Abstract
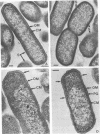
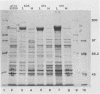
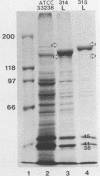
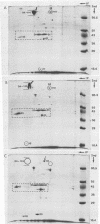
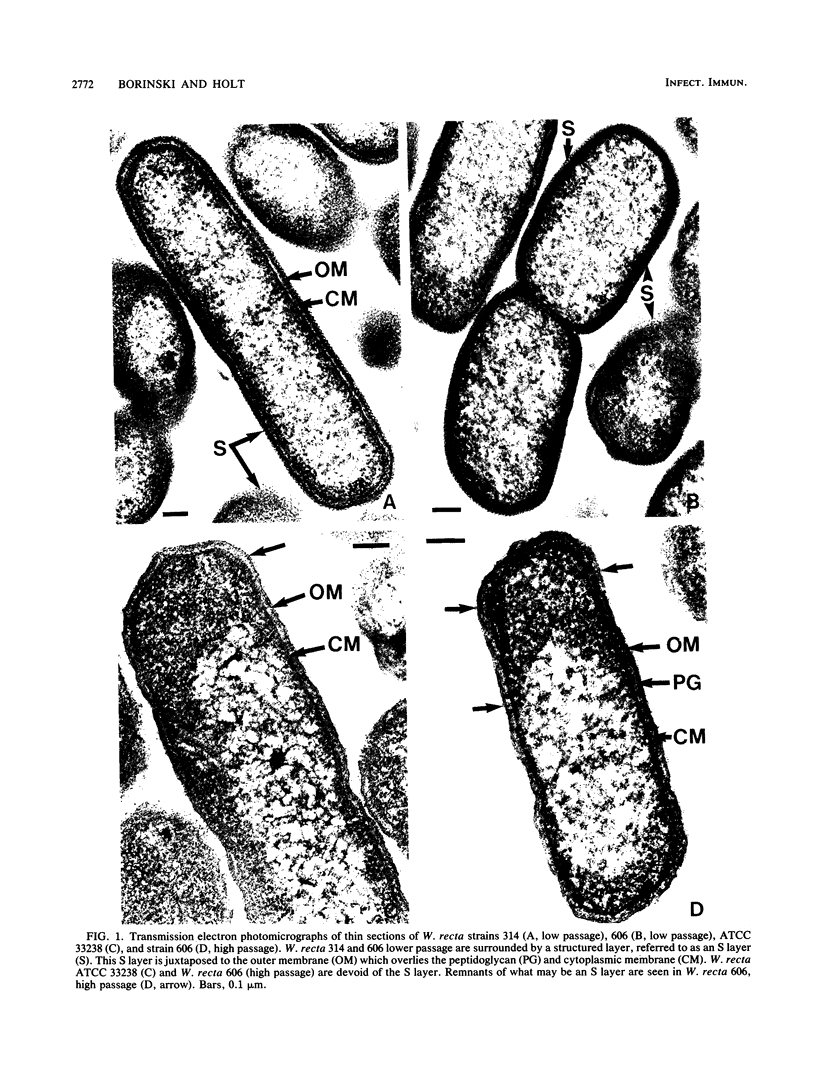
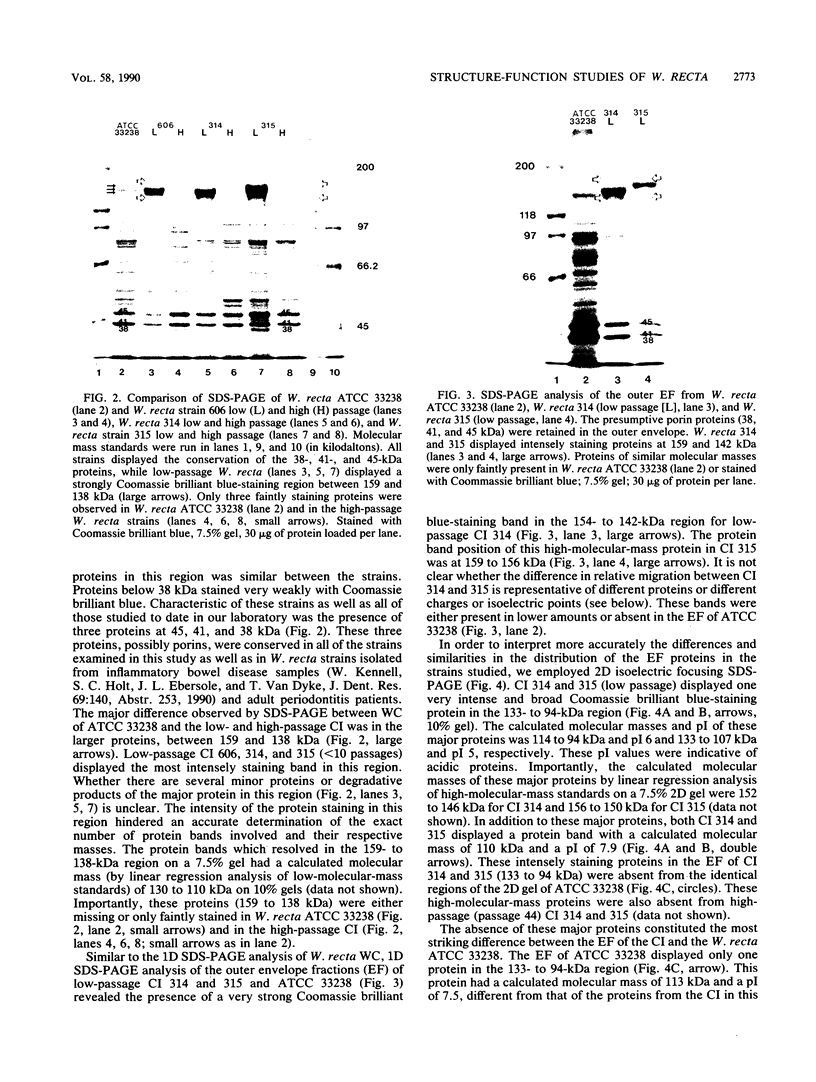
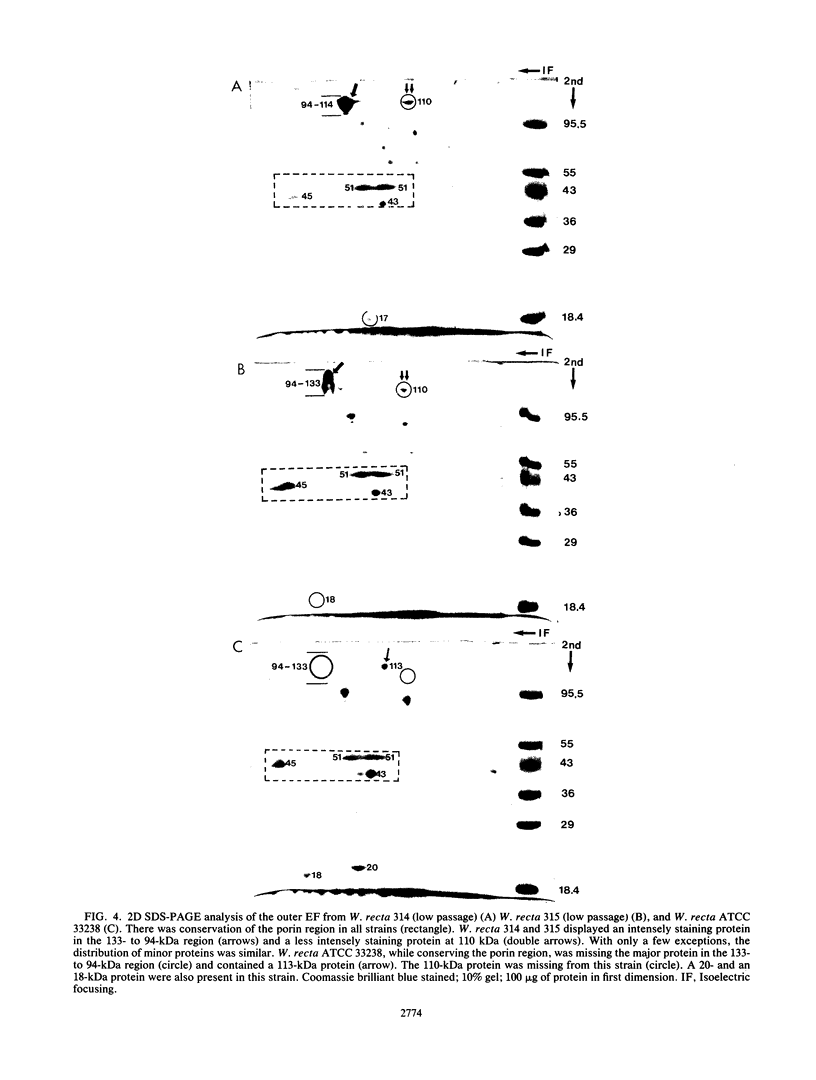
Selected characteristics of the surface of Wolinella recta ATCC 33238 and three W. recta clinical isolates (CI) were studied as well as the adherence of these strains to human gingival fibroblasts (HGF). W. recta ATCC 33238 and the CI were examined by electron microscopy, electrophoresis, isoelectric focusing, and adherence to HGF. Electron microscopic examination of CI revealed the presence of a periodic paracrystalline layer external to and associated with the outer membrane. This surface layer (S layer) was not observed on ATCC 33238. Whole cells and outer envelope protein profiles of the CI revealed major bands of 159- to 138-kilodalton proteins which were barely detectable in ATCC 33238. Repeated in vitro subculturing of the CI on solid or liquid medium resulted in both the physical loss of this layer and the loss of the high-molecular-weight proteins. Low-passage-number CI demonstrated 40 to 60% less adherence to HGF than ATCC 33238. These observations suggest that short term in vitro-subcultured W. recta strains