anti-HO-1 (1 : 1000) and goat anti-COX-2 (1 : 5000). Membranes were washed six times for 5 min with TBST. The appropriate dilutions of secondary antibodies (diluted 1 : 1000) were incubated for 1 h. After six washes with TBST, the protein bands were detected with the enhanced chemiluminescence reagent (PerkinElmer Life and Analytical Sciences).
2.10. Measurement of Intracellular Reactive Oxygen Species
The level of intracellular reactive oxygen species (ROS) was quantified by fluorescence with H2DCF-DA. Following incubations with the indicated treatments, microglial BV-2 cells or neuronal SH-SY5Y cells were loaded with 10 μM of H2DCF-DA for 30 min at 37°C. Cells were then washed three times with phosphate-buffer saline, pH 7.4, and the relative levels of fluorescence were analyzed by a spectrophotofluorimeter (FLUOstar OPTIMA, BMG LABTECH, Germany, 495 nm excitation and 520 nm emission). Intracellular ROS-containing cells were identified as those with increased FITC fluorescence of oxidized H2DCF.
2.11. Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was measured by the incorporation of a cationic fluorescent dye rhodamine 123. The reduction in fluorescent intensity of rhodamine 123 staining represented a fall in the mitochondrial membrane potential. After 24 h of incubation in normal medium with or without treatment, the cells were changed to serum-free medium containing 10 μM rhodamine 123, there they were incubated for 15 min at 37°C. The cells were then collected, and the fluorescence intensity was analyzed within 15 min by a spectrophotofluorimeter (FLUOstar OPTIMA, BMG LABTECH, Germany, 490 nm excitation and 515 nm emission).
2.12. Data Analysis
Data were expressed as mean ± SEM. Analysis of variance (ANOVA) was used to assess the statistical significance of the differences followed by Tukey's test for all pair's comparisons. A value of P < .05 was considered statistically significant. The data were analyzed with the Statistical Package for Social Sciences (SPSS, Chicago, IL).
3. Results
3.1. Effects of SHXT on LPS-Activated Microglial BV-2 Cell- and 6-OHDA-Induced Loss of SH-SY5Y Cell Viability
Cells were incubated with or without SHXT for 24 h. According to the results of MTT assay, SHXT (25, 50, 100 and 200 μg/mL) did not effect the cell viability of BV-2 cells (Figure 1(a)) and SH-SY5Y cells (Figure 1(b)). In order to examine the effect of SHXT on LPS-activated microglia-mediated cell viability, co-culture of BV-2 cells with non-differentiated or differentiated SH-SY5Y cells was investigated. As shown in Figures 1(c) and 1(d), SHXT attenuated LPS (100 ng/mL)-activated BV-2 cells-induced cell death in non-differentiated and differentiated SH-SY5Y cells. Moreover, exposure of non-differentiated (Figure 1(e)) and differentiated (Figure 1(f)) SH-SY5Y cells to 6-OHDA induced significant cell death. SHXT increased cell viability in 6-OHDA treated non-differentiated (Figure 1(e)) and differentiated (Figure 1(f)) SH-SY5Y cells. SHXT protected against activated microglia- and 6-OHDA-induced differentiated neuronal cell death at the concentrations from 100 to 200 μg/mL and 50 to 200 μg/mL, respectively. In addition, we examined dopamine receptor expression of differentiated SH-SY5Y cells using western blotting, and marked increase of dopamine receptor expression was observed (online Supplementary FigureS1).
Figure 1.
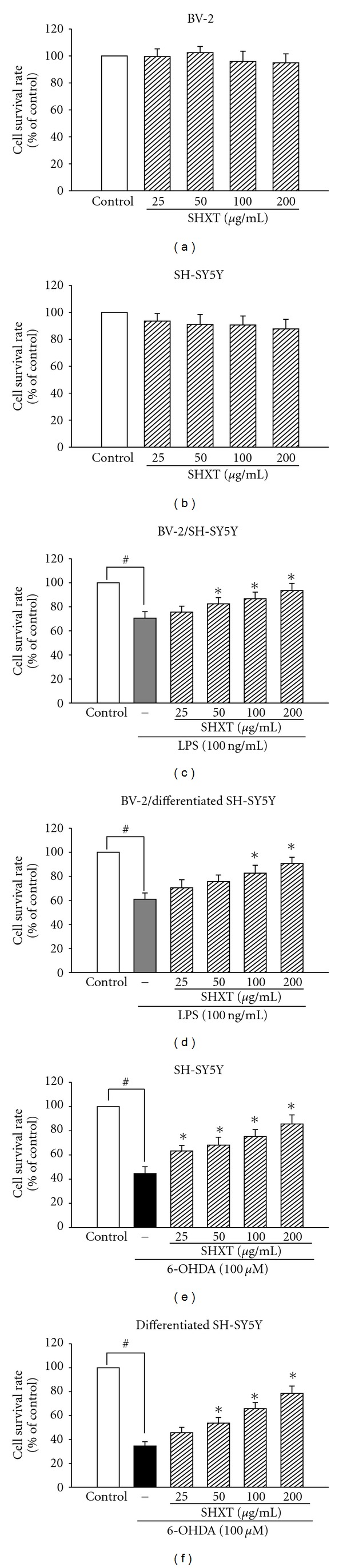
Effects of SHXT on microglial BV-2 cells (a), neuroblastoma SH-SY5Y cells (b), lipopolysaccharide (LPS, 100 ng/mL) activated BV-2 treated non-differentiated (c) and differentiated (d) SH-SY5Y cells, and 6-hydroxydopamine (6-OHDA, 100 μM) treated non-differentiated (e) and differentiated (f) SH-SY5Y cells. Cells were treated with SHXT (25–200 μg/mL) for 24 h. Cell viability was determined by MTT assay. Bars represent the mean ± SEM from six independent experiments. # P < .05 versus control, *P < .05 versus LPS or 6-OHDA only.
