Abstract
Impulsivity is widely regarded as a multidimensional trait that encompasses two or more distinct patterns of behavior, and dopaminergic systems are implicated in the expression of impulsive behavior in both humans and animals. Impulsive choice, or the tendency to choose rewards associated with relatively little or no delay, has been extensively studied in humans and animals using delay discounting tasks. Here, delay discounting procedures were used to assess the effects of receptor-selective dopaminergic agonists, antagonists, and dopamine transporter ligands on choices of immediate versus delayed sucrose pellets. The effects of d-amphetamine, GBR 12909, apomorphine, SKF 81297, sumanirole, pramipexole, ABT-724, SCH 23390, L-741,626, PG01037, and L-745,870 were assessed in 24 Sprague Dawley rats. The only drugs to affect impulsive choice selectively without altering undelayed choice were the D1-like antagonist SCH 23390 (0.01 mg/kg) and the D4 partial agonist ABT-724 (3.2 mg/kg), which both increased impulsive choice. The shared effects of these compounds may be explained by their localization within the prefrontal cortex on different groups of neurons. None of the selective agonists and antagonists tested reduced impulsive choice, so further research is needed to determine if direct dopaminergic agonists or antagonists may be therapeutically useful in the treatment of impulse-control disorders.
Keywords: Delay discounting, inter-temporal choice, impulsive choice, impulsivity, self control, dopamine, SCH 23390, ABT-724, rat
Impulsivity and self control are constructs used to describe what is increasingly apparent to be more than one class of behaviors. Based on operant and neurobiological experiments in humans and animals, a growing consensus largely agrees on two types of impulsive behavior: impulsive choice or inter-temporal choice and what is termed impulsive action or behavioral disinhibition (Dalley et al., 2008; de Wit, 2009; Evenden, 1999; Perry & Carroll, 2008; Winstanley et al., 2006). Impulsive choice is the tendency to choose rewards associated with little or no delay to their delivery, while impulsive action refers to the inability to withhold or inhibit a prepotent response. In addition to these two, a third component of impulsivity has been proposed by some. Impulsive preparation or reflection impulsivity, acting before gathering and processing all necessary information, has been argued to encompass impulsive-like responding on a variety of cognitive tasks used in humans and a rodent task called the uncertain visual discrimination task (Evenden, 1999).
Impulsive choice is typically modeled using delay discounting procedures that provide choice opportunities between a smaller amount of a reinforcer delivered after little or no delay and large amount of the same reinforcer delivered after a longer delay (Ainslie, 1975). Impulsive choice on these procedures is defined as the tendency to choose the smaller reinforce over the larger, more delayed reinforcer, while self-control is defined as the tendency choose the larger, delayed reinforcer. Variants of this task are used in both humans and animals, and in humans extensive evidence links delay discounting to impulse-control disorders such as attention deficit hyperactivity disorder (ADHD) (Schweitzer & Sulzer-Azaroff, 1995; Solanto et al., 2001; Sonuga-Barke et al., 1992, 1996) and substance abuse (Audrain-McGovern, 2009; Baker et al., 2003; Bickel et al., 1999; Bobova et al., 2009; Coffey et al., 2003; Dom et al., 2006; Heyman & Gibbs, 2006; Johnson et al., 2007; Jones et al., 2009; Kirby & Petry, 2004; Kirby et al., 1999; Madden et al., 1997, 1999; Mitchel, 1999; Monterosso et al., 2007; Odum et al., 2000, 2002; Petry, 2001; Petry & Casarella, 1999; Reynolds, 2006; Reynolds et al., 2007; Vuchinich & Simpson, 1998). Animal research on impulsive choice could provide valuable insight into these and other impulse-control disorders. For example, altering impulsive choice as expressed on a delay discounting task with drug administrations could provide preclinical pharmacological evidence for possible treatment medications for impulse-control disorders in humans.
As amphetamine and methylphenidate are the two most common pharmaceutical treatments for ADHD, it is not surprising that these have been extensively studied in experiments with rodents behaving on delay discounting tasks. Systemic methylphenidate treatment typically reduces impulsive choice (i.e., animals choose the larger reinforcer at longer delays) (e.g., Perry et al., 2008; Pitts & Febbo, 2004; Pitts & McKinney, 2005; van Gaalen et al., 2006), while treatment with d-amphetamine shows mixed results. In intact animals, d-amphetamine has been shown to reduce impulsive choice (Floresco et al., 2008; van den Bergh et al., 2006; van Gaalen et al., 2006; Wade et al., 2000; Winstanley et al., 2005), increase impulsive choice (Evenden & Ryan, 1996; Helms et al., 2006), or have no significant effect (Stanis et al., 2008; Uslaner & Robinson, 2006). Others have explored these discrepancies further, noting that the effects of amphetamine may depend on whether there is a stimulus present during the delay to the larger reinforcer (Cardinal et al., 2000), environmental enrichment (Perry et al., 2008), or baseline level of delay discounting (Barbelivien et al., 2008).
Compounds selective for a subset of the five dopamine receptors have been administered to animals responding on delay discounting tasks, but sometimes with mixed results. The nonselective dopamine antagonist flupenthixol has been shown to increase impulsive choice (Floresco et al., 2008; Wade et al., 2000). This effect may be due to D1-like antagonism or D2-like antagonism, as some reports show that the D1-like antagonist SCH 23390 increases impulsive choice while the D2-like antagonists haloperidol and eticlopride have no effect (Evenden & Ryan, 1996; van Gaalen et al., 2006), while another found an increase in impulsive choice with the D2-like antagonist raclopride and no effect with SCH 23390 (Wade et al., 2000). To the authors’ knowledge, the only direct dopamine agonist examined for effects on impulsive choice is the D3-preferring agonist 7-OH-DPAT, which increased impulsive choice (van den Bergh et al., 2006). In humans, the direct D3-preferring agonist pramipexole is known to increase impulsive choice in individuals with Parkinson’s disease, but not in healthy controls (Hamidovic et al., 2008; Voon et al., 2010)
As the effects of systemic injections of selective dopamine receptor agonists and antagonists are largely unknown, we administered the most selective dopamine receptor agonists and antagonists readily available to male Sprague Dawley rats responding on a slight variation of the delay discounting task described by Evenden and Ryan (1996). The drugs administered are listed in Table 1, along with dose ranges and pretreatment durations for each.
Table 1.
