Abstract
Sapropterin dihydrochloride, a synthetic, stable form of the tetrahydrobiopterin cofactor of phenylalanine hydroxylase, has been shown to reduce plasma phenylalanine (Phe) levels in a significant portion of patients with phenylketonuria (PKU). When we undertook introducing this medication to our PKU clinic population, the challenges of recalling and reconnecting with a variably treated and variably compliant patient population became apparent. We offered a trial of sapropterin to all of our clinic patients with PKU. In order to determine responsiveness, we used a 2 tier dose escalation protocol. After diet records were taken, and baseline plasma Phe levels were established, a 7-day trial of sapropterin at 10 mg/kg/day was started. At day 8, plasma phenylalanine levels were measured. Patients were considered to be responders if they had a 30% reduction in plasma Phe. If they did not respond, the dose of sapropterin was increased to 20 mg/kg/day, and levels were rechecked again in 8 days. Patients who were not responders at this time continued sapropterin for a total of 30 days and had Phe levels checked one last time. Patients who were responders and who were on a Phe restricted diet underwent gradual liberalization of their diet to the maximum tolerated natural protein intake while still maintaining plasma levels in the acceptable treatment range of 120–360 µmol/L. In our population, 36/39 patients with hyperphenylalaninemia (HPA) who were offered a trial of sapropterin elected to start sapropterin. Five of 36 patients were non-adherent with diet records and/or medication doses and we were unable to determine if they were responders. We were unable to categorize 2 of 31 of the patients who completed the trial as responders due to dietary issues, though they were probably responders. Of the 29 patients who completed the sapropterin trial and we could categorize, 18/29 (62%) were determined to be responders. Patients were classified based on their off-diet diagnostic plasma phenylalanine levels as classical PKU (>1200 µmol/L) and variant PKU (>400and <1200 µmol/L). The group with variant PKU had a 100% response rate, and patients with classical PKU had a 27% response rate. For the patients in the responder group who were on Phe restricted diet, we were able to liberalize most diets, in two cases to unrestricted protein intake. We also had unexpected beneficial findings in our clinic experience, including positive behavioral improvements in an adult severely affected by untreated PKU. Even in patients who were not considered to be responders, the introduction of sapropterin provided a tool to reconnect with patients and re-introduce beneficial dietary measures.
Keywords: phenylketonuria, sapropterin dihydrochloride, tetrahydrobiopterin
1. Introduction
Phenylketonuria (PKU) is an autosomal recessive inborn error of metabolism resulting in most cases from a deficiency of phenylalanine hydroxylase, the enzyme that catalyzes the hydroxylation of phenylalanine to tyrosine. The remaining cases are caused by defects in tetrahydrobiopterin metabolism [1].
The association between elevated plasma phenylalanine and mental retardation was first described by Asbjorn Folling in 1934. Untreated HPA will result in impaired postnatal cognitive development due to the neurotoxic effects of elevated plasma Phe [2]. Other clinical features in untreated patients include a 'mousy' odor, hypopigmentation, seizure disorder and eczema.
“Classic PKU” (plasma Phe greater than 1200 µmol/L) leads to the most severe clinical outcome, and outcomes from variant PKU (plasma Phe >400 µmol/L and <1200 µmol/L) and benign HPA (plasma Phe <400µmol/L) are more variable. The classifications of classical PKU, variant HPA (which can also be referred to as “moderate” or “mild” PKU) and benign HPA are controversial. Our clinic standard of care is to treat patients with a baseline plasma Phe of >400 µmol/L, a level above which neurologic consequences can be seen, and therefore the level is not truly "benign". At levels <400 µmol/L we maintain annual contact to monitor levels and to alert our female patients about pregnancy and the need for weekly monitoring of levels during pregnancy.
The mainstay of treatment has been dietary management with a combination of restricted natural protein intake and synthetic protein supplementation to maintain the plasma phenylalanine levels between 120–360 µmol/L for life [3]. This diet is especially important in early childhood to prevent irreversible neurocognitive complications. However, it has been shown that discontinuation of a Phe restricted diet at later stages in life has negative cognitive outcomes including loss of executive functioning skills [4], behavioral problems [5], and emotional difficulties including depression [6]. Therefore, lifelong dietary management through adulthood is considered the best course of treatment for optimal neurocognitive functioning.