3.2. LPS-Induced Inflammation and Associated Oxidative Stress-Related Factors in BV-2 Cells were Reduced by SHXT
To investigate whether SHXT inhibited LPS-activated microglia-induced inflammatory and oxidative stress factors, the effects of SHXT on the associated noxious factors of LPS-treated BV-2 cells were examined. As shown in Figure 2, LPS (100 ng/mL) increased expression of iNOS, COX-2 and gp91phox in BV-2 cells, which were inhibited by SHXT treatment. Pairwise comparison at each concentration by Tukey's test indicated that SHXT concentration-dependently inhibited iNOS at a concentration of 50 and 200 μg/mL and inhibited COX-2 at a concentration of 25, 50, 100 and 200 μg/mL. Additionally, SHXT upregulated HO-1 expression in LPS treated BV-2 cells in a concentration-dependent manner from 25 to 200 μg/mL (Figure 2(d)). SHXT also reduced LPS-induced NO, PGE2 and iROS production (Table 1). Furthermore, SHXT (50–200 μg/mL) concentration-dependently attenuated IκBα degradation and NF-κBp65 translocation in LPS-treated BV-2 cells (Figure 3).
Figure 2.
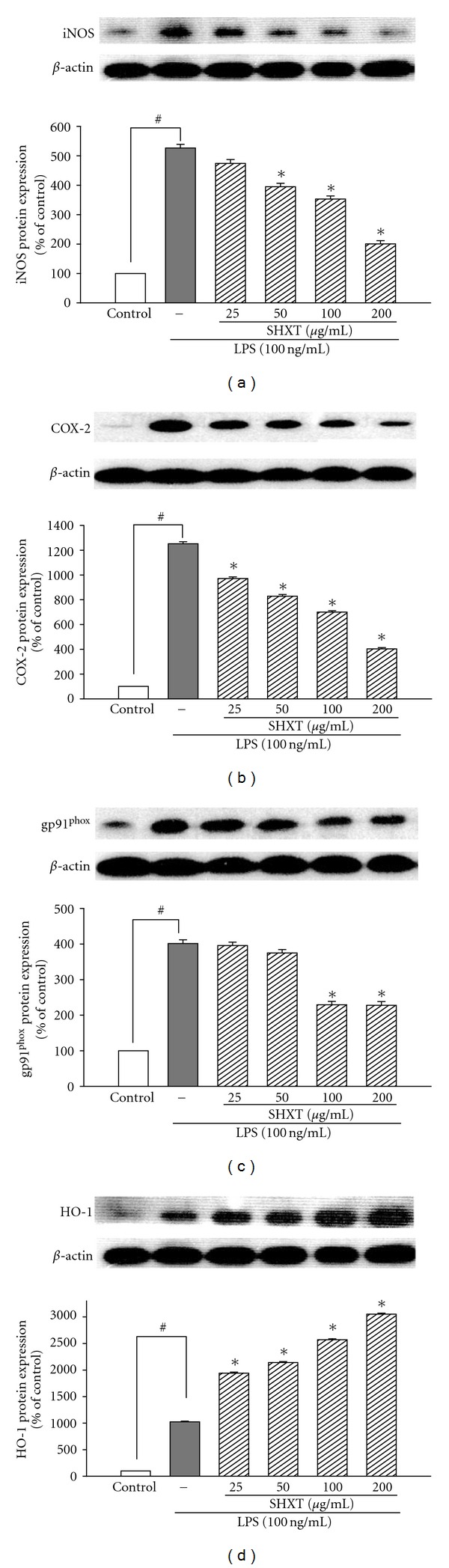
Effects of SHXT on expression of iNOS, COX-2, gp91phox and HO-1 in BV-2 cells treated with lipopolysaccharide (LPS, 100 ng/mL) for 24 h. Cultures were pretreated with SHXT for 30 min before the addition of LPS treatment. Bars represent the mean ± SEM from six independent experiments. Densitometry analyses are presented as the relative ratio of protein/β-actin protein, and are represented as percentages of the control group. # P < .05 versus control, *P < .05 versus LPS only.
Table 1.