Compounds assessed, including the mechanism of action and selectivity profile of each. Selectivity refers to the difference in affinity between the first receptor or transporter listed and the second.
| Compound | Mechanism | Dose Range (mg/kg) | Pretreatment Duration |
|---|---|---|---|
| d-Amphetamine | DAT/NET blockade | 0.032 – 1.0 | 5 min |
| GBR 12909 | DAT blockade | 1.0 – 10 | 5 min |
| Apomorphine | D1/D2/D3/D4/D5 agonist | 0.032 – 0.32 | 5 min |
| SKF 81297 | D1-like agonist | 0.1 – 1.0 | 5 min |
| SCH 23390 | D1-like antagonist | 0.001 – 0.032 | 5 min |
| Pramipexole | D3-preferring agonist | 0.032 – 0.32 | 5 min |
| Sumanirole | D2-preferring agonist | 1.0 – 3.2 | 5 min |
| ABT-724 | D4 partial agonist | 1.0 – 3.2 | 5 min |
| Haloperdol | D2-like antagonist | 0.01 – 0.1 | 30 min |
| L-741,626 | D2-preferring antagonist | 0.32 – 3.2 | 30 min |
| PG01037 | D3-preferring antagonist | 10 – 56 | 30 min |
| L-745,870 | D4 antagonist | 0.32 – 3.2 | 30 min |
DAT: dopamine transporter, NET: norepinephrine transporter
Method
Subjects
Twenty-four male Sprague Dawley rats served as subjects (Harlan, Indianapolis, IN). Rats were approximately 10 weeks old at the start of the experiment. A food restriction protocol was in place to maintain the rats at approximately 325 g throughout the experiment. This weight was chosen as it is approximately 85% of the mean adult weight supplied by the manufacturer for this strain, and this weight was not changed once established. When not in session, rats were housed in accordance with institutional animal care and use guidelines in polycarbonate cages with fresh water continuously available. The lights in the housing colony were on from 07:00 to 19:00 h, and sessions were conducted between 09:00 and 15:00 h. These protocols were approved by the University of Michigan Committee on the Use and Care of Animals and conformed to the guidelines established by the NIH Guide for the Use of Laboratory Animals.
Apparatus
Sessions were conducted in rodent operant conditioning chambers with an area of 30.5 cm x 24.1 cm x 21.0 cm and stainless steel grid floors (ENV-008; Med Associates Inc., St. Albans, VT). Both sides of the front panel of the chamber held a retractable lever (E23-17, Coulbourn Instruments, Whitehall, PA). Between the levers was a food tray connected to a 45 mg pellet dispenser (ENV-200R1AM and ENV-203M-45, Med Associates, Inc.). Above both of the levers and the food tray were triple stimulus lights containing a red, green, and yellow LED (ENV-222M, Med Associates, Inc.). A houselight was located near the top of the opposite wall to provide illumination to the chamber (ENV-215M, Med Associates, Inc.). Chambers were connected to a computer running Med-PC IV software (Med Associates, Inc.) to control experimental events and record data.
Procedure
Rats were trained to respond on a conjoint fixed-time 60 s fixed ratio (FR) 1 schedule of reinforcement, with the active lever alternating each session between the left and right levers. This schedule arranged one sucrose pellet to be delivered every 60 s independent of behavior, with every lever press also producing a pellet. This was continued for four sessions, at which point the schedule was switched to a FR 1 with no response-independent pellet deliveries. Rats were allowed to respond on this schedule until 80 responses or more were recorded on two consecutive 20-min sessions.
The sessions were then extended to 75 min and split into five components of ten discrete-choice trials each. Total trial duration was 90 s and began with one or both levers extending into the chamber and illumination of the triple-stimulus lights above the lever(s). If a single response was made within 20 s, the levers retracted, the lights were extinguished, and the consequence programmed for that lever was delivered. If no response was made within 20 s, that trial was recorded as an omission and the levers retracted for the remaining 70 s of that trial. The first two trials of each component were always forced-choice trials where only one lever was extended into the chamber, forcing the subject to sample the contingencies for that component. These forced-choice trials remained in place throughout the experiment, and the contingencies for each set matched those of the free-choice trials in the same component. The remaining eight trials were free-choice trials where both levers were extended into the chamber, allowing the rat to respond on either. The three stimulus lights above each lever were lit whenever that lever was inserted in the chamber, and the stimulus lights above the pellet tray were lit during sucrose pellet deliveries. Initially, the consequences for both levers were immediate deliveries of either one or three 45-mg sucrose pellets, with the side associated with each amount counterbalanced across subjects. This condition was continued until rats chose the three-pellet option on at least 85% of free-choice trials. The three-pellet and one-pellet levers were then switched twice, with each new lever assignments in place until rats responded on the three-pellet option on at least 85% of trials. This process took a mean of 21 sessions (SD = 8.7 sessions). When this training regimen was completed, delays were introduced between responses made on the three-pellet lever and the delivery of the three pellets. During these delays, both levers were retracted and the stimulus lights above the levers were off. The delays to the three-pellet option were 0, 10, 20, 40, or 60 s and were always presented in ascending order with one delay in effect in each of the five 10-trial components.
Drug testing began after there was an effect of delay on choices (i.e., choice of the three-pellet option was less in the 60-s condition than in the 0-s condition), and no increasing or decreasing trend in choices was apparent over a period of five sessions. These criteria approximate those proposed by Johnson & Bickel (2008), which do not presuppose anything about discounting data, other than the idea that delayed rewards should be less reinforcing than immediate rewards. (Other patterns of behavior may suggest that behavior is being controlled by an aspect of the environment other than the delay to the reinforcer.) Sessions were generally conducted five days per week with vehicle injections administered on the first and fourth days of the week, drugs administered on the second and fifth days, and no injections given on the third day, but there was always a minimum of two days between drug administrations. Vehicle injections always corresponded to the vehicle for the scheduled drug injection or injections for the following day in number, substance, and time relative to the experimental session. Each session was preceded by a vehicle or drug injection 5 min before the start of the session, with the rat then immediately placed in the darkened experimental chamber. On some days, an antagonist or vehicle injection was administered 30 min prior to the session, with the rat placed back in his home cage for the intervening 25 min before the agonist or vehicle injection was given, as appropriate. All agonists and the corresponding vehicle injections were administered 5 min before the session. All antagonists and the corresponding vehicle injections were administered 30 min before the session start, except SCH 23390 which was administered 5 min before session start due to its relatively rapid onset and short duration of action (Hietala et al., 1992). All subjects did not receive all drugs, but each subject did receive all doses for those drugs that were administered. Each drug was tested in 12 subjects except SKF 81207, which was administered to 13 subjects due to experimenter error. The drugs were generally administered in the following order with only slight and infrequent exceptions: pramipexole, sumanirole, d-amphetamine, haloperidol, ABT-724, PG01037, L-741,626, SKF 81297, SCH 23390, SKF 81297 and SCH 23390 combinations, apomorphine, GBR 12909, and L-745,870. Subjects received an average of 6.5 drug conditions each (range 4–13).