possess surface characteristics which are significantly different from those of their long-term in vitro-subcultured counterparts. These differences may have significant effects on host cell interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Roberts I., Boulnois G. J., Saunders J. R., Hart C. A. Contribution of capsular polysaccharide and surface properties to virulence of Escherichia coli K1. Infect Immun. 1987 Nov;55(11):2662–2668. doi: 10.1128/iai.55.11.2662-2668.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bramanti T. E., Holt S. C. Iron-regulated outer membrane proteins in the periodontopathic bacterium, Bacteroides gingivalis. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1146–1154. doi: 10.1016/0006-291x(90)90986-w. [DOI] [PubMed] [Google Scholar]
- Dzink J. L., Tanner A. C., Haffajee A. D., Socransky S. S. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985 Sep;12(8):648–659. doi: 10.1111/j.1600-051x.1985.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Evenberg D., Lugtenberg B. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. II. Purification and characterization of a major cell envelope protein related to autoagglutination, adhesion and virulence. Biochim Biophys Acta. 1982 Jan 22;684(2):249–254. doi: 10.1016/0005-2736(82)90013-x. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Umeda A., Takade A., Murata K., Amako K. Hexagonal surface layer of Campylobacter fetus isolated from humans. Infect Immun. 1989 Aug;57(8):2563–2565. doi: 10.1128/iai.57.8.2563-2565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J., Holt S. C. Growth studies of Wolinella recta, a gram-negative periodontopathogen. Oral Microbiol Immunol. 1987 Sep;2(3):105–111. doi: 10.1111/j.1399-302x.1987.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J., Weintraub S. T., Wong G. G., Holt S. C. Chemical and biological characterization of the lipopolysaccharide of the oral pathogen Wolinella recta ATCC 33238. Infect Immun. 1988 Aug;56(8):2028–2035. doi: 10.1128/iai.56.8.2028-2035.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Kay W. W., Ainsworth T., Chamberlain J. B., Austen R. A., Buckley J. T., Trust T. J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981 Oct;148(1):333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerosuo E., Haapasalo M., Lounatmaa K. Ultrastructural relationship of cell envelope layers in Wolinella recta. Scand J Dent Res. 1989 Feb;97(1):54–59. doi: 10.1111/j.1600-0722.1989.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Kinder S. A., Holt S. C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989 Nov;57(11):3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]