As with the restrictive diets for many inborn errors of metabolism, this diet is cumbersome and less palatable than a regular diet. When many patients enter adolescence they abandon strict adherence, with 75% of adult patients essentially off diet [5]. Control of PKU during adulthood is not only important for cognitive performance of the affected adult, but is essential in pregnant women. Uncontrolled maternal PKU during pregnancy can have devastating outcomes for the fetus including congenital heart defects, microcephaly, mental retardation, and intrauterine growth retardation [7].
Since January 2008, an additional approach for managing PKU in the United States is to supplement the diet with a synthetic form of the tetrahydrobiopterin cofactor for PAH. Controlled phase II and III clinical trials have established that sapropterin dihydrochloride, a synthetic, stable form of the tetrahydrobiopterin cofactor, is effective in reducing phenylalanine levels in a significant proportion of patients with HPA. [8,9]. Sapropterin is approved in the United States for use to lower phenylalanine levels in patients with tetrahydrobiopterin responsive PKU.
Various methods have been proposed to determine a patient’s responsiveness to sapropterin. In the phase II clinical trial, the subjects were on unrestricted diets, and responsiveness was determined to be a >30% decrease in plasma phenylalanine level after an eight day trial of 10 mg/kg/day of sapropterin [9]. Other groups have proposed BH4 loading trials with measurements at several time periods before and after a dose of BH4 [10]. It has also been suggested that there is a genotype/phenotype correlation between residual PAH activity, and the genotypes can be used to predict BH4 responsiveness [11], however this correlation is weak [12]. Therefore, as the use of sapropterin becomes standard of care in the treatment of responsive patients with PKU, the question arises regarding the best way to determine if a patient is a responder in a realistic clinic setting treating all-comers diagnosed with PKU.
A protocol to implement routine sapropterin use and assessment for efficacy in the PKU population at the Johns Hopkins Institute of Genetic Medicine has been in place since January, 2008. Our protocol aims to evaluate both the response in individual patients, as well as to clarify the assessment process in less straight forward patients, such as children on controlled diets and older adults with untreated classical PKU.
2. Materials and Methods
This was a therapeutic trial available to any patient with variant PKU or classical PKU as diagnosed by off-diet diagnostic plasma Phe levels who has been seen in the Johns Hopkins Metabolic Genetics clinic. Protocol enrollment began in January 2008, and this study reports patients assessed through September 2009. No dietary or trial baseline plasma Phe concentration criteria were used to limit enrollment.
2.1 Patients
Baseline protein intake was calculated from 3 day diet records, and baseline plasma amino acid levels were established as a single 3–5 hour fasting plasma phenylalanine concentration determined from a quantitative plasma amino acid profile prior to starting the trial. The only dietary changes made prior to beginning sapropterin dihydrochloride were in patients with inadequate protein intake as determined from their dietary records by a metabolic dietician (C.K.). These diets were altered to provide adequate protein intake, and a new baseline Phe concentration was determined. Otherwise, patients were not required to be on a Phe controlled diet, and no enrollment restrictions were made based on initial plasma Phe level.
Patients were classified as classical or variant PKU based on their off-diet diagnostic plasma phenylalanine levels as ascertained from clinical medical records. Classical PKU was defined as a plasma phenylalanine of >1200 µmol/L, and variant PKU as a plasma phenylalanine of 401–1199 µmol/L.
2.2 Study design
Eligible subjects received an open label 7-day course of sapropterin at the full sized 100 mg pill dose closest to 10 mg/kg taken once daily. Subjects were asked to keep a stable diet throughout the study, and to keep a diary for three days of each week containing a record of all foods and beverages ingested including synthetic and low protein containing foods. Dietary records were reviewed by a single qualified metabolic dietician and monitored for calorie intake, natural protein intake, synthetic protein intake, and total Phe intake.
A positive response was defined as a reduction of at least 30% in the plasma phenylalanine or reduction to treatment range of less than 360 µmol/L after day 7. If no response was observed after 7 days at a dose of 10 mg/kg/day, the dose was increased to the full sized 100 mg pill dose closest to 20 mg/kg/day. If no response was seen at this dose, the patient continued on 20 mg/kg/day of sapropterin (with no dietary changes made) until day 30, and plasma Phe was checked one last time. Plasma Phe levels were assayed on days 0, 8,16 and 30 in a single central laboratory with the aminoacid analyzer method for this phase of the trial.