Drugs
Drugs and dose ranges are listed in Table 1. Pramipexole was generously provided by Drs. Jianyong Chen and Shaomeng Wang (University of Michigan, Ann Arbor, MI), sumanirole by Benjamin Greedy and Dr. Stephen Husbands (University of Bath, Bath, UK), GBR 12909 by Novo Industri (Bagsvaerd, Denmark), ABT-724 by Dr. Kenner Rice (Chemical Biology Research Branch, National Institute on Drug Abuse, Bethesda, MD), and PG01037 was synthesized according to the procedure in the literature (Grundt et al., 2005) by Dr. Peter Grundt (University of Minnesota – Duluth, Duluth, MN) and Ms. J Cao (Medicinal Chemistry Section – National Institute on Drug Abuse, Baltimore, MD). Haloperidol, SKF 81297, SCH 23390, and apomorphine were obtained from Sigma-Aldrich (St. Louis, MO), L-741,626 and L-745,870 were obtained from Tocris (Ellisville, MO), and d-amphetamine was obtained from the National Institute on Drug Abuse (Bethesda, MD). All drugs were dissolved in sterile saline except L-741,626, which was dissolved in 5% ethanol, and PG01037, which was dissolved in 20% β-cyclodextrin. All injections were administered s.c. in a volume of 1.0 ml/kg except 56 mg/kg PG01037 which was administered in of volume of 1.75 ml/kg due to solubility limits.
Data Analysis
If a subject responded within the limited hold period on at least four of the eight free-choice trials of any component, those data were included in data analyses. Percent choice of the three-pellet lever was compared across delays to the three-pellet option and drug dose with a two-way repeated measures analysis of variance (ANOVA), using Systat SigmaStat 3.5 (San Jose, CA). When data were excluded in some components for some subjects due to the stated inclusion criterion, SigmaStat used a Mixed Models ANOVA to assess within- and between-subjects effects on the incomplete data set. Response latency was measured from the insertion of the response lever or levers into the chamber to a response on either lever within the limited hold period. Latencies were compared across trial type (free- or forced-choice) and drug dose with a two-way repeated measures ANOVA with GraphPad Prism 5 (La Jolla, CA). If a subject did not respond on either lever during the limited hold period, that trial was recorded as an omission. Omitted free-choice trials were compared across drug doses with a one-way repeated measures ANOVA with Prism 5. Results of statistical tests that were not significant at α = .05 are not reported.
Results
A two-way ANOVA was conducted to assess the main effects of delay to three pellets, drug dose, and the interaction of the two for each drug tested. All ANOVAs revealed a highly significant main effect of delay on choices (F range = 23 to 45, all p < 0.001), indicating that choice of the 3 pellets decreased as the delay to this option increased. Individual F values will not be reported for brevity.
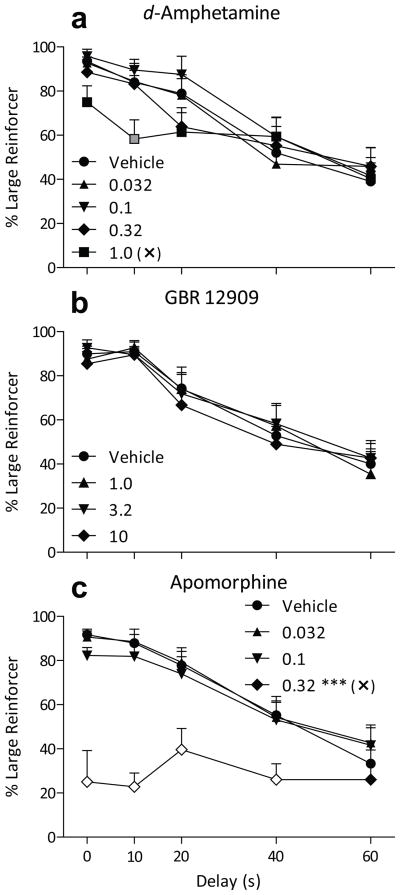
Acute pretreatments of d-amphetamine tended to decrease choice of the three-pellet option, but only at shorter delays to the three pellets (Fig. 1a; dose by delay interaction F16,176 = 2.3, p < 0.005). Bonferroni-adjusted post hoc tests revealed a significant reduction in choices of three pellets after 1.0 mg/kg d-amphetamine when the delay was 10 s (p < 0.01). Response latency (Table 2) was not significantly different between forced- and free-choice trials and was not affected by pretreatments of d-amphetamine up to doses of 1.0 mg/kg. d-Amphetamine also did not increase trials omitted (Table 2).
Figure 1.
Percent choice of the three-pellet lever (+ SEM) when that option was delayed from 0 to 60 s as a function of dose of d-amphetamine (a), GBR 12909 (b), or apomorphine (c) pretreatment. Each symbol shape represents a pretreatment dose, and the symbol fill color represents statistical significance of a Bonferroni-adjusted post hoc test comparing that point to the corresponding Vehicle point at the same delay (black = n.s.; gray = p < .05; white = p < .001). Asterisks appearing near a dose in the legend represent a significant difference from the Vehicle condition, independent of delay (* p < .05; ** p < .01; *** p < .001). Selective effects on behavior corresponding to an increase (↑) in impulsive choice, or a disruption in behavior (✕), are also indicated in the legend.
Table 2.
Mean (± SEM) omissions and response latency after administration of d-amphetamine, GBR 12909, apomorphine, SKF 81297, SCH 23390, and the combination of SKF 81297 and SCH 23390.
| Drug | Dose (mg/kg) | Omitted Trials | Response Latency (s) | |
|---|---|---|---|---|
| Forced Choice | Free Choice | |||
| d-Amphetamine | Vehicle | 0.02 (±0.02) | 1.45 (±0.20) | 1.14 (±0.29) |
| 0.032 | 0 (±0) | 1.64 (±0.30) | 1.27 (±0.38) | |
| 0.1 | 0 (±0) | 1.15 (±0.21) | 1.12 (±0.28) | |
| 0.32 | 0.17 (±0.17) | 1.41 (±0.22) | 1.02 (±0.25) | |
| 1.0 | 0.58 (±0.58) | 1.63 (±0.32) | 1.32 (±0.32) | |
| GBR 12909 | Vehicle | 0.11 (±0.08) | 1.59 (±0.23) | 1.27 (±0.34) |
| 1.0 | 0 (±0) | 1.45 (±0.33) | 1.17 (±0.32) | |
| 3.2 | 0.08 (±0.08) | 1.50 (±0.30) | 1.17 (±0.28) | |
| 10 | 0 (±0) | 1.19 (±0.22) | 1.13 (±0.33) | |
| Apomorphine | Vehicle | 0.08 (±0.04) | 1.44 (±0.33) | 1.03 (±0.30) |
| 0.032 | 0 (±0) | 1.50 (±0.34) | 1.19 (±0.42) | |
| 0.1 | 0.08 (±0.08) | 1.31 (±0.21) | 1.10 (±0.36) | |
| 0.32 | 6.5 (±2.0) *** | 2.83 (±0.47) *** | 1.77 (±0.38) ** | |
| SKF 81297 | Vehicle | 0.05 (±0.05) | 1.49 (±0.18) | 0.97 (±0.15) |
| 0.1 | 0.08 (±0.08) | 1.54 (±0.22) | 1.00 (±0.19) | |
| 0.32 | 0.08 (±0.08) | 1.47 (±0.15) | 0.90 (±0.13) | |
| 1.0 | 6.3 (±3.1) * | 1.93 (±0.30) | 1.77 (±0.32) ** | |
| SCH 23390 | Vehicle | 0.10 (±0.10) | 1.40 (±0.18) | 1.00 (±0.15) |
| 0.001 | 0.08 (±0.08) | 1.15 (±0.14) | 0.88 (±0.13) | |
| 0.0032 | 0.08 (±0.08) | 1.42 (±0.21) | 0.92 (±0.12) | |
| 0.01 | 1.6 (±1.0) | 1.64 (±0.27) | 1.24 (±0.15) | |
| 0.032 | 20.8 (±4.0) *** | 3.06 (±0.67) *** | 2.81 (±0.71) *** | |
| 0.01 SCH 23390 + SKF 81297 | Vehicle | 0.02 (±0.02) | 1.39 (±0.18) | 0.99 (±0.16) |
| 0.01 SCH | 1.6 (±1.0) | 1.64 (±0.27) | 1.24 (±0.15) | |
| + 0.1 SKF | 6.6 (±3.2) | 1.96 (±0.34) | 1.62 (±0.24) | |
| + 0.32 SKF | 3.1 (±3.0) | 1.93 (±0.22) | 1.13 (±0.13) | |
| + 1.0 SKF | 5.0 (±2.3) | 2.65 (±0.43) †† | 1.96 (±0.26) † | |
SCH = SCH 23390. SKF = SKF 81297.
