Abstract
Cells comprising a tissue migrate as part of a collective. How collective processes are coordinated over large multi-cellular assemblies has remained unclear, however, because mechanical stresses exerted at cell-cell junctions have not been accessible experimentally. We report here maps of these stresses within and between cells comprising a monolayer. Within the cell sheet there arise unanticipated fluctuations of mechanical stress that are severe, emerge spontaneously, and ripple across the monolayer. This stress landscape becomes increasingly rugged, sluggish, and cooperative with increasing system density. Within that landscape, local cellular migrations follow local orientations of maximal principal stress. Migrations of both endothelial and epithelial monolayers conform to this behavior, as do breast cancer cell lines before but not after the epithelial-mesenchymal transition. Collective migration in these diverse systems is seen to be governed by a simple but unifying physiological principle: neighboring cells join forces to transmit appreciable normal stress across the cell-cell junction, but migrate along orientations of minimal intercellular shear stress.
A variety of fundamental processes in development, health, and disease depend upon the coordinated motion of cell groups.1–10 To describe coordinated cellular motions in these processes, high-throughput genomic approaches have identified molecular players and mapped their interaction into comprehensive signaling networks.11, 12 But even with detailed signaling and structural information in hand, the role of intercellular adhesion in collective migration is disputed13, 14, and our understanding of collective cellular migration lacks predictive power and remains largely descriptive. Central to these limitations is the absence of a physical picture that links cell motion to mechanical stresses exerted within the cell body and at cell-cell boundaries, for these stresses have never before been measured. Here we report high resolution maps of these stress components everywhere within an advancing monolayer sheet, which serves as a simple experimental model system. These stress maps reveal that the local cellular trajectory follows local stress fields that are severely heterogeneous and dramatically cooperative over distances spanning many cell bodies. Together, these findings reveal an unanticipated but unifying physiological principle, namely, that each cell tends to migrate and remodel so as to maintain minimal local intercellular shear stress. Detailed knowledge of the biology of the cell-cell junction, the cryptic lamellipodium (online supplement 6), or any specific molecular event could never predict such a unifying principle because it is an emergent property of a multicellular collective system. By analogy to the well known guidance mechanisms of chemotaxis, durotaxis and haptotaxis, we call this distinct but innately collective mechanism plithotaxis, from the Greek “plithos” denoting crowd, swarm, or throng.