We were also careful not to cause sub-physiologic plasma Phe levels in patients who were starting sapropterin while on a protein controlled diet, and had normal range Phe levels at the start of the trial. In these cases, we brought the patients in earlier (one or two days after starting sapropterin) to make sure that we were not causing unacceptably low plasma Phe levels.
2.3 Dietary Modification
If a positive response was seen, then the dietary natural protein or phenylalanine intake was gradually increased to obtain a maximum liberalization of the diet while maintaining a fasting plasma Phe level of 120–360 µmol/L. In some cases, if the original response dose was 10 mg/kg/day, the dose of sapropterin was increased to 20 mg/kg/day in an attempt to liberalize the diet further. During this phase of dietary modification, plasma Phe levels were checked on a weekly basis either via blood spots sent to the Maryland State screening laboratory or by the central clinical laboratory where the initial trial analysis was done. Once a stable, maximally liberalized diet was obtained, we resumed standard of care for monitoring Phe levels in our patients by checking Phe on a monthly basis either via blood spots sent to the Maryland State screening laboratory or by the central clinical laboratory where the initial trial analysis was done
3. Results
Thirty-nine patients were offered a trial of sapropterin; 20 were females, and 19 were males. Patient ages ranged from 3 years to 58 years with an average of 23.4 years, and a median of 19 years. Of the PKU patients who were offered a trial of sapropterin, 36 of 39 patients (92.3%) elected to start taking it. Of the patients who started the trial, 20 had classical PKU, and 16 had variant PKU (Table 1).
Table 1.
Characteristics of patients who completed the sapropterin trial.
| n (%) | |
|---|---|
| completed trial * | 29 |
| Males | 12 (41%) |
| females | 17 (59%) |
| protein restricted diet | 17 (59%) |
| classical PKU | 15 (52%) |
| variant PKU | 14 (48%) |
| Responders | 18 (62%) |
| Non responders | 11 (38%) |
Only patients who were adherent to the trial and who we could categorize as responders or non responders are described in this table.
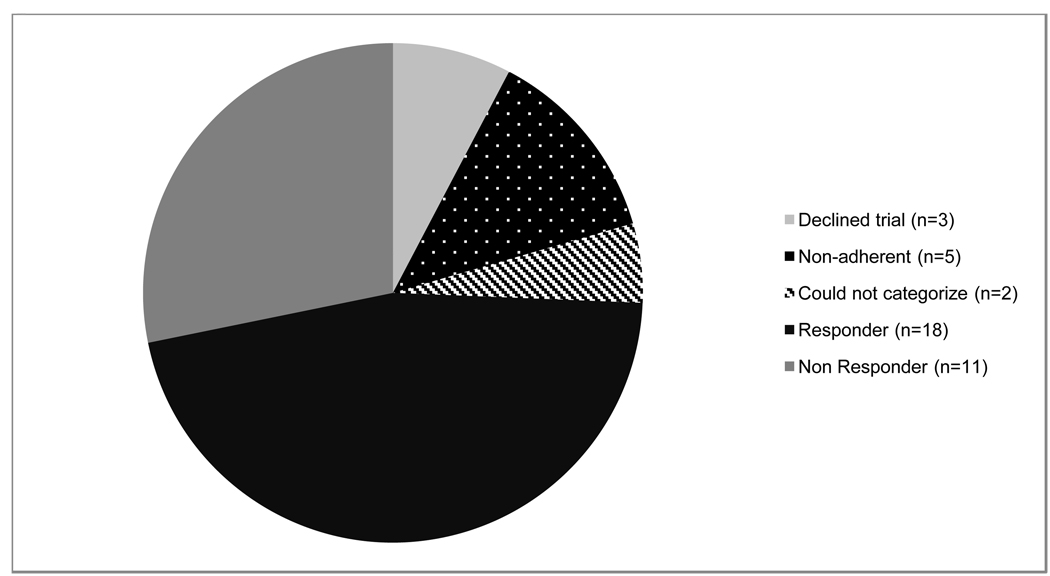
Five of 36 patients (four patients with classical PKU and one with variant PKU) were non-adherent to the requirement of diet records and/or medication doses and we were unable to determine whether they were responders. Four of these patients were male and one was female. We were unable to categorize two of the patients who completed the trial because they had low starting plasma Phe levels and inconsistent and poor oral intake (Fig. 1). These patients are addressed in the discussion section. Of the patients who completed the trial and we could categorize, 12/29 (41.4%) patients were not following protein restricted diets. The average starting plasma phenylalanine level among these patients was 1372.6 µmol/L with a range of 444 µmol/L to 1847 µmol/L. Seventeen patients were on some form of restricted diet, though not all patients maintained Phe in acceptable treatment ranges with an average baseline Phe of 587.0 µmol/L and a range of 225 µmol/L to 1363 µmol/L. In this group the diet ranged from 6–35 mg/kg/day of Phe.
