Abstract
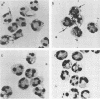
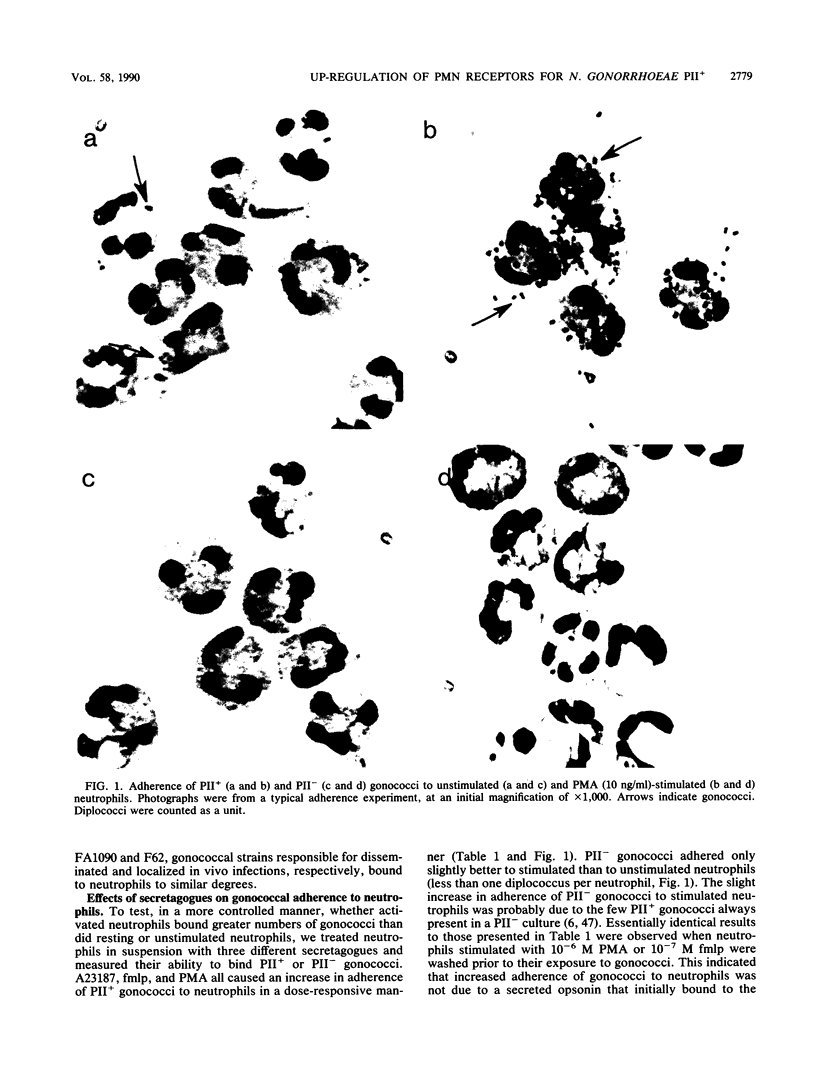
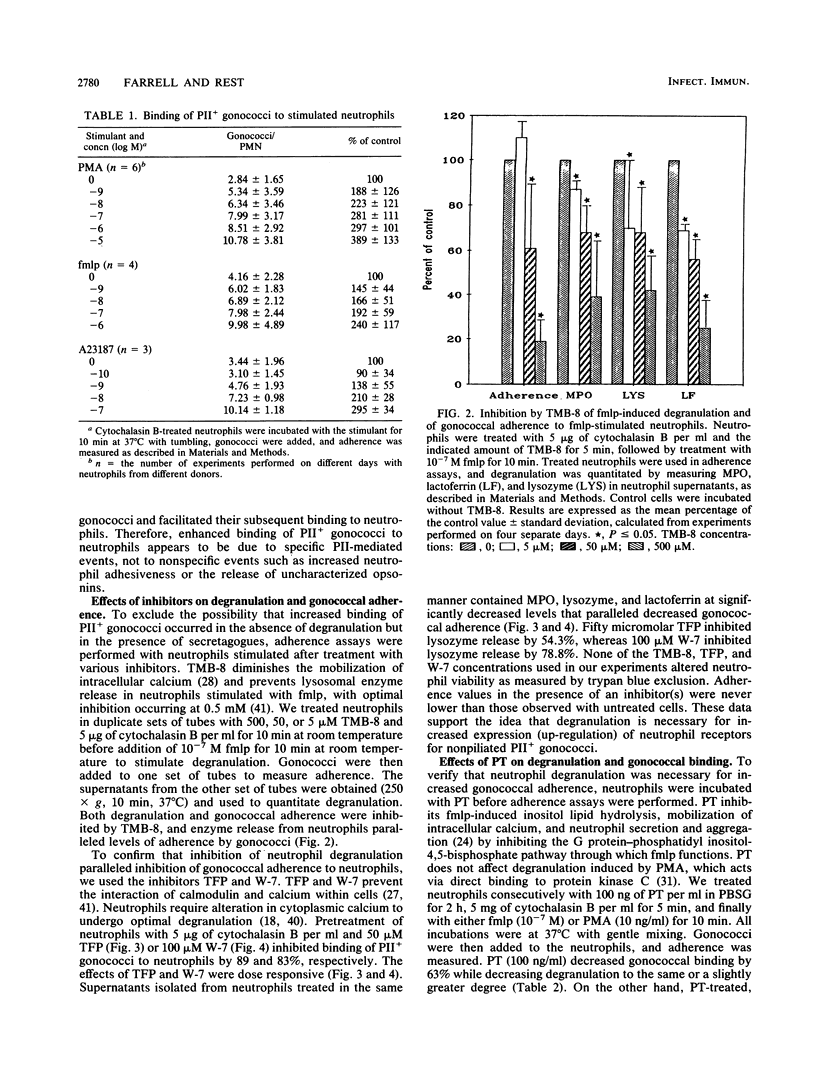
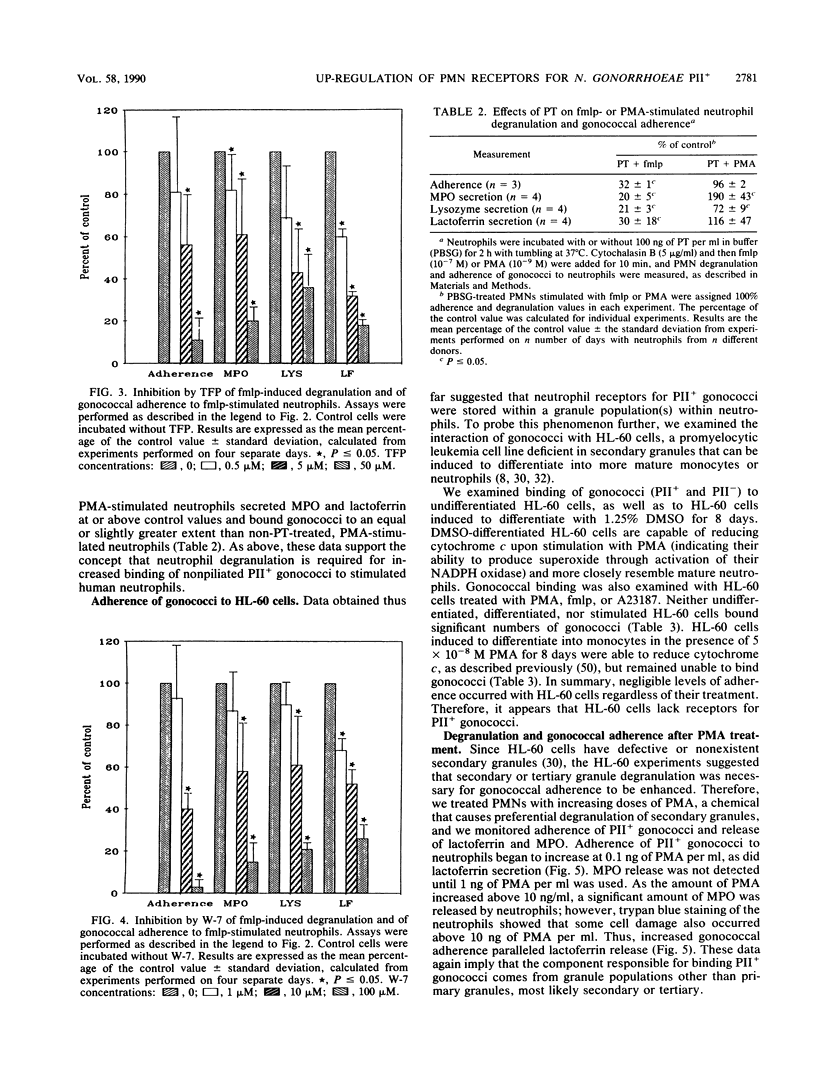
In the absence of serum, nonpiliated gonococci expressing PII outer membrane proteins (PIIs) adhere to human neutrophils whereas non-PII-expressing (PII-) gonococci do not. After an observation that neutrophils in monolayers bound more gonococci than neutrophils in suspension, we treated neutrophil suspensions with known stimulants of degranulation and measured subsequent gonococcal adherence to suspended neutrophils. The chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fmlp), the potent secretagogue phorbol myristate acetate, and the calcium ionophore A23187 all caused increased adherence of PII+ gonococci, but not PII- gonococci, to neutrophils in a dose-responsive manner. Increased adherence of gonococci to neutrophils was paralleled by increased degranulation of neutrophil myeloperoxidase, lysozyme, and lactoferrin. Inhibition of fmlp-induced neutrophil degranulation by pertussis toxin, the calmodulin inhibitors trifluoperazine and N-5-chloronaphthalene sulfonamide, or the intracellular calcium-binding agent trimethoxybenzoic acid also inhibited fmlp-induced gonococcal adherence to neutrophils. Neither undifferentiated nor myelocytically differentiated HL-60 cells, which possess primary but defective or nonexistent secondary granules, bound PII+ or PII- gonococci. Gonococci did not adhere to human monocytes, monocyte-derived macrophages, lymphocytes, platelets, or erythrocytes, indicating that several receptors, such as the complement receptors CR1, CR3 (CD11b/CD18), and CR4 (CD11c/CD18) or the adherence complex LFA-1 (CD11a/CD18), were probably not involved in gonococcal adherence to human neutrophils.