Effects of SHXT (25, 50, 100 and 200 μg/mL) on LPS-induced nitrite, PGE2, iROS, TNF-α and IL-1β production in BV-2 cells.
| Nitrite (μM) | PGE2 (pg/mL) | iROS (%) | TNF-α (pg/mL) | IL-1β (pg/mL) | |
|---|---|---|---|---|---|
| Control | 0.25 ± 0.03 | 14.45 ± 1.53 | 100 | 70.41 ±8. 51 | 10.51 ± 3.57 |
| LPS only | 3.76 ± 0.20# | 41.83 ± 2.53# | 201.52 ± 9.52# | 704.61 ± 30.53# | 68.51 ± 5.65# |
| LPS + SHXT 25 μg/mL | 2.96 ± 0.41 | 27.39 ± 2.14* | 192.51 ± 8.52 | 650.25 ± 20.56 | 58.52 ± 7.24 |
| LPS + SHXT 50 μg/mL | 1.89 ± 0.34* | 22.52 ± 1.95* | 182.57 ± 8.50 | 552.53 ± 10.51* | 50.14 ± 4.65* |
| LPS + SHXT 100 μg/mL | 1.63 ± 0.21* | 21.62 ± 1.52* | 130.73 ± 6.25* | 430.56 ± 12.86* | 42.51 ± 4.64* |
| LPS + SHXT 200 μg/mL | 1.50 ± 0.14* | 14.68 ± 1.35* | 109.64 ± 6.63* | 263.50 ± 15.58* | 26.52 ± 5.63* |
Values represent the mean ± SEM from six independent experiments. # P < .05 versus control, *P < .05 versus LPS only.
Figure 3.
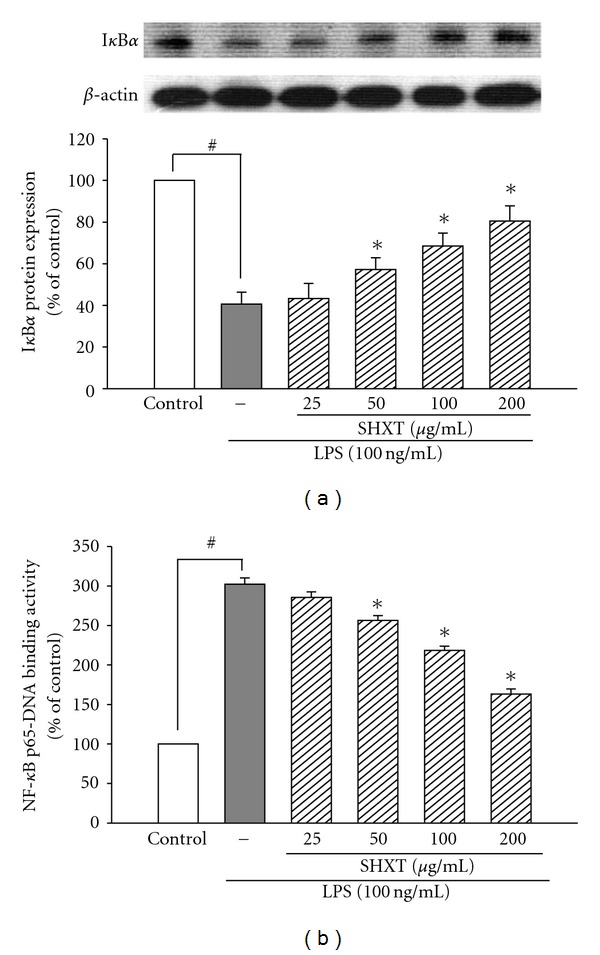
Effects of SHXT on the IκBα expression (a) and NF-κB activation (b) in lipopolysaccharide (LPS, 100 ng/mL)-treated BV-2 cells. Cultures were pretreated with SHXT for 30 min before the addition of LPS. Then the cells were collected at 1 h for NF-κB activity assay and at 24 h for IκBα protein analyses. Densitometry analyses are presented as the relative ratio of protein/β-actin protein, and they are represented as percentages of the control group. Changes of NF-κBp65 translocation levels are represented as percentages of the control group. Bars represent the mean ± SEM from six independent experiments. # P < .05 versus control, *P < .05 versus LPS only.
3.3. SHXT Inhibited Overproduction of TNF-α and IL-1β in LPS-Treated BV-2 Cells and Overexpression of nNOS, COX-2 and gp91phox in SH-SY5Y Cells Co-Cultured with BV-2 Cells
As shown in Table 1, LPS (100 ng/mL) caused a marked increase of TNF-α at 2 h and IL-1β at 24 h in BV-2 cells. Results indicated SHXT concentration-dependently attenuated LPS-induced overproduction of TNF-α and IL-1β from 25 to 200 μg/mL and 50 to 200 μg/mL, respectively. Under co-culture conditions, LPS exposure induced upregulation of nNOS, COX-2 and gp91phox of SH-SY5Y cells. However, SHXT (50–200 μg/mL) concentration-dependently attenuated LPS-activated BV-2 cell-induced overexpression of nNOS (Figure 4(a)), COX-2 (Figure 4(b)) and gp91phox (Figure 4(c)) on SH-SY5Y cells.
Figure 4.