Figure 1.
Trial adherence and response rate of patients offered a trial of sapropterin.
3.1 Efficacy
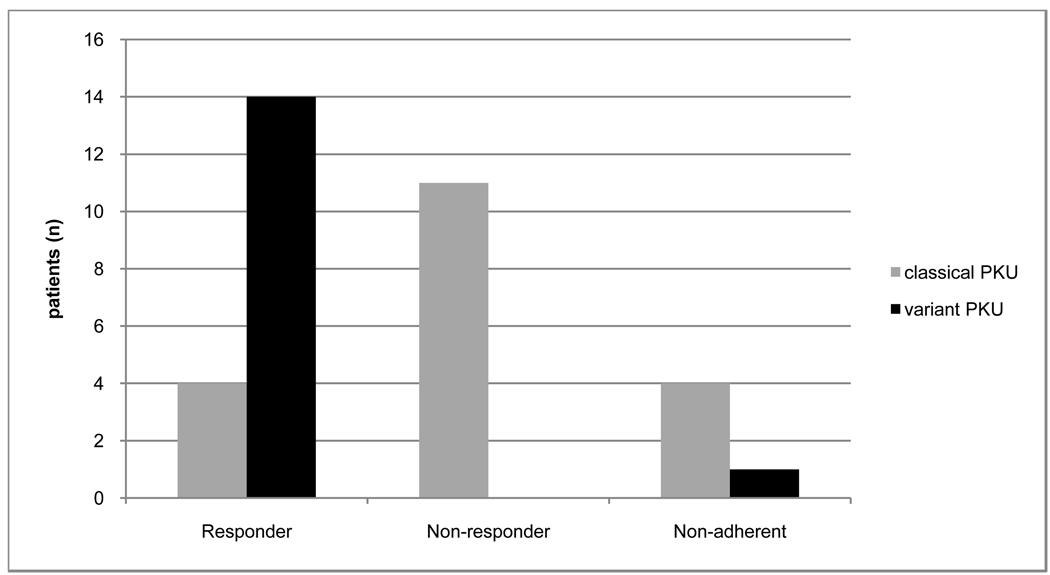
In this secion we will only discuss those patients we could categorize. Eighteen of the 29 (62%) patients who completed the sapropterin trial and we could categorize were determined to be responders. The doses of sapropterin dihydrochloride required for clinical response varied, with 14 patients requiring a dose of 7–15 mg/kg/day, and 4 patients requiring a dose of 15–20 mg/kg/day. Four of 15 (26.7%) patients with classical PKU were determined to be responders, and 14/14 (100%) variant PKU patients were responders (Fig. 2).
Figure 2.
Trial adherence and response rate of patients with different diagnostic plasma Phenylalanine levels who were offered a trial of sapropterin.