p < .05,
p < .01,
p < .001 compared to vehicle in Bonferroni-adjusted post hoc tests.
p < .05,
p < .01 compared to agonist alone in Bonferroni-adjusted post hoc tests.
Pretreatments of the dopamine transporter blocker GBR 12909 up to 10 mg/kg did not significantly alter choices (Fig. 1b). Response latency and omissions (Table 2) were also not altered.
The nonselective dopamine agonist apomorphine had little effect on impulsive choice until a dose of 0.32 mg/kg, at which a sizeable decrease in choice of the large reinforcer was observed (Fig. 1c; p < 0.001, dose main effect F3,128 = 43, p < 0.001, dose by delay interaction F12,128 = 6.1, p < 0.001). That dose of 0.32 mg/kg apomorphine decreased large-reinforcer choice to below 50%, such that a majority of responses were allocated to the small-reinforcer option at all delays. This decrease was significantly different from vehicle choice data at delays ranging from 0 to 40 s (all p < 0.001). This pattern of choice was accompanied by an increase in response latency in both the forced- and free-choice trials (Table 2; both p < 0.01, dose main effect F3,66 = 22, p < 0.001, dose by trial type interaction F3,66 = 2.9, p < 0.05) and an increase in omissions (Table 2, F3,33 = 10, p < 0.001).
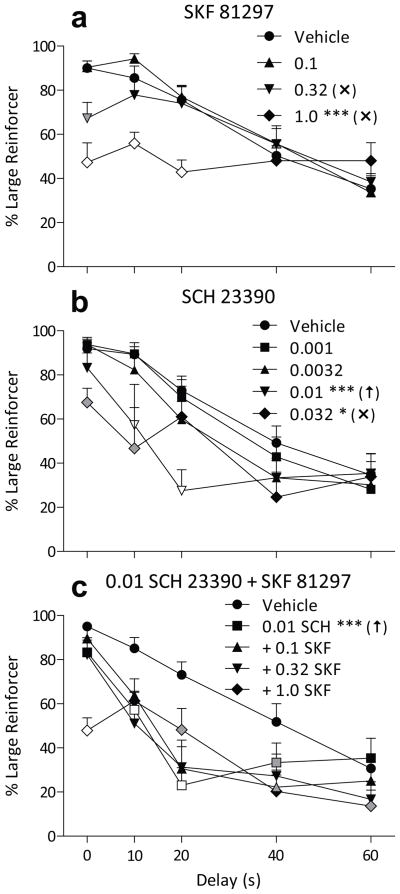
The D1-like agonist SKF 81297 dose-dependently decreased large-reinforcer choice, but this effect was limited to the shorter delays (Fig. 2a). This tendency resulted in a significant main effect of SKF 81297 dose (F3,138 = 11, p < 0.001) and a significant dose by delay interaction (F12,138 = 7.6, p < 0.001). A dose of 0.32 mg/kg SKF 81297 decreased large-reinforcer choice only at the 0 s delay condition (p < .002), while choice after 1.0 mg/kg hovered around 50% at all delays, significantly decreasing choice from 0 to 20 s (all p < 0.001). Omissions (Table 2, F3,36 = 4.1, p < .02) and response latency (dose main effect F3,72 = 6.1, p < 0.001, trial type main effect F1,72 = 4.5, p < .05) were slightly increased at 1.0 mg/kg, this effect being most notable during free-choice trials (p < 0.01).
Figure 2.
Percent choice of the three-pellet lever (+ SEM) when that option was delayed from 0 to 60 s as a function of dose of SKF 81297 (a) or SCH 23390 (b), or 0.01 mg/kg SCH 23390 administered with varying doses of SKF 81297 (c). For the combinations of SCH 23390 and SKF 81297, the symbol fill color represents statistical comparison to the corresponding SCH 23390 alone point at the same delay. All other details are as in Figure 1.
Administration of the D1-like antagonist SCH 23390 produced a selective increase in impulsive choice, with 0.01 mg/kg decreasing choice at moderate delays without affecting choice in the 0 s delay condition (Fig. 2b). A significant main effect of dose was observed (F4,152 = 6.7, p < 0.001), with both 0.01 (p < 0.001) and 0.032 (p < 0.05) SCH 23390 decreasing large-reinforcer choice. A significant dose by delay interaction was also noted (F16,152 = 3.1, p < 0.001). The effects of 0.01 mg/kg were selective to the 10 and 20 s delays (both p < 0.001), while 0.032 mg/kg resulted in more indifferent choice and a significant reduction in large-reinforcer choice at the 0 and 10 s delays (both p < 0.05). This move toward indifference at 0.032 mg/kg SCH 23390 was accompanied by a large increase in trials omitted (Table 2, F4,44 = 26, p < 0.001) and a large increase in both forced- and free choice latency (both p < 0.001, dose main effect F4,88 = 13, p < 0.001).
A range of doses of the D1-like agonist SKF 81297 were co-administered with 0.01 mg/kg of the D1-like antagonist SCH 23390 to determine if the effects seen with SCH 23390 were reversible by a D1-like agonist. Little systematic reversal was found with doses of SKF 81297 up to 1.0 mg/kg (Fig. 2c). There was a significant main effect of dose on large-reinforcer choice (F4,156 = 23, p < 0.001), with 0.01 mg/kg SCH 23390 alone decreasing choice (p < 0.001). No dose of SKF 81297 significantly reversed this effect, although there were some effects of SKF 81297 dose that depended on delay (F16,156 = 5.1, p < 0.001). A dose of 0.01 mg/kg SCH 23390 alone decreased large-reinforcer choice relative to vehicle at delays ranging from 10 s to 40 s (all p < 0.05). When co-administered with SCH 23390, compared to the effects of 0.01 mg/kg SCH 23390 alone, 0.1 mg/kg SKF 81297 further decreased large-reinforcer choice at a 40 s delay (p < 0.05) and 1.0 mg/kg SKF 81297 increased large-reinforcer choice at a 20 s delay (p < 0.05), but decreased it at a 0 s (p < 0.001) and 60 s (p < 0.05) delay. Adding 1.0 mg/kg SKF 81297 to 0.01 mg/kg SCH 23390 increased the response latency over that observed with 0.01 mg/kg SCH 23390 alone in both the forced- and free-choice trials (Table 2; both p < 0.05, dose main effect F4,88 = 7.8, p < 0.001, trial type main effect F1,88 = 5.0, p < 0.05). No significant effect on trials omitted was observed across these dosing conditions (Table 2).