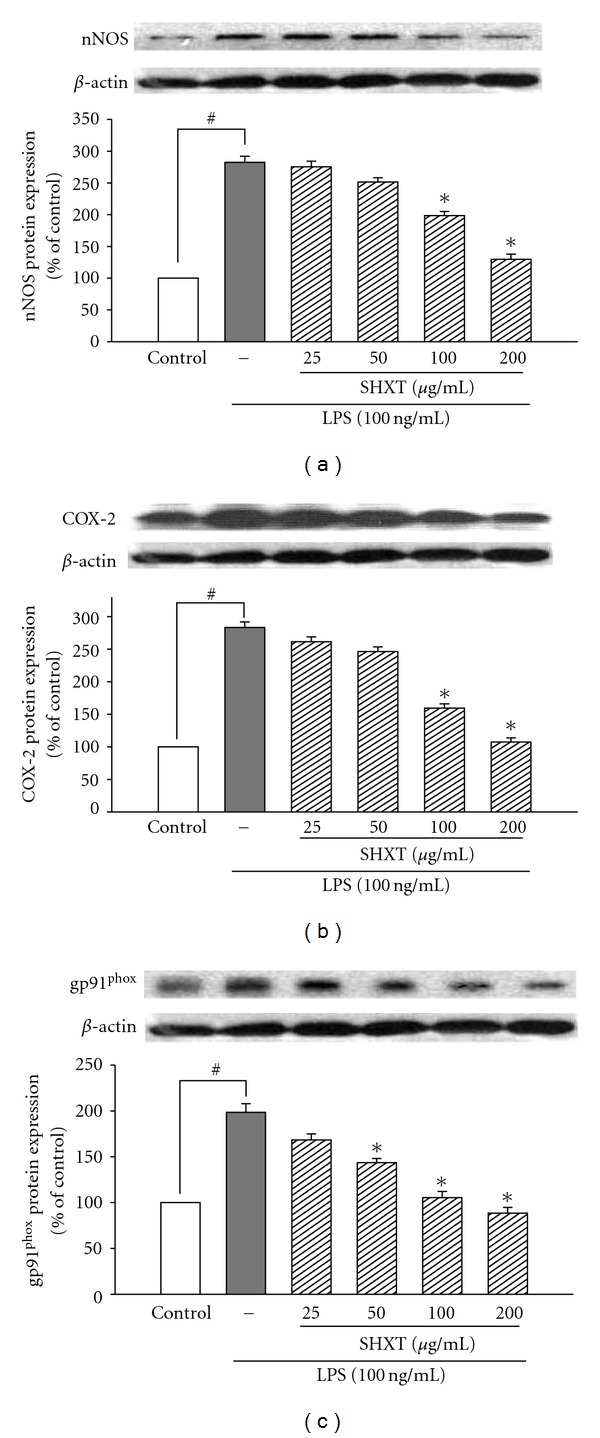
Inhibitory effects of SHXT on the expressions of nNOS (a), COX-2 (b) and gp91phox (c) in SH-SY5Y cells under co-culture with lipopolysaccharide (LPS, 100 ng/mL)-treated BV-2 cells. Cultures were pretreated with SHXT for 30 min followed by LPS treatment for 24 h. Densitometry analyses are presented as the relative ratio of protein/β-actin protein, and are represented as percentages of the control group. Bars represent the mean ± SEM from six independent experiments. # P < .05 versus control, *P < .05 versus LPS only.
3.4. 6-OHDA-Induced Inflammatory Factors and Mitochondrial Membrane Potential Changes in SH-SY5Y Cells Were Attenuated by SHXT
SH-SY5Y cells treated with 6-OHDA (100 μM) for 24 h resulted in significant increases in expression of nNOS (Figure 5(a)) and COX-2 (Figure 5(b)), which were inhibited by SHXT (25–200 μg/mL). SHXT also inhibited 6-OHDA-induced IκBα degradation (Figure 5(c)) and NF-κBp65 translocation (Figure 5(d)) at a concentration of 100 μg/mL and 200 μg/mL. SHXT (25–200 μg/mL) attenuated 6-OHDA-induced increases of gp91phox (Figure 6(a)) and iROS (Figure 6(b)). Furthermore, SHXT (50–200 μg/mL) significantly attenuated 6-OHDA-induced decrease in mitochondria membrane potential (Figure 6(c)).
Figure 5.
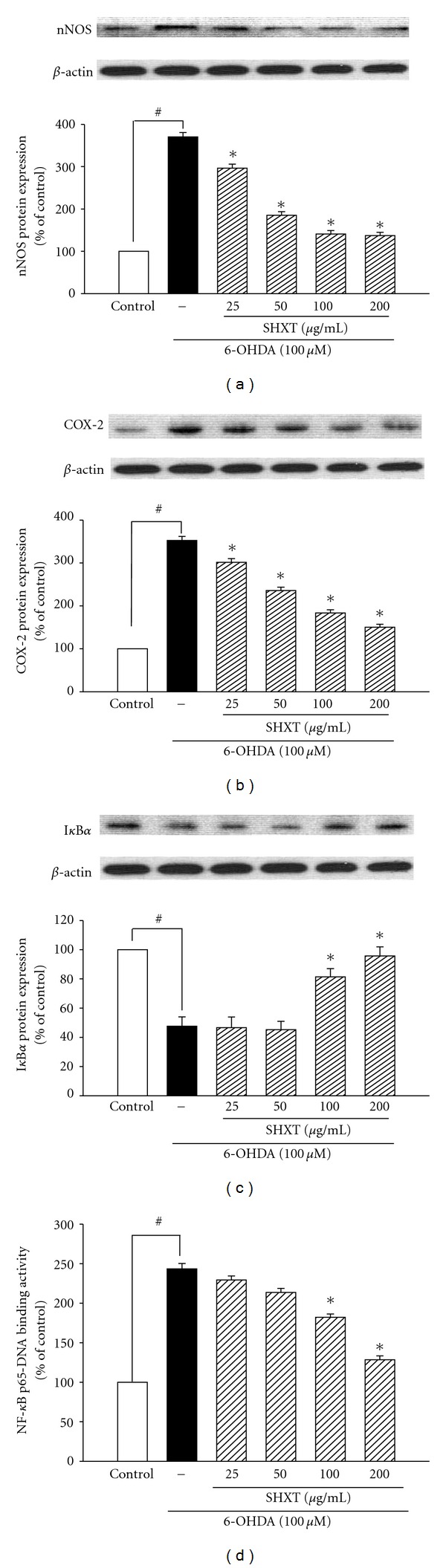
Inhibitory effects of SHXT on the expressions of nNOS (a), COX-2 (b), IκBα(c) and NF-κB activation (d) on 6-hydroxydopamine (6-OHDA, 100 μM) treated SH-SY5Y cells. Cultures were pretreated with SHXT for 30 min before the addition of 6-OHDA. Then the cells were collected at 1.5 h for NF-κB activity assay and at 24 h for nNOS, COX-2 and IκBα protein analyses. Densitometry analyses are presented as the relative ratio of protein/β-actin protein, and are represented as percentages of the control group. Changes of NF-κB p65 translocation levels are represented as percentages of the control group. Bars represent the mean ± SEM from six independent experiments. # P < .05 versus control, *P < .05 versus 6-OHDA only.