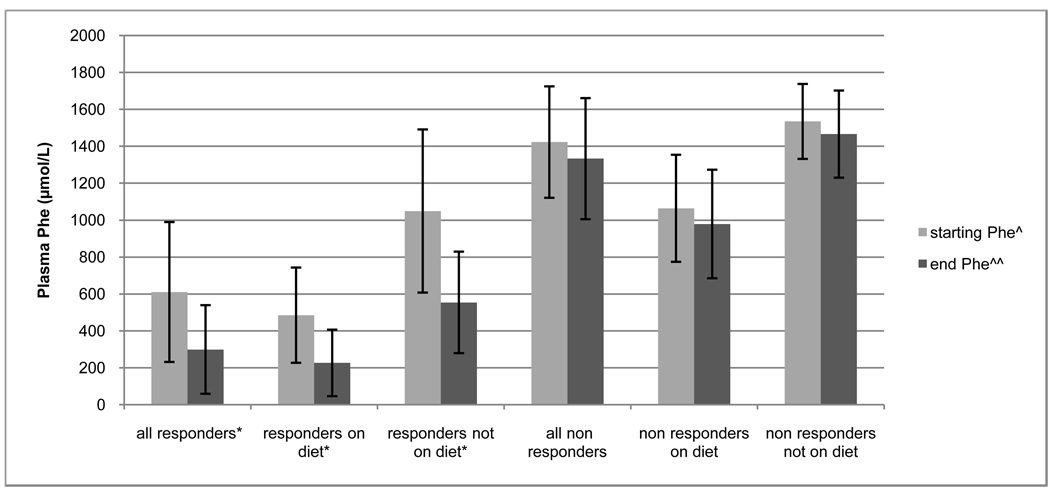
Four of the 12 (33.3%) adults who were not on a Phe-restricted diet were responders. The average baseline starting Phe in this group was 1049.0 µmol/L with a range of 444 to 1461 µmol/L. The average Phe while on sapropterin in this group was 553.7 µmol/L with a range of 162 µmol/L to 793 µmol/L. The P value obtained using a student’s paired T-test was significant at 0.035 (Fig. 3).
Figure 3.
Start and end phenylalanine levels of patients with different diagnostic plasma phenylalanine levels who completed a trial of sapropterin.
*P value <0.001 ^Plasma phenylalanine level taken the day prior to starting sapropterin. ^^Response plasma phenylalanine level when 30% decrease from baseline was observed (in responders), or plasma phenylalanine when the patient reached the maximum sapropterin dose of 20 mg/kg/day (in non responders).
Fourteen of the 17 (82.3%) patients who were on a Phe-restricted diet were responders. The average baseline plasma phenylalanine level of these patients was 484.9 µmol/L with a range of 225 µmol/L to 1061 µmol/L. The average response Phe in this group was 226.1 µmol/L with a range of 28 µmol/L to 696 µmol/L. The P value obtained using a student’s paired T-test was highly significant at < 0.001.
Eleven patients were non-responders. The average starting plasma Phe of these patients was 1422.3 µmol/L with a range of 783 to 1847 µmol/L. The average end trial plasma Phe of these patients was 1332.6 µmol/L with a range of 731–1798 µmol/L. Of these patients, 3 started out on some form of protein restricted diet and had an average plasma Phe of 1063.7 µmol/L with a range of 783–1363 µmol/L and an average end trial Phe of 978.7 µmol/L with a range of 731–1304 µmol/L. Eight patients were on unrestricted diets and had an average starting plasma Phe of 1534.4 µmol/L with a range of 1363–1847 µmol/L and an average end trial plasma Phe of 1465.4 µmol/L with a range of 1148–1798 µmol/L.
3.2 Dietary modifications
Within the responder group, a wide range of dietary modifications were tolerated ranging from a mild increase in Phe intake from 20 mg/kg/day to 22 mg/kg/day, and with the benefit of improved plasma Phe levels, to an increase in daily dietary Phe allowances up to a non- protein restricted diet. In responder patients who started on some form of Phe restricted diet, the average Phe tolerance was increased from 21.0 mg/kg/day to 41.0 mg/kg/day (Table 2).
Table 2.
Dietary modifications in patients who started the sapropterin trial on a protein restricted diet.
| patient | start diet (Phe mg/kg/d) |
Response dose of sapropterin (mg/kg/d) |
Liberalized Diet (Phe mg/kg/d) |
Sapropterin dose Adjusted with diet (mg/kg/d) |
percent increase in Phe intake |
|---|---|---|---|---|---|
| 1 | 31 | 9 | 47 | 20 | 52% |
| 2 | 36 | 12 | 60 | 67% | |
| 3 | 31 | 10 | 100 | 223% | |
| 4 | 17 | 9 | 100 | 16.5 | 488% |
| 5 | 12 | 11 | 20 | 20 | 67% |
| 6 | 10 | 10 | 15 | 20 | 50% |
| 7 | 33.5 | 20 | 39 | 16% | |
| 8 | 6 | 15.5 | 9 | 75% | |
| 9 | 20 | 20 | 22 | 10% | |
| 10 | 25 | 11 | 44 | 76% | |
| 11 | 30 | 13 | 48 | 60% | |
| 12 | 13 | 11 | 20.5 | 20 | 58% |
| 13 | 12.3 | 11 | 19.5 | 19 | 59% |
| 14 | 17 | 11 | 30 | 20 | 76% |
During the dietary liberalization phase, we increased the sapropterin dose in five patients who initially responded to a lower dose of sapropterin. This modification allowed for further liberalization of the diet.