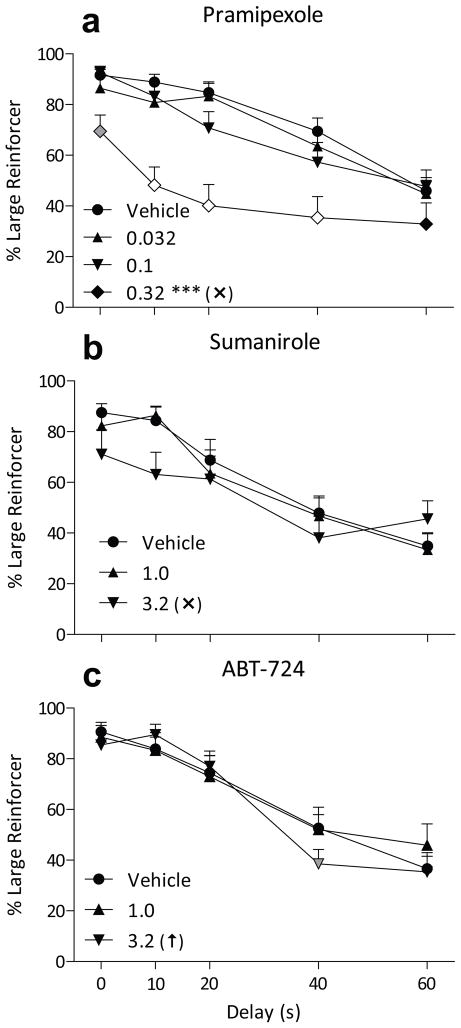
A dose of 0.32 mg/kg the D3-preferring agonist pramipexole decreased large-reinforcer choice across a range of delays, leading to a significant effect of pramipexole dose (F3,129 = 21, p < 0.001) and a significant dose by delay interaction (F12,129 = 1.9, p < 0.05; Fig. 3a). A dose of 0.32 mg/kg pramipexole reduced large-reinforcer choice as a whole (p < 0.001), and specifically at delays from 0 to 40 (all p < 0.01). Response latency (Table 3; dose main effect F3,66 = 4.7, p < 0.005) and omissions (F3,33 = 3.4, p < 0.05) were also increased at 0.32 mg/kg pramipexole, with response latency most increased during forced-choice trials at 0.32 mg/kg (p < 0.05).
Figure 3.
Percent choice of the three-pellet lever (+ SEM) when that option was delayed from 0 to 60 s as a function of pretreatment dose of pramipexole (a), sumanirole (b), or ABT-724 (c). All other details are as in Figure 1.
Table 3.
Mean (± SEM) omissions and response latency after administration of pramipexole, sumanirole, ABT-724, haloperidol, L-741,626, PG01037, and L-745,870.
| Drug | Dose (mg/kg) | Omissions | Response Latency | |
|---|---|---|---|---|
| Forced Choice | Free Choice | |||
| Pramipexole | Vehicle | 0.03 (±0.03) | 1.16 (±0.28) | 1.01 (±0.39) |
| 0.032 | 1.8 (±1.6) | 1.21 (±0.28) | 0.83 (±0.29) | |
| 0.1 | 0.25 (±0.18) | 1.19 (±0.17) | 0.97 (±0.36) | |
| 0.32 | 4.5 (±1.8) * | 1.90 (±0.42) * | 1.43 (±0.28) | |
| Sumanirole | Vehicle | 0.08 (±0.08) | 1.52 (±0.27) | 1.09 (±0.31) |
| 1.0 | 0.25 (±0.18) | 1.26 (±0.19) | 1.07 (±0.22) | |
| 3.2 | 8.3 (±3.4) * | 1.86 (±0.35) | 1.82 (±0.34) * | |
| ABT-724 | Vehicle | 0.08 (±0.08) | 1.39 (±0.27) | 0.96 (±0.26) |
| 1.0 | 0.08 (±0.08) | 1.21 (±0.22) | 0.99 (±0.27) | |
| 3.2 | 0 (±0) | 1.34 (±0.24) | 0.86 (±0.23) | |
| Haloperidol | Vehicle | 0.11 (±0.11) | 1.14 (±0.12) | 0.81 (±0.18) |
| 0.01 | 0 (±0) | 1.21 (±0.29) | 0.84 (±0.26) | |
| 0.032 | 0.08 (±0.08) | 1.20 (±0.12) | 0.89 (±0.23) | |
| 0.1 | 5.1 (±3.6) | 1.87 (±0.32) * | 1.93 (±0.63) ** | |
| L-741,626 | Vehicle | 0 (±0) | 1.32 (±0.18) | 1.30 (±0.39) |
| 0.32 | 0 (±0) | 1.39 (±0.19) | 1.36 (±0.45) | |
| 1.0 | 0.25 (±0.18) | 1.81 (±0.35) | 1.24 (±0.33) | |
| 3.2 | 2.2 (±1.7) | 2.71 (±0.29) *** | 2.19 (±0.28) * | |
| PG01037 | Vehicle | 0.17 (±0.09) | 1.21 (±0.15) | 0.84 (±0.23) |
| 10 | 0 (±0) | 1.16 (±0.17) | 0.90 (±0.34) | |
| 32 | 0.17 (±0.17) | 1.14 (±0.12) | 0.99 (±0.31) | |
| 56 | 0.08 (±0.08) | 1.46 (±0.27) | 1.08 (±0.21) | |
| L-745,870 | Vehicle | 0.02 (±0.02) | 1.31 (±0.20) | 1.03 (±0.31) |
| 0.32 | 0 (±0) | 1.49 (±0.29) | 1.14 (±0.35) | |
| 1.0 | 0 (±0) | 1.42 (±0.24) | 1.04 (±0.31) | |
| 3.2 | 0 (±0) | 1.33 (±0.20) | 1.09 (±0.35) | |
p < .05,
p < .01,
p < .001 compared to vehicle in Bonferroni-adjusted post hoc tests.
Large-reinforcer choices were not significantly altered by the D2-preferring agonist sumanirole up to 3.2 mg/kg (Fig. 3b). Trials omitted (Table 3, F2,22 = 5.9, p < 0.01) and free-choice response latency (dose main effect F2,44 = 6.5, p < 0.005) were increased at 3.2 mg/kg (p < 0.05).