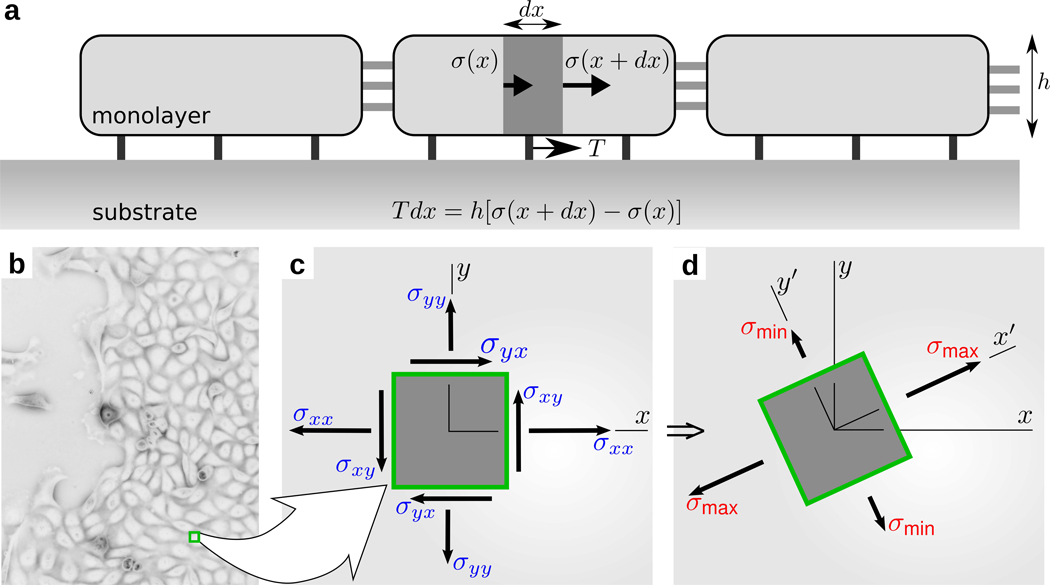
To measure the local state of stress within a monolayer (Fig. 1), we developed Monolayer Stress Microscopy, MSM (online supplement 1). On an inverted optical microscope, we record cell-generated displacements of fluorescent markers embedded near the surface of a collagen-coated polyacrylamide gel substrate on which the cells are adherent. We use a novel approach for stage drift compensation (online supplement 1), and then use resulting dedrifted gel deformations to compute a map of the traction forces, T, exerted by the monolayer upon the gel.15 Finally, from these traction forces measured directly at the interface between the cell and its substrate (Fig. S3), a straightforward and rigorous two-dimensional balance of forces as demanded by Newton’s laws is then used to obtain the distribution of the mechanical line forces everywhere within the cell sheet (Fig. 1 a); for convenience, these measured line forces (in units of force per unit length) are converted to stresses (force per unit area) using the average monolayer height, h (Fig. 1 b; online supplement 3, Fig. S4). Gradients of these line forces and stresses within the cell sheet are attributable to the pileup of traction forces applied on the underside of the cells. At each point within the sheet the local coordinate system (Fig. 1 c) can be rotated in the cell plane in order to find those special orientations along which the local normal stress is maximal and minimal, respectively, thus defining the two principal stress components (σmax and σmin) and the two corresponding, mutually perpendicular, principal orientations (Fig. 1 d; Online Supplement 1). As such, the associated MSM result displays at high resolution, and maps separately, each individual component of the in-plane stress tensor.
Figure 1.
(a) Simplified representation of the physical relationship between cell-substrate tractions, T, which have been reported previously15, and intercellular stresses, σ, which are reported for the first time here. Intercellular stresses arise from the accumulation of unbalanced cell-substrate tractions. At any point within the monolayer (b), the intercellular stresses, defined in laboratory frame (x, y), (c), have shear (σxy, and σyx) and normal (σxx, and σyy) components. This frame can be rotated locally to obtain the principal frame (x', y'), (d), where shear stresses vanish and the resulting normal stresses are called principal stresses (σmax and σmin). The corresponding axes are called maximum, aligned with x', and minimum, aligned with y', principal orientations.