Figure 6.
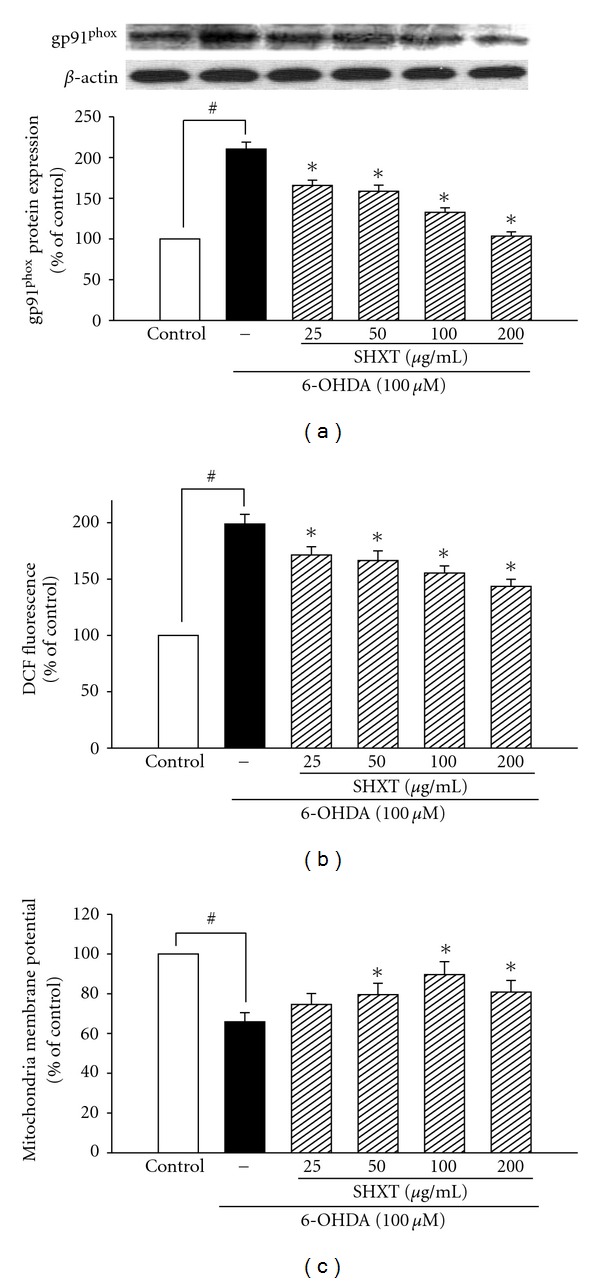
Effects of SHXT on 6-hydroxydopamine (6-OHDA, 100 μM)-induced changes of gp91phox expression (a), iROS formation (b) and mitochondria membrane potential (c) in SH-SY5Y cells. The iROS was measured by using H2DCF-DA staining and mitochondrial membrane potential was measured by rhodamine 123. Densitometry analyses are presented as the relative ratio of protein/β-actin protein, and are represented as percentages of the control group. Bars represent the mean ± SEM from six independent experiments. # P < .05 versus control group, *P < .05 versus 6-OHDA only.
4. Discussion
The present study demonstrated that SHXT could inhibit LPS-activated microglia- and neurotoxin 6-OHDA-induced neurotoxicity. The major findings showed that SHXT significantly reduced LPS-activated microglia- and 6-OHDA-induced neuronal cell death. In LPS-activated microglia-like BV-2 cells, SHXT attenuated inflammatory cytokine TNF-α and IL-1β, decreased the production of NO, PGE2 and iROS, downregulated overexpression of iNOS, COX-2 and gp91phox, decreased IκBα degradation and NF-κBp65 translocation, and upregulated HO-1 expression. Moreover, SHXT downregulated overexpression of nNOS, COX-2, gp91phox on neuronal SH-SY5Y cells co-cultured with activated microglia BV-2 cells. In 6-OHDA-induced neurotoxicity, the protective effects of SHXT are mediated by downregulating nNOS, COX-2 and gp91phox, decreased iROS level, IκBα degradation and NF-κBp65 translocation and increased mitochondria membrane potential of SH-SY5Y cells (Figure 7).