There was no significant main effect of the D4 partial agonist ABT-724 dose on large-reinforcer choice, but there was a significant dose by delay interaction (F8,88 = 2.1, p < 0.05, Fig. 3c). This was due to a small, but significant decrease in large-reinforcer choice after 3.2 mg/kg ABT-724 with a delay of 40 s (p < 0.025). Response latency and omissions (Table 3) were not altered by ABT-724 at the doses tested.
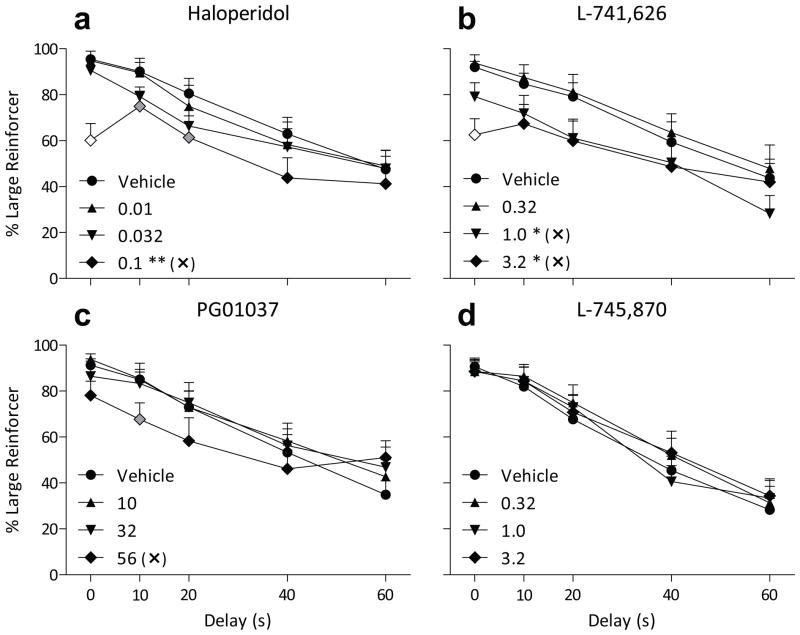
The D2-like antagonist haloperidol reduced large-reinforcer choice at a dose that also increased response latency (Fig. 4a, Table 3). There was a significant main effect of dose on choice (F3,126 = 5.8, p < 0.005), but no significant dose by delay interaction. A dose of 0.1 mg/kg haloperidol reduced large-reinforcer choice (p < 0.002), and this same dose increased response latency (Table 3; dose main effect F3,66 = 8.0, p < 0.001). This increase was observed during both forced- and free-choice trials (both p < 0.05). Omissions were not significantly increased at doses up to 0.1 mg/kg.
Figure 4.
Percent choice of the three-pellet lever (+ SEM) when that option was delayed from 0 to 60 s as a function of pretreatment dose of haloperidol (a), L-741,626 (b), PG01037 (c), or L-745, 870 (d). All other details are as in Figure 1.
The D2-preferring antagonist L-741,626 dose-dependently decreased large-reinforcer choice (Fig. 4b; F3,130 = 6.5, p < 0.001) with no significant dose by delay interaction. L-741,626 decreased large-reinforcer choice across delays when administered at 1.0 or 3.2 mg/kg (both p < 0.05). Response latency was increased after administration of 3.2 mg/kg L-741,626 in both forced- (p < 0.001) and free-choice (p < 0.05) trials (Table 3; dose main effect F3,66 = 12, p < 0.001). Omissions were not increased at the doses tested (Table 3).
The D3-preferring antagonist PG01037 slightly decreased large-reinforcer choice at the highest dose tested (Fig. 4c). No significant main effect of dose was found, but there was a significant dose by delay interaction (F12,132 = 1.9, p < 0.05). The dose of 56 mg/kg PG01037 significantly reduced large-reinforcer choice at a delay of 10 s (p < 0.05). Response latency and omissions (Table 3) were not affected by PG01037 at the doses tested.
The D4 antagonist L-745,870 had little effect on behavior at the doses tested. Large-reinforcer choice was not altered (Fig. 4d), nor was response latency or omissions (Table 3).
Discussion
In general, doses of drugs that increased response latency or trials omitted also moved choice data toward indifference (50% large-reinforcer choice). A decrease or increase in choice that is independent of delay is not necessarily an increase or decrease, respectively, in impulsive choice. Changes in choice behavior that occur independent of delay may reflect a change in impulsive choice, but have also been conceptualized as an effect on sensitivity to the amount of the reinforcer or an inability to discriminate or adapt to the consequences of responding (Acheson et al., 2006; Pitts & Febbo, 2004). For these reasons, we viewed effects coinciding with changes in choices in the 0-s delay condition as somewhat ambiguous, and took a cautious approach to identifying selective, potentially clinically-relevant effects. Those effects that coincided with a decrease in large reinforcer choice at delay = 0s or that were accompanied by a significant increase in response latency were interpreted more cautiously and noted in the legend of each graph (✕). Doses of compounds that selectively increased impulsive choice by these standards are also indicated (↑). Two drugs did affect choice of the large reinforcer as a function of delay without altering response latency or undelayed choice. These drugs are SCH 23390 and ABT-724, and are discussed in more detail below.
The D1-like antagonist SCH 23390 selectively increased impulsive choice at 0.01 mg/kg (Fig. 2b). This effect has been reported previously at a similar dose (van Gaalen et al., 2006), but not on an adjusting-amount procedure over the same dose range (Wade et al., 2000). The D4 partial agonist ABT-724, which has not been previously assessed on a model of impulsive choice, also selectively increased choice of the smaller reward when the larger reward was delayed 40 s. The importance of dopaminergic systems in impulsive choice is supported by evidence from a variety of experimental approaches. Dopaminergic pathways from the basal ganglia to the prefrontal cortex have been identified as abnormal in people with ADHD, as well as involved in choices on the delay discounting task in animals (for recent reviews see Bickel et al., 2007; Winstanley et al., 2006). Functional magnetic resonance imaging (fMRI) scans of people choosing between delayed or immediate rewards show activation of prefrontal cortex (PFC) and the striatum, with delayed choices or choices where preference is near to indifference associated with more PFC activation (Ballard & Knutson, 2009; Hoffman et al., 2008; McClure et al., 2004; Shamosh et al., 2008). Lesion studies in animals support the involvement of these structures, with the nucleus accumbens (NAc) core and PFC involved in sensitivity to reinforcer size and delay (Acheson et al., 2006; Bezzina et al., 2007, 2008; Cardinal et al., 2001; Kheramin et al., 2002; Winstanley et al., 2004). Given that ADHD is associated with lower PFC dopamine activity (Ernst et al., 1998) and lower PFC activation during a task involving delayed reward (Rubia et al., 1999), and that methamphetamine abusers also show lower PFC activity during the delay discounting task (Monterosso et al., 2007), this neural pathway is a plausible target for treatment of impulse-control disorders.