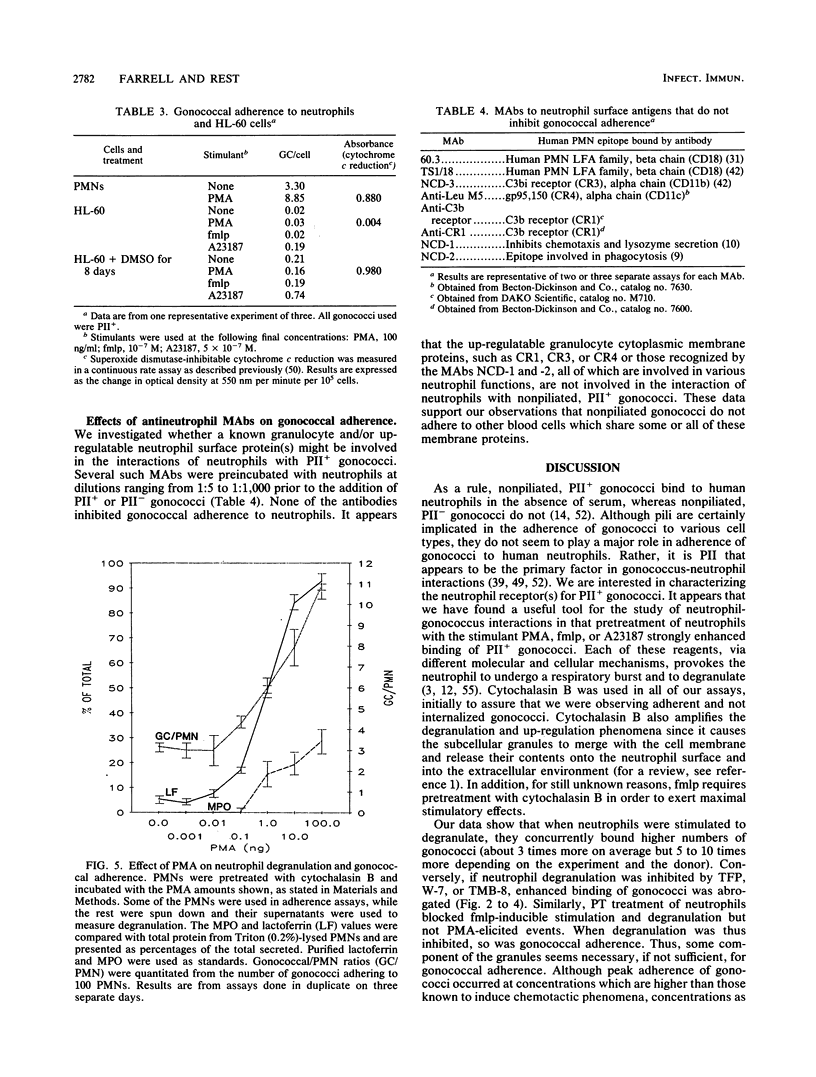
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Birgens H. S. Lactoferrin in plasma measured by an ELISA technique: evidence that plasma lactoferrin is an indicator of neutrophil turnover and bone marrow activity in acute leukaemia. Scand J Haematol. 1985 Apr;34(4):326–331. doi: 10.1111/j.1600-0609.1985.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Black W. J., Schwalbe R. S., Nachamkin I., Cannon J. G. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984 Aug;45(2):453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläckberg L., Hernell O. Isolation of lactoferrin from human whey by a single chromatographic step. FEBS Lett. 1980 Jan 14;109(2):180–183. doi: 10.1016/0014-5793(80)81081-7. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter T. G., Keeling P. J., Henson P. M. A monoclonal antibody-inhibiting FMLP-induced chemotaxis of human neutrophils. J Immunol. 1981 Dec;127(6):2241–2245. [PubMed] [Google Scholar]
- Cotter T. G., Spears P., Henson P. M. A monoclonal antibody inhibiting human neutrophil chemotaxis and degranulation. J Immunol. 1981 Oct;127(4):1355–1360. [PubMed] [Google Scholar]
- Elkins C., Rest R. F. Monoclonal antibodies to outer membrane protein PII block interactions of Neisseria gonorrhoeae with human neutrophils. Infect Immun. 1990 Apr;58(4):1078–1084. doi: 10.1128/iai.58.4.1078-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., White J. G., Holmes B. Specific degranulation of human polymorphonuclear leukocytes. Nature. 1974 Mar 22;248(446):347–348. doi: 10.1038/248347a0. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., George W. J. Mediation of immunologic discharge of lysosomal enzymes from human neutrophils by guanosine 3',5'-monophosphate. Requirement of calcium, and inhibition by adenosine 3',5'-monophosphate. J Exp Med. 1974 Jul 1;140(1):225–238. doi: 10.1084/jem.140.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D. F., Weir D. M., Blackwell C. C., Winstanley F. P. Binding of Neisseria gonorrhoeae by lectin-like receptors on human phagocytes. J Clin Lab Immunol. 1984 Mar;13(3):107–110. [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Smiley P. A. Association of the N-formyl-Met-Leu-Phe receptor in human neutrophils with a GTP-binding protein sensitive to pertussis toxin. Proc Natl Acad Sci U S A. 1985 Feb;82(3):869–873. doi: 10.1073/pnas.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Malagodi M. H., Chiou C. Y. Pharmacological evaluation of a new Ca2+ antagonist, 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in smooth muscles. Eur J Pharmacol. 1974 Jun;27(1):25–33. doi: 10.1016/0014-2999(74)90198-8. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Greenberger J. S., Cohen H. J. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979 Aug;82(2):315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Olofsson T. Induction of differentiation in a human promyelocytic leukemic cell line (HL-60). Production of granule proteins. Exp Cell Res. 1981 Jan;131(1):225–230. doi: 10.1016/0014-4827(81)90422-5. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytowski B., Easton T. G., Valinsky J. E., Calderon T., Sun T., Christman J. K., Wright S. D., Michl J. A monoclonal antibody to a human neutrophil-specific plasma membrane antigen. Effect of the antibody on the C3bi-mediated adherence by neutrophils and expression of the antigen during myelopoiesis. J Exp Med. 1988 Feb 1;167(2):421–439. doi: 10.1084/jem.167.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querinjean P., Masson P. L., Heremans J. F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur J Biochem. 1971 Jun 11;20(3):420–425. doi: 10.1111/j.1432-1033.1971.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Lee N., Bowden C. Stimulation of human leukocytes by protein II+ gonococci is mediated by lectin-like gonococcal components. Infect Immun. 1985 Oct;50(1):116–122. doi: 10.1128/iai.50.1.116-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ortega M., Ofek I., Sharon N. Membrane glycoproteins of human polymorphonuclear leukocytes that act as receptors for mannose-specific Escherichia coli. Infect Immun. 1987 Apr;55(4):968–973. doi: 10.1128/iai.55.4.968-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Rest R. F. Interactions of gonococci with phagocytic cells. Annu Rev Microbiol. 1989;43:121–145. doi: 10.1146/annurev.mi.43.100189.001005. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg N., Deal C., Nyberg G., Normark S., So M., Karlsson K. A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Eden C., Andersson B., Aniansson G., Leffler H., Lomberg H., Mestecky J., Wold A. E. Glycoconjugate receptors for bacteria attaching to mucosal sites: examples for Escherichia coli and Streptococcus pneumoniae. Adv Exp Med Biol. 1987;216B:931–939. [PubMed] [Google Scholar]
- Swanson J., Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983 May 1;157(5):1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. Y., Sivam G., Britigan B. E., Rosen G. M., Cohen M. S. Oxygen metabolism of the HL-60 cell line: comparison of the effects of monocytoid and neutrophilic differentiation. J Leukoc Biol. 1988 Feb;43(2):140–147. doi: 10.1002/jlb.43.2.140. [DOI] [PubMed] [Google Scholar]
- Trust T. J., Lambden P. R., Watt P. J. The cohesive properties of variants of Neisseria gonorrhoeae strain P9: specific pilus-mediated and non-specific interactions. J Gen Microbiol. 1980 Jul;119(1):179–187. doi: 10.1099/00221287-119-1-179. [DOI] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986 Feb;132(2):503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988 Feb;56(2):363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Jong M. T. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986 Dec 1;164(6):1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]