Figure 7.
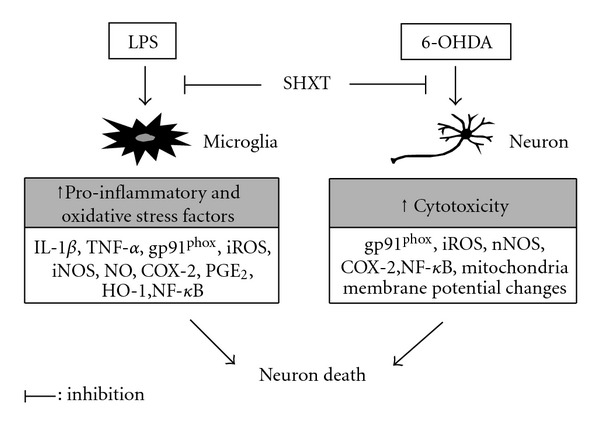
Hypothetical protective mechanisms of SHXT on microglia-mediated and 6-OHDA-induced toxicity in neuronal cells.
Microglial-mediated neurotoxicity has been implicated in numerous neurodegenerative diseases [29]. Activated microglia increase oxidative stress [36] and inflammatory mediators [37]. Pro-inflammatory cytokines TNF-α and IL-1β are thought to play a major role in inducing neuronal death [38]. In this study, SHXT significantly attenuated LPS-induced increase of pro-inflammatory cytokines (TNF-α and IL-1β) on BV-2 cells. Moreover, pro-inflammatory cytokines can induce iNOS expression and subsequently induce large amounts of NO production in activated microglia [7]. NO, together with iNOS and nNOS, is known to be involved in the pathogenesis of PD [39, 40]. Excessive accumulation of NO has long been known to be toxic to neurons [41]. Oxygen free radicals such as superoxide can react with NO to form much more deadly intermediates such as peroxynitrite [42]. The current results indicated that SHXT not only reduced LPS-activated microglia-induced increase of iNOS and NO, but also decreased the overexpression of nNOS in microglia-stimulated neuronal SH-SY5Y cells. SHXT also attenuated the overexpression of nNOS induced by 6-OHDA in SH-SY5Y cells. COX-2 is believed to be a critical enzyme in the inflammatory response and has been implicated in neuronal survival and death [43]. Cytokines (particularly TNF-α) activate COX-2 enzymes, and inhibition of COX-2 has been shown to provide neuroprotection [44]. Our results showed that SHXT not only decreased TNF-α production but also attenuated COX-2 expression and PGE2 production on LPS-treated microglia BV-2 cells. SHXT also inhibited overexpression of COX-2 on activated microglia-treated or 6-OHDA-treated neuronal SH-SY5Y cells. It is well known that HO-1 can inhibit iNOS expression and NO production in activated macrophages via inactivation of NF-κB [45, 46]. Our results showed that SHXT inhibited LPS-induced iNOS expression, NO production, IκBα degradation and NF-κB p65 translocation. SHXT also enhanced HO-1 expression.
Phagocyte NADPH oxidase (PHOX) is a major source of superoxide and the catalytic center of this oxidase is the membrane-integrated protein gp91phox [47, 48]. NADPH oxidase is deeply involved with microglia-induced neurotoxicity as a result of either generating extracellular ROS or by increasing microglia intracellular ROS production, which activates the generation of pro-inflammatory mediators and subsequently induces damages to neurons [1]. Microglia are critical to NADPH oxidase-mediated neurotoxicity in LPS-induced inflammation-mediated neurodegeneration [49]. Our study showed that SHXT decreased gp91phox overexpression and iROS levels on LPS-activated BV-2 cells. SHXT also downregulated gp91phox overexpression on SH-SY5Y cells co-cultured with LPS-activated microglia BV-2 cells. Additionally, NADPH oxidase-generated ROS are involved in the signaling event leading to COX-2 expression and PGE2 production in microglia treated with LPS [50]. The present results indicated SHXT inhibited gp91phox, iROS, COX-2 and PGE2. Therefore, the protective effects of SHXT on LPS-activated microglia-induced neuronal cell damage might be due to its anti-inflammatory and anti-oxidative effects.
It has been reported that the auto-oxidation of dopamine to 6-OHDA generates ROS and reactive quinones [31, 32], which subsequently induces lipid peroxidation, damages mitochondrial membrane and results eventually in the collapse of mitochondrial membrane potential, leading to cell death [51]. The present results indicated that SHXT decreased gp91phox overexpression and iROS levels in SH-SY5Y cells exposed to 6-OHDA. SHXT also attenuated the decrease of mitochondrial membrane potential and cell death induced by 6-OHDA. Furthermore, SHXT also inhibited 6-OHDA-induced increases of nNOS, COX-2, IκBα degradation and NF-κBp65 translocation. Therefore, the prevention of SHXT on 6-OHDA-induced neuronal cell damage might be partly mediated via decreasing production of iROS, increasing mitochondrial membrane potential and attenuating overexpression of inflammatory protein.