Both D1-like (D1 and D5) and D2-like (D2, D3, and D4) dopamine receptors, as well as dopamine transporters, are known to exist in the dopaminergic pathways connecting the striatum to the PFC (Ciliax et al., 1995; Gaspar et al., 1995; Lévesque et al., 1992; Mrzljak et al., 1996; Muly III et al., 1998; Revay et al., 1996), and both D4 and D1-like receptors are located in the frontal cortex. D1-like receptors are located both on GABAergic interneurons (Muly III et al., 1998) and on pyramidal neurons with some projections back to the striatum, among other areas (Gaspar et al., 1995). D4 receptors are located primarily on GABAergic interneurons in the monkey cortex (Mrzljak et al., 1996), but have been located on both GABAergic interneurons and pyramidal neurons in the rat cortex (Wędzony et al., 2000). As GABA is an inhibitory neurotransmitter, D4 agonism and D1-like antagonism in the prefrontal cortex may functionally have the same effect depending on relative influence of binding sites on GABAergic and pyramidal sites. This complex organization of the prefrontal cortex, and the fact that the D4 antagonist L-745,870 and the D1-like agonist SKF 81297 did not have the opposite result to ABT-724 and SCH 23390 in the present study, may result from the hypothesized notion that moderate stimulation of D1-like receptors results in optimal cell firing (Muly III et al., 1998). While inconsistent drug effects with this task have been observed in the literature, it is somewhat encouraging that, of all the dopaminergic compounds tested in the current experiment, only those that act at receptor subtypes with significant prevalence in the prefrontal cortex selectively altered impulsive choice. Given the increasing body of evidence suggesting a critical role of the prefrontal cortex in impulsive choice, these results add to the construct validity of this task. Future experiments, perhaps incorporating microinfusions of compounds selective for D1-like and D4 receptros into the prefrontal cortex, could help illuminate the role of this brain area in this task.
D4 receptors are an intriguing target for ADHD medications. D4 polymorphisms in humans are associated with ADHD (Faraone et al., 2005), and D4 receptor distribution in the brain is relatively limited, but includes the prefrontal cortex (Van Tol et al., 1991). Methylphenidate has been shown to increase dopamine and norepinephrine in the prefrontal cortex to a greater extent and at lower doses than in the nucleus accumbens (dopamine) or medial septal area (norepinephrine) (Berridge et al., 2006). Added to the finding that D4 receptors have high affinity for both dopamine and norepinephrine (Wedemeyer et al., 2007) and dopaminergic and noradrenergic mechanisms are involved in the current commonly used ADHD treatments, the D4 receptor is an appealing target for ADHD treatment. The effect seen with ABT-724 in the present study was small in magnitude, but this could be due to the relatively low intrinsic efficacy of this compound (Brioni et al., 2004). The selective increase in impulsive choice was also in the opposite direction than would be clinically relevant. Further research is needed to determine if a D4 ligand could produce a reliable, therapeutically-relevant effect.
Both the agonists (pramipexole and sumanirole) and the antagonists (haloperidol, PG01037, and L-741,626) acting through D2 and/or D3 receptors had similar effects. As a whole, these drugs tended to decrease choice of the large reinforcer independent of delay. None had a selective effect on impulsive choice, without affecting choice of the large reinforcer at 0-s delay, although 0.32 mg/kg pramipexole did approach this pattern. The largest decreases in large-reinforcer choice were observed at the intermediate delays (10 to 40 s), while choice in the 0-s delay condition was less affected. If these effects are interpreted as in increase in impulsive choice, they confirm research in humans that found an increase in impulsive choice with acute pramipexole administration to Parkinson’s patients (Voon et al., 2010). Healthy volunteers do not show increases in impulsive choice after pramipexole administration (Hamidovic et al., 2008; Voon et al., 2010), suggesting that the intact rats employed in the present study should not necessarily show an analogous effect of pramipexole. In the brain, D2 and D3 receptors are found in large numbers in the nucleus accumbens, but are also found in prefrontal cortex (Bouthenet et al., 1991). The core of the nucleus accumbens has been shown to be involved in accurately assessing reinforcer value in delay discounting tasks (Acheson et al., 2006; Cardinal et al., 2001). It is unknown why stimulation and blockade of D2 or D3 receptors would have similar effects, however.
Apomorphine had a unique profile of effects on choice, with the first active dose producing a bias toward the lever producing the small reinforcer at all delays to the large reinforcer. Choice of the large reinforcer after administration of 0.32 mg/kg apomorphine was even below 50%, which would indicate indifference. Apomorphine has been shown to produce a robust anorectic effect at this dose (Willner et al., 1985). However, if apomorphine was causing the sucrose pellets to be unpalatable, it would seem that responding would cease rather than one pellet being preferred. Apomorphine has also been shown to induce perseverative responding that appears disconnected from response consequences (Robbins et al., 1983) or that is punished (Koffarnus & Woods, unpublished observation), which could potentially explain these data. Instances of persistent responding on the lever associated with the smaller, immediate reinforcer have been reported by others (e.g., Cardinal et al., 2001) after manipulations that may have interfered with the ability of subjects to adapt to rapidly changing contingencies (Acheson et al., 2006). The effect seen in the present study appeared to be a transient drug effect, however, as choice patterns quickly returned to typical baseline levels in subsequent drug-free sessions. Why the subjects tended to perseverate on the response option producing fewer reinforcers in this instance is unknown.
Neither d-amphetamine nor the selective dopamine transporter blocker GBR 12909 selectively increased or decreased impulsive choice at the doses tested. At 1.0 mg/kg, d-amphetamine decreased large-reinforcer choice at shorter delays. Previous research has found an increase, decrease, or lack of effect with d-amphetamine. The one study to previously test GBR 12909 found a decrease in impulsive choice, the same effect that was found with d-amphetamine in that report (van Gaalen et al., 2006). The absence of consistent effects with these drugs is curious, although environmental conditions are known to affect the effects of d-amphetamine on this task (Cardinal et al., 2000; Perry et al., 2008).
In conclusion, of the five dopamine receptors D1-like and D4 receptors appear to be most selectively involved in mediating impulsive choice. Both the D1-like antagonist SCH 23390 and the D4 partial agonist ABT-724 increased impulsive choice, which may be explained by their differing locations within the PFC, an area known to be involved in impulsive choice. None of the selective agonists and antagonists tested reduced impulsive choice, however, so further research is needed to determine if direct dopaminergic agonists or antagonists may be therapeutically useful in the treatment of impulse-control disorders.