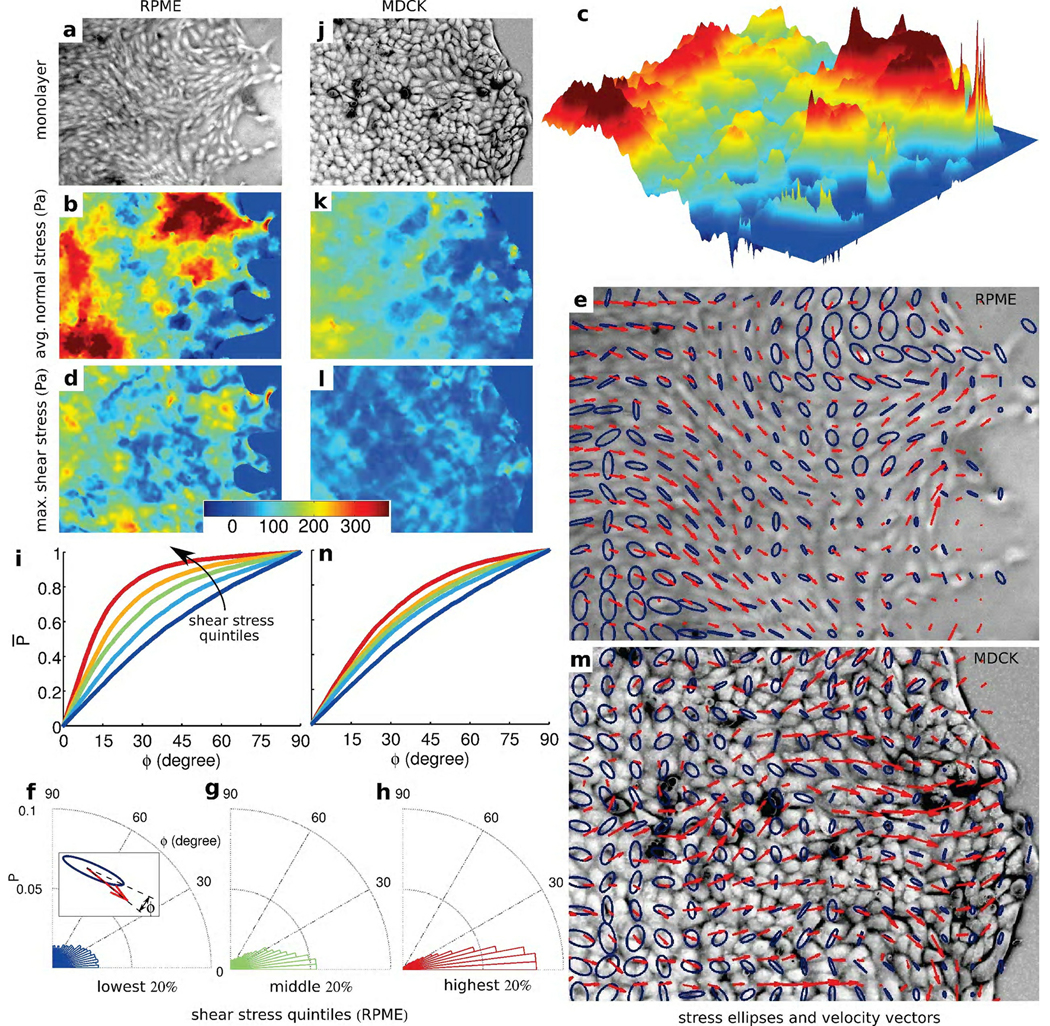
We consider first the average local normal stress, simply defined as σ̅ = (σmax + σmin) /2, and its spatial heterogeneity. A traditional image of an advancing monolayer of rat pulmonary microvascular endothelial (RPME) cells is unremarkable (Fig. 2 a). The underlying distribution of local normal stress, by contrast, is severely heterogeneous; normal stresses are mostly positive (tensile) with values exceeding 300 Pa in regions spanning tens of cells. These regions of predominantly tensile stresses alternate with regions of weakly negative (compressive) stresses (Fig. 2 b). These fluctuations occur steadily over distances spanning multiple cell widths and define a stress landscape that is rugged (Figs. 2b, k), by which we mean that the spatial fluctuations over these relatively short distances are comparable in magnitude to the spatial mean values. We consider next the distribution of the intercellular shear stress (Fig. S1) which is not to be confused with any additional shear stress that might be imposed by flow over the monolayer surface16, which in this case is everywhere zero. As in the case of the normal stress, the shear stress at a point within a material varies with orientation and attains its maximal value, μ = (σmax − σmin) /2, at 45° from the principal orientations (Fig. 1 d). The local maximal shear stress was systematically smaller than the local normal stress, but was also characterized by a rugged landscape (Fig. 2 c). As the monolayer advances, these respective stress landscapes evolve continuously in time (supplemental movie SM1). Finally, dependence of local stresses upon orientation signifies stress anisotropy. To visualize this anisotropy, we plotted ellipses whose major axis corresponds to the local σmax and minor axis corresponds to the local σmin, each aligned with corresponding principal orientations. Where σmax = σmin the stress field is isotropic, the ellipse becomes a circle, μ is zero, and there exists no preferred stress orientation. But where σmax ≫ σmin the local stress field is highly anisotropic, the ellipse becomes spindle-like, μ is nonzero, and there exists a strongly preferred and well-defined stress orientation. From region-to-region, we found that ellipse size, ellipse shape, and ellipse orientation varied extensively, but with strong local correlations (Fig. 2 e).