4. Discussion
We introduced sapropterin dihydrochloride as standard of care in our clinic, and followed a 2 tier medication administration protocol to determine if patients were responders. Our protocol was less straightforward than those discussed in the clinical trials since we accepted all comers who have been seen in our clinic at any time including those on controlled diets, young children and severely affected untreated adults.
Overall, we determined that 62% of our patients who completed the trial and who we could categorize were responders. This is similar to the 56% response rate seen in a phase III study examining sapropterin efficacy in increasing phenylalanine tolerance [13]. This is higher than the 20% response rate seen by Burton et al in 2007 in their phase II open label screening study. This difference is possibly due to the fact that the phase II trial used only a lower dose of 10 mg/kg/day, whereas we escalated the dose to 20 mg/kg/day when applicable. However, similar to this study, we found that patients with the highest baseline levels of plasma phenylalanine were less likely to respond to sapropterin than patients with the lowest baseline plasma phenylalanine. This finding emphasizes the likely importance of residual PAH enzyme activity in sapropterin’s ability to lower plasma Phe levels. Nevertheless, we still observed a 27% response rate to sapropterin in classical PKU patients. This insinuates that some patients with high plasma Phe levels must retain some baseline residual enzymatic function or enzyme transcription.
We were able to liberalize diets in a majority of patients, in 2 cases to a non-protein restricted diet (Patients 3 and 4 in Table 2). These findings are in agreement with previous studies indicating that sapropterin is a useful adjunct to dietary therapy in patients with PKU, and this has the potential to improve quality of life. We found that over time, in some cases, there was a lack of compliance with diet or sapropterin administration, because after the initial trial was completed, levels of Phe became more variable and out of treatment range (supplementary table1). One patient even started the Atkins diet, which we did not advise. One responder electively discontinued sapropterin because he felt that the small increase in tolerated Phe intake in his diet was not significant to him, and a second patient discontinued for unknown reasons.
We had 2 patients who we were unable to categorize as responders due to low starting phenylalanine levels and inconsistent oral intake. One of these patients has variant PKU and a baseline Phe of 646 umol/L when he was initially diagnosed. At the time he started the sapropterin trial, he was on a protein-restricted diet with a Phe intake of 38 mg/kg/day and had a plasma Phe of 130 umol/L. He was started on a sapropterin dose of 8 mg/kg/day and did not have a decrease in his plasma Phe when measured 8 days later. However, due to poor oral intake, the milk content in his formula was increased at that time. It was noted that he did not have an appreciable increase in his plasma Phe even with increases in his natural protein intake at the next visit 8 days later. We therefore continued to liberalize his diet. His most recent plasma Phe was 196 µmol/L with a Phe intake of 65 mg/kg/day and a sapropterin dose of 8 mg/kg/day. We were unable to include these findings in the above results, because while he doesn’t fit the strict definition of a responder (he did not have a 30% decrease in plasma Phe), he is obviously responsive to sapropterin due to the 70% increase in his tolerated Phe intake. This finding highlights the observation that using a strict definition for a responder (30% decrease in plasma Phe) could potentially cause us to miss patients who might benefit from this medication. Therefore, we feel that all patients with variant PKU should be thoroughly studied before being labeled non- responders.
The other patient we were unable to categorize had many extenuating circumstances. He is an ex 28-week premature infant with a history of severe intrauterine growth retardation as well as classical PKU. He developed chronic lung disease as an infant, and a subsequent oral food aversion. He had three separate sapropterin trials, and in 2 of 3 trials he showed a response to 10 mg/kg/day of sapropterin. In one of these trials he had an 87% decrease in plasma Phe (352 µmol/L to 45 µmol/L), and in the other trial he had a 77% decrease in plasma Phe (213µmol/L to 49 µmol/L). However, subsequent levels while on the same dose of sapropterin were out of treatment range. We were unable to explain this variability because he was entirely G-tube fed on a consistent Phe controlled diet. Therefore we were unable to classify him as a responder.