In conclusion, this study suggests that SHXT can effectively attenuate LPS-activated microglia- and neurotoxin 6-OHDA-induced neurotoxicity. Considering the importance of inflammation and ROS in neurodegeneration, SHXT might have a protective potential in the microglia-mediated or oxidative stress-related neurodegenerative disorders.
Supplementary Data
Supplementary data are available at eCAM online.
Funding
National Science Council, Taiwan (NSC-96-2320-B-037-039-MY3).
Supplementary Material
Dopamine receptor expression of differentiated SH-SY5Y cells using western blotting.
Acknowledgment
The authors thank Belinda Wilson for her editorial assistance.
References
- 1.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. British Journal of Pharmacology. 2007;150(8):963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Bernhardi R. Glial cell dysregulation: a new perspective on Alzheimer disease. Neurotoxicity Research. 2007;12(4):215–232. doi: 10.1007/BF03033906. [DOI] [PubMed] [Google Scholar]
- 3.Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: Lessons from multiple sclerosis. Current Medicinal Chemistry. 2007;14(27):2925–2936. doi: 10.2174/092986707782360015. [DOI] [PubMed] [Google Scholar]
- 4.Kim SU, De Vellis J. Microglia in health and disease. Journal of Neuroscience Research. 2005;81(3):302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- 5.Gao H-M, Jiang J, Wilson B, Zhang W, Hong J-S, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. Journal of Neurochemistry. 2002;81(6):1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 6.Gao H-M, Hong J-S, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. Journal of Neuroscience. 2003;23(4):1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iravani MM, Leung CCM, Sadeghian M, Haddon CO, Rose S, Jenner P. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. European Journal of Neuroscience. 2005;22(2):317–330. doi: 10.1111/j.1460-9568.2005.04220.x. [DOI] [PubMed] [Google Scholar]
- 8.Merrill JE. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Developmental Neuroscience. 1992;14(1):1–10. doi: 10.1159/000111642. [DOI] [PubMed] [Google Scholar]
- 9.Mandel S, Grünblatt E, Riederer P, Gerlach M, Levites Y, Youdim MBH. Neuroprotective strategies in Parkinson’s disease: an update on progress. CNS Drugs. 2003;17(10):729–762. doi: 10.2165/00023210-200317100-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lo Y-C, Liu Y, Lin Y-C, Shih Y-T, Liu C-M, Burka LT. Neuronal effects of 4-t-butylcatechol: a model for catechol-containing antioxidants. Toxicology and Applied Pharmacology. 2008;228(2):247–255. doi: 10.1016/j.taap.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YC, Uang HW, Lin RJ, Chen IJ, Lo YC. Neuroprotective effects of glyceryl nonivamide against microglia-like cells and 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. Journal of Pharmacology and Experimental Therapeutics. 2007;323:877–887. doi: 10.1124/jpet.107.125955. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Qin L, Li G, et al. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Journal of Pharmacology and Experimental Therapeutics. 2003;305(1):212–218. doi: 10.1124/jpet.102.043166. [DOI] [PubMed] [Google Scholar]
- 13.Wu DC, Jackson-Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. Journal of Neuroscience. 2002;22(5):1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suk K, Lee H, Kang SS, Cho GJ, Choi WS. Flavonoid baicalein attenuates activation-induced cell death of brain microglia. Journal of Pharmacology and Experimental Therapeutics. 2003;305(2):638–645. doi: 10.1124/jpet.102.047373. [DOI] [PubMed] [Google Scholar]
- 15.Chen LW, Wang YQ, Wei LC, Shi M, Chan YS. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson’s disease. CNS & Neurological Disorders Drug Targets. 2007;6:273–281. doi: 10.2174/187152707781387288. [DOI] [PubMed] [Google Scholar]
- 16.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian green propolis against in vitro and in vivo ischemic neuronal damage. Evidence-Based Complementary and Alternative Medicine. 2005;2:201–207. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho YS, Yu MS, Lai CS, So KF, Yuen WH, Chang RC. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on beta-amyloid peptide neurotoxicity. Brain Research. 2007;1158:123–134. doi: 10.1016/j.brainres.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 18.Li F-Q, Wang T, Pei Z, Liu B, Hong J-S. Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. Journal of Neural Transmission. 2005;112(3):331–347. doi: 10.1007/s00702-004-0213-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Kim YO, Kim H, et al. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. The FASEBJ Journal. 2003;17:1943–1944. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- 20.Lin W-C, Tan T-W. The role of gastric muscle relaxation in cytoprotection induced by San-huang-xie-xin-tang in rats. Journal of Ethnopharmacology. 1994;44(3):171–179. doi: 10.1016/0378-8741(94)01184-2. [DOI] [PubMed] [Google Scholar]
- 21.Shih YT, Wu DC, Liu CM, Yang YC, Chen IJ, Lo YC. San-Huang-Xie-Xin-Tang inhibits Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. Journal of Ethnopharmacology. 2007;112:537–544. doi: 10.1016/j.jep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Lo Y-C, Tsai P-L, Huang Y-B, et al. San-Huang-Xie-Xin-Tang reduces lipopolysaccharides-induced hypotension and inflammatory mediators. Journal of Ethnopharmacology. 2005;96(1-2):99–106. doi: 10.1016/j.jep.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Lo YC, Lin YL, Yu KL, et al. San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. Journal of Ethnopharmacology. 2005;101(1–3):68–74. doi: 10.1016/j.jep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Heo HJ, Kim D-O, Choi SJ, Shin DH, Lee CY. Potent inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid β protein-induced neurotoxicity. Journal of Agricultural and Food Chemistry. 2004;52(13):4128–4132. doi: 10.1021/jf049953x. [DOI] [PubMed] [Google Scholar]