Acknowledgments
The authors would like to thank Aaron Chadderdon, Alexa Cohen, Bruce Kaczmarek, Elizabeth Kossak, Jenny Montgomery, Collette Rothe, Sarah Snider, Nhu Truong, and J. Cao (Medicinal Chemistry Seection, NIDA-IRP) for technical assistance, and Gail Winger, Jeremiah Bertz, and Nhu Truong for advice and help with manuscript preparation. This research was supported by USPHS/NIDA grants R01 DA015449, R01 DA020669, and T32 DA007267, and a portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Acheson A, Ferrar AM, Patak M, Hausknecht KA, Kieres AK, Choi S, de Wit H, Richards JB. Nucleus accumbens lesions decrease sensitivity to rapid changes in the delay to reinforcement. Behavioural Brain Research. 2006;173:217–228. doi: 10.1016/j.bbr.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: Similarities and differences across commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbelivien A, Billy E, Lazarus C, Kelche C, Majchrzak M. Rats with different profiles of impulsive choice behavior exhibit differences in responses to caffeine and d-amphetamine and in medial prefrontal cortex 5-HT utilization. Behavioural Brain Research. 2008;187:273–283. doi: 10.1016/j.bbr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bezzina G, Cheung THC, Asgari K, Hampson CL, Body S, Bradshaw CM, et al. Effects of quinolinic acid-induced lesions of the nucleus accumbens core on inter-temporal choice: A quantitative analysis. Psychopharmacology. 2007;195:71–84. doi: 10.1007/s00213-007-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G, Body S, Cheung THC, Hampson CL, Bradshaw CM, Szabadi E, et al. Effect of disconnecting the orbital prefrontal cortex from the nucleus accumbens core on inter-temporal choice behaviour: A quantitative analysis. Behavioural Brain Research. 2008;191:272–279. doi: 10.1016/j.bbr.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence. 2007;90S:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: Personality and cognitive correlates. Experimental and Clinical Psychopharmacology. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: Comparison with dopamine D2 receptor mRNA. Brain Research. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Moreland RB, Cowart M, Hsieh GC, Stewart AO, Hedlund P, et al. Activation of dopamine D4 receptors by ABT-724 induces penile erection in rats. Proceedings of the National Academy of Sciences. 2004;101:6758–6763. doi: 10.1073/pnas.0308292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in the rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, et al. The dopamine transporter: immunochemical characterization and localization in brain. The Journal of Neuroscience. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacology, Biochemistry and Behavior. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, D’haene P, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: Differences in self-report measures and a discounting task. Addiction. 2006;101:50–59. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. The Journal of Neuroscience. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: A discussion of clinical and experimental findings. Journal of Psychopharmacology. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MTL, Chods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: Cellular localization in different classes of efferent neurons. The European Journal of Neuroscience. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Goewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, et al. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. Journal of Medicinal Chemistry. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. Journal of Clinical Psychopharmacology. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and d-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology. 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Hietala J, Seppäla T, Lappalainen J, Syvälahti E. Quantification of SCH, 39166, a novel selective D1 dopamine receptor antagonist, in rat brain and blood. Psychopharmacology. 1992;106:455–458. doi: 10.1007/BF02244814. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Gibb SP. Delay discounting in cigarette chippers. Behavioural Pharmacology. 2006;17:669–679. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Experimental and Clinical Psychopharmacology. 2008;16:264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Experimental and Clinical Psychology. 2007;15:187–194. doi: 10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- Jones BA, Landes RD, Yi R, Bickel WK. Temporal horizon: Modulation by smoking status and gender. Drug and Alcohol Dependence. 2009;104:S87–93. doi: 10.1016/j.drugalcdep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheramin S, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, et al. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: A quantitative analysis. Psychopharmacology. 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proceedings of the National Academy of Sciences. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, Jacobs EA. Discounting of delayed rewards in opioid-dependent outpatients: Exponential or hyperbolic discounting functions. Experimental and Clinical Psychopharmacology. 1999;7:284–293. doi: 10.1037//1064-1297.7.3.284. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Muly EC, III, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: Distribution and subcellular localization. The Journal of Neuroscience. 1998;18:10553–10565. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Madden GL, Badger GJ, Bickel WK. Needle sharing in opioid-dependent outpatients: Psychological processes underlying risk. Drug and Alcohol Dependence. 2000;60:259–266. doi: 10.1016/s0376-8716(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Odum AL, Madden GL, Bickel WK. Discounting of delayed health gains and losses by current, never- and ex-smokers of cigarettes. Nicotine & Tobacco Research. 2002;4:295–303. doi: 10.1080/14622200210141257. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behavioural Brain Research. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. Journal of Abnormal Psychology. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug and Alcohol Dependence. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Febbo SM. Quantitative analyses of methamphetamine’s effects on self-control choices: Implications for elucidating behavioral mechanisms of drug action. Behavioural Processes. 2004;66:213–233. doi: 10.1016/j.beproc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP. Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. Journal of the Experimental Analysis of Behavior. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revay R, Vaughan R, Grant S, Kuhar MJ. Dopamine transporter immunohistochemistry in median eminence, amygdala, and other areas of the rat brain. Synapse. 1996;22:93–99. doi: 10.1002/(SICI)1098-2396(199602)22:2<93::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Reynolds B. The Experiential Discounting Task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behavioural Pharmacology. 2006;17:133–142. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2007;15:264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Watson BA, Gaskin W, Ennis Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine, and other drugs with conditioned reinforcement. Psychopharmacology. 1983;80:113–119. doi: 10.1007/BF00427952. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. The American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Sulzer-Azaroff B. Self-control in boys with attention deficit hyperactivity disorder: Effects of added stimulation and time. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1995;36:671–686. doi: 10.1111/j.1469-7610.1995.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, et al. Individual differences in delay discounting: Relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: A supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion – I. The effect of delay on choice. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Williams E, Hall M, Saxton T. Hyperactivity and delay aversion. III: The effect on cognitive style of imposing delay after errors. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1996;37:189–194. doi: 10.1111/j.1469-7610.1996.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Avila HM, White MD, Gulley JM. Dissociation between long-lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay-discounting task. Psychopharmacology. 2008;199:539–548. doi: 10.1007/s00213-008-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature Neuroscience. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice – mediation by enhanced incentive motivation? European Journal of Neuroscience. 2006;24:2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- van den Bergh FS, Bloemarts E, Groenink L, Olivier B, Oosting RS. Delay aversion: Effects of 7-OH-DPAT, 5-HT1A/1B-receptor stimulation and d-cycloserine. Pharmacology Biochemistry & Behavior. 2006;85:736–743. doi: 10.1016/j.pbb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer ANM, Vanderschuren LJMJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biological Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Van Tol HHM, Bunzow JR, Guan H-C, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology. 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wedemeyer C, Goutman JD, Avale ME, Franchini LF, Rubinstein M, Calvo DJ. Functional activation by central monoamines of human dopamine D4 receptor polymorphic variants coupled to GIRK channels in Xenopus oocytes. European Journal of Pharmacology. 2007;562:165–173. doi: 10.1016/j.ejphar.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Wędzony K, Chocyk A, Maćkowiak M, Fijał K, Czyrak A. Cortical localization of dopamine D4 receptors in the rat brain – immunocytochemical study. Journal of Physiology and Pharmacology. 2000;51:205–221. [PubMed] [Google Scholar]
- Willner P, Towell A, Muscat R. Apomorphine anorexia: A behavioural and neuropharmacological analysis. Psychopharmacology. 1985;87:351–356. doi: 10.1007/BF00432720. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of Neuroscience. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: Therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology. 2007;15:176–186. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]