We had some unexpected positive experiences with several patients in our clinic. One patient is a 46 year old man with untreated PKU and the expected severe mental retardation and behavioral problems. His baseline plasma Phe was 1255 µmol/L on an unrestricted diet, and after taking 20 mg/kg/day of sapropterin his plasma Phe fell to 308 µmol/L. His caretakers in the facility where he resides noticed significant behavioral improvements, resulting in fewer behavioral incidents. He was able to have increased social interactions, and for the first time in his life was able to take a vacation with the other residents in his facility. A similar behavioral improvement was previously reported in an individual with PKU who was placed on a Phe restricted diet at 65 years of age [14].
Behavioral improvements had been previously suggested in a study using a controlled diet in adults severely affected by untreated PKU, but this group found that there was a very low compliance with the diet [15]. Our observation indicates that a trial of sapropterin is worthwhile even in severely affected PKU patients, and can have beneficial improvements on quality of life in this challenging population in whom dietary modifications may not be possible.
We also found that the introduction of this medication provided a means to reconnect with a patient population who had not been seen in the clinic in many years, and these new clinical interactions were beneficial even for patients who were not determined to be sapropterin responders. One patient is a 50 year old late treated woman living in a semi-supervised group home. She had an an initial plasma Phe of 1519 µmol/L, and was on an unrestricted diet. She suffered from chronic headaches and depression. She was not a sapropterin responder, but she restarted a Phe restricted diet with natural protein restriction and synthetic supplements, and her plasma Phe was reduced to 127 µmol/L. She noted that she felt “great” at subsequent visits, and had a substantial reduction in her headaches. Since her re-establishment of care with our clinic, she has regular assessments with a metabolic dietician and dietary adjustments.
Plasma Phe control is influenced by a wide range of factors including dietary adherence, intercurrent illness, weight loss or gain, and now Sapropterin administration and compliance. Controlled escalation of protein intake proved to be challenging for both the patients and the clinicians since Phe intake determinations relied on patient and parent reports, and in many cases we increased diet gradually by as little as 5 mg/kg/day of Phe. However, considering the potential improvement for quality of life, we feel that this should be the goal of subsequent treatment once a patient is determined to be a sapropterin responder.
In conclusion, our experience is a good representation of the challenges and benefits of introducing sapropterin as a new standard of care to our patients. This includes further confirmation of the substantial response rate to sapropterin, the successful implementation of a 2 tier medication response trial, the positive outcomes in severely affected untreated PKU patients, and the reestablishment of care in patients lost to follow up.
Supplementary Material
Personal characteristics, Phe levels, and dietary information on all patients who were responders or who we were unable to characterize.
^ patients we were unable to characterize, either due to inadequate oral intake or low starting Phe levels, described in the discussion section.
*Phe levels ascertained during the dietary modification portion of the trial.
** Follow up monthly “standard of care” surveillance Phe levels obtained after the trial was complete.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
HJ Vernon, Email: hvernon1@jhmi.edu.
C Koerner, Email: ckoerne1@jhmi.edu.
M Johnson, Email: mjohn174@jhmi.edu.
A Bergner, Email: abergne1@jhmi.edu.
A Hamosh, Email: ahamosh@jhmi.edu.