Figure 2. Intercellular stress maps and mechanical guidance of collectively migrating monolayers.
(a) Transmitted light image of rat pulmonary microvascular endothelial (RPME) cell monolayer. Corresponding to this image are the maps of average normal stress (b), which is predominately tensile but forms a rugged stress landscape (c), the maximum shear stress (d), principal stress ellipses (blue) and cell velocity vectors (red) (e). The alignment angle, ϕ, between major axis of the principal stress ellipse and direction of the cellular motion (f, inset) shows that the greater the local shear stress the narrower is the distribution of ϕ (f, g, h). The cumulative probability distribution P̅ (ϕ) varied strongly and systematically with stress anisotropy (i); curves from blue, to red are in the order of higher quintiles. Comparable maps are found for the Madin-Darby canine kidney (MDCK) cell monolayer (j–n). Note that the average tensile stress (k) increased systematically with increasing distance from the advancing front thus contributing to the state of global tug-of-war15. Vertical size of the images of cell monolayer: RPME − 545 µm, MDCK − 410 µm. Each curve in (i) and (n) and distributions in (f), (g), and (h) have more than 8,000 observations.
As cells extend cryptic lamellipodia17 (Fig. S7) and advance within the monolayer, stresses at every point and at every instant of time must be in mechanical balance. Nonetheless, no mechanistic framework or physical picture yet exists that might link these stresses to cellular orientation, remodeling, or migration. Here we ask, to what extent are these intercellular stresses meaningful biologically and useful predictively? The answer to this question is suggested by two pieces of experimental evidence. First, since phase-contrast images and stress maps are mutually independent measurements, the coincidence between orientation of the cell body versus orientation of the maximal principal stress is striking (Fig. 2 e, and Fig. S5). Further, because the maximal principal orientation corresponds to the local axis of highest normal stresses and zero shear stress, this result suggests that the cell-cell junction, as well as the cell body, support high normal stresses, which are overwhelmingly tensile, but only minimal shear stresses. One would predict, therefore, that major organized actin structures that span the cell, as would be imaged at low resolution, might align with maximal principal orientations, and for the spindle-like RPME cells this is in fact seen to be the case (Fig. 2 e, and Fig. S6). Second, cells not only align with the maximal principal orientation, but also migrate along that orientation (Fig. 2 e, red arrows; supplemental movie SM2). Appreciable portions of the stress field are approximately isotropic, however, and therefore the local orientation of cell motion would not be expected to correlate with a stress field possessing no preferred orientation.
As such, these observations lead naturally to the following prediction: regions of higher stress anisotropy will exhibit stronger alignment between the direction of local maximal principal stress and that of local cellular migration velocity. To test this prediction, we reasoned as follows. Since the maximum local shear stress is given by μ = (σmax − σmin) /2, we took μ as a direct and quantitative index of stress anisotropy. We then rank-ordered this stress anisotropy by quintiles. For each point within the cellular monolayer falling within any given quintile, we measured the alignment angle ϕ between the orientation of the local maximal principal stress and the orientation of the local cellular migration velocity vector (Fig. 2 f, inset). The greater was the local shear stress, the narrower was the distribution of ϕ (Fig. 2 f, g, h). We then constructed the cumulative probability distribution function, P̅(ϕ), reasoning that if there were perfect alignment between the orientation of local cellular migration velocity and that of local maximal principal stress, then all angles ϕ would be 0° and the cumulative probability distribution would be a step function from probability 0 to probability 1 occurring at 0°. If there were no alignment, however, then all angles between 0° and 90° would be equally likely, and the cumulative probability function would be a straight line from probability 0 at 0° to probability 1 at 90°. In the regions with lowest stress anisotropy (blue), the angular distribution was broad but not uniform. In regions with highest stress anisotropy (red), the angular distribution was quite narrow; the orientation of cellular velocity and the orientation of maximal principal stress were coupled strongly, but were unrelated to the magnitude of local average stress (Fig. S10). The stronger was the stress anisotropy the greater was the overall degree of alignment.