- 25.Ge QF, Hu X, Ma ZQ, Liu JR, Zhang WP, Chen Z, et al. Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons. Pharmacological Research. 2007;55:148–157. doi: 10.1016/j.phrs.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Gu J-W, Hasuo H, Takeya M, Akasu T. Effects of emodin on synaptic transmission in rat hippocampal CA1 pyramidal neurons in vitro. Neuropharmacology. 2005;49(1):103–111. doi: 10.1016/j.neuropharm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.An H, Xu H, Yu Y, et al. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunology Letters. 2002;81:165–169. doi: 10.1016/s0165-2478(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 28.Qian L, Block ML, Wei S-J, et al. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. Journal of Pharmacology and Experimental Therapeutics. 2006;319(1):44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- 29.Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews Neuroscience. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 30.Block ML, Hong J-S. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Progress in Neurobiology. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Progress in Neurobiology. 2001;65(2):135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 32.Hanrott K, Gudmunsen L, O’Neill MJ, Wonnacott S. 6-hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cδ . Journal of Biological Chemistry. 2006;281(9):5373–5382. doi: 10.1074/jbc.M511560200. [DOI] [PubMed] [Google Scholar]
- 33.Benotmane AM, Hoylaerts MF, Collen D, Belayew A. Nonisotopic quantitative analysis of protein-DNA interactions at equilibrium. Analytical Biochemistry. 1997;250(2):181–185. doi: 10.1006/abio.1997.2231. [DOI] [PubMed] [Google Scholar]
- 34.Baumgarten G, Knuefermann P, Nozaki N, Sivasubramanian N, Mann DL, Vallejo JG. In vivo expression of proinflammatory mediators in the adult heart after endotoxin administration: the role of toll-like receptor-4. The Journal of Infectious Diseases. 2001;183:1617–1624. doi: 10.1086/320712. [DOI] [PubMed] [Google Scholar]
- 35.Shen Z, Peedikayil J, Olson GK, Siebert PD, Fang Y. Multiple transcription factor profiling by enzyme-linked immunoassay. BioTechniques. 2002;32(5):1168–1177. doi: 10.2144/02325dd07. [DOI] [PubMed] [Google Scholar]
- 36.Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7(1):75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 37.Chao CC, Hu S, Peterson PK. Glia, cytokines, and neurotoxicity. Critical Reviews in Neurobiology. 1995;9(2-3):189–205. [PubMed] [Google Scholar]
- 38.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 39.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(10):4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klivenyi P, Andreassen OA, Ferrante RJ, Lancelot E, Reif D, Beal MF. Inhibition of neuronal nitric oxide synthase protects against MPTP toxicity. NeuroReport. 2000;11(6):1265–1268. doi: 10.1097/00001756-200004270-00024. [DOI] [PubMed] [Google Scholar]
- 41.Xie Z, Wei M, Morgan TE, et al. Peroxynitrite mediates neurotoxicity of amyloid β-peptide 1-42- and lipopolysaccharide-activated microglia. Journal of Neuroscience. 2002;22(9):3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estévez AG, Jordán J. Nitric oxide and superoxide, a deadly cocktail. Annals of the New York Academy of Sciences. 2002;962:207–211. doi: 10.1111/j.1749-6632.2002.tb04069.x. [DOI] [PubMed] [Google Scholar]
- 43.Vijitruth R, Liu M, Choi D-Y, Nguyen XV, Hunter RL, Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. Journal of Neuroinflammation. 2006;3 doi: 10.1186/1742-2094-3-6. Article ID 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teismann P, Ferger B. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse. 2001;39(2):167–174. doi: 10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 45.Oh G-S, Pae H-O, Lee B-S, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radical Biology and Medicine. 2006;41(1):106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Kim KM, Pae H-O, Zhung M, et al. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264.7 macrophages. Biomedicine and Pharmacotherapy. 2008;62(9):630–636. doi: 10.1016/j.biopha.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Takeya R, Ueno N, Sumimoto H. Regulation of superoxide-producing NADPH oxidases in nonphagocytic cells. Methods in Enzymology. 2006;406:456–468. doi: 10.1016/S0076-6879(06)06034-4. Article ID 34. [DOI] [PubMed] [Google Scholar]
- 48.Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochemical and Biophysical Research Communications. 2005;338(1):677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 49.Qin L, Liu Y, Wang T, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. Journal of Biological Chemistry. 2004;279(2):1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 50.Wang T, Qin L, Liu B, et al. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. Journal of Neurochemistry. 2004;88(4):939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- 51.Guo S, Bezard E, Zhao B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radical Biology and Medicine. 2005;39(5):682–695. doi: 10.1016/j.freeradbiomed.2005.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dopamine receptor expression of differentiated SH-SY5Y cells using western blotting.