References
- 1.Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edn. New York: McGraw-Hill; 2001. pp. 1667–1709. (Assoc eds.) [Google Scholar]
- 2.Sarkissian CN, Gámez A, Scriver CR. What we know that could influence future treatment of phenylketonuria. J. Inherit. Metab. Dis. 2009;32(1):3–9. doi: 10.1007/s10545-008-0917-7. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health Consensus Developemnt Conference Statement, Phenylketonuria: screening and management, October 16–18, 2000. Pediatrics. 2001;108:972–982. doi: 10.1542/peds.108.4.972. [DOI] [PubMed] [Google Scholar]
- 4.VanZutphen KH, Packman W, Sporri L, Needham MC, Morgan C, Weisiger K, Packman S. Executive functioning in children and adolescents with phenylketonuria. Clin. Genet. 2007;72(1):13–18. doi: 10.1111/j.1399-0004.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 5.Koch R, Burton B, Hoganson G, Peterson R, Rhead W, Rouse B, Scott R, Wolff J, Stern AM, Guttler F, Nelson M, de la Cruz F, Coldwell J, Erbe R, Geraghty MT, Shear C, Thomas J, Azen C. Phenylketonuria in adulthood: a collaborative study. J. Inherit. Metab. Dis. 2002;25(5):333–346. doi: 10.1023/a:1020158631102. [DOI] [PubMed] [Google Scholar]
- 6.Levy HL, Waisbren SE. PKU in adolescents: rationale and psychosocial factors in diet continuation. Acta. Paediatr. 1994;407:92–97. doi: 10.1111/j.1651-2227.1994.tb13463.x. [DOI] [PubMed] [Google Scholar]
- 7.Levy HL, Ghavami M. Maternal phenylketonuria: a metabolic teratogen. Teratology. 1996;53(3):176–184. doi: 10.1002/(SICI)1096-9926(199603)53:3<176::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, Whitley CB, Feillet F, Feigenbaum AS, Bebchuk JD, Christ-Schmidt H, Dorenbaum A. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomized placebo-controlled study. Lancet. 2007;370(9586):504–510. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 9.Burton BK, Grange DK, Milanowski A, Vockley G, Feillet F, Crombez EA, Abadie V, Harding CO, Cederbaum S, Dobbelaere D, Smith A, Dorenbaum A. The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydrobiopterin): a phase II, multicentre, open-label, screening study. J. Inherit. Metab. Dis. 2007;30(5):700–707. doi: 10.1007/s10545-007-0605-z. [DOI] [PubMed] [Google Scholar]
- 10.Blau N, Bélanger-Quintana A, Demirkol M, Feillet F, Giovannini M, MacDonald A, Trefz FK, van Spronsen FJ. Optimizing the use of sapropterin (BH4) in the management of phenylketonuria. Mol. Genet. Metab. 2009;96:158–163. doi: 10.1016/j.ymgme.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Karacić I, Meili D, Sarnavka V, Heintz C, Thöny B, Ramadza DP, Fumić K, Mardesić D, Barić I, Blau N. Genotype-predicted tetrahydrobiopterin (BH4)-responsiveness and molecular genetics in Croatian patients with phenylalanine hydroxylase (PAH) deficiency. Mol. Genet. Metab. 2009;97(3):165–171. doi: 10.1016/j.ymgme.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Blau N, Erlandsen H. The metabolic and molecular bases of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Mol. Genet. Metab. 2004;82(2):101–111. doi: 10.1016/j.ymgme.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Trefz FK, Burton BK, Longo N, Casanova MM, Gruskin DJ, Dorenbaum A, Kakkis ED, Crombez EA, Grange DK, Harmatz P, Lipson MH, Milanowski A, Randolph LM, Vockley J, Whitley CB, Wolff JA, Bebchuk J, Christ-Schmidt H, B J Sapropterin Study Group. Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J. Pediatr. 2009;154(5):700–707. doi: 10.1016/j.jpeds.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Merrick J, Aspler S, Schwarz G. Phenylalanine-restricted diet should be life long. A case report on long-term follow-up of an adolescent with untreated phenylketonuria. Int J Adolesc Med Health. 2003;15(2):165–168. doi: 10.1515/ijamh.2003.15.2.165. [DOI] [PubMed] [Google Scholar]
- 15.Lee PJ, Amos A, Robertson L, Fitzgerald B, Hoskin R, Lilburn M, Weetch E, Murphy G. Adults with late diagnosed PKU an dsevere challenging behavior: a randomized placebo-controlled trial of a phenylalanine restricted diet. J. Neurol. Neurosurg. Psychiatry. 2009;80:631–635. doi: 10.1136/jnnp.2008.151175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Personal characteristics, Phe levels, and dietary information on all patients who were responders or who we were unable to characterize.
^ patients we were unable to characterize, either due to inadequate oral intake or low starting Phe levels, described in the discussion section.
*Phe levels ascertained during the dietary modification portion of the trial.
** Follow up monthly “standard of care” surveillance Phe levels obtained after the trial was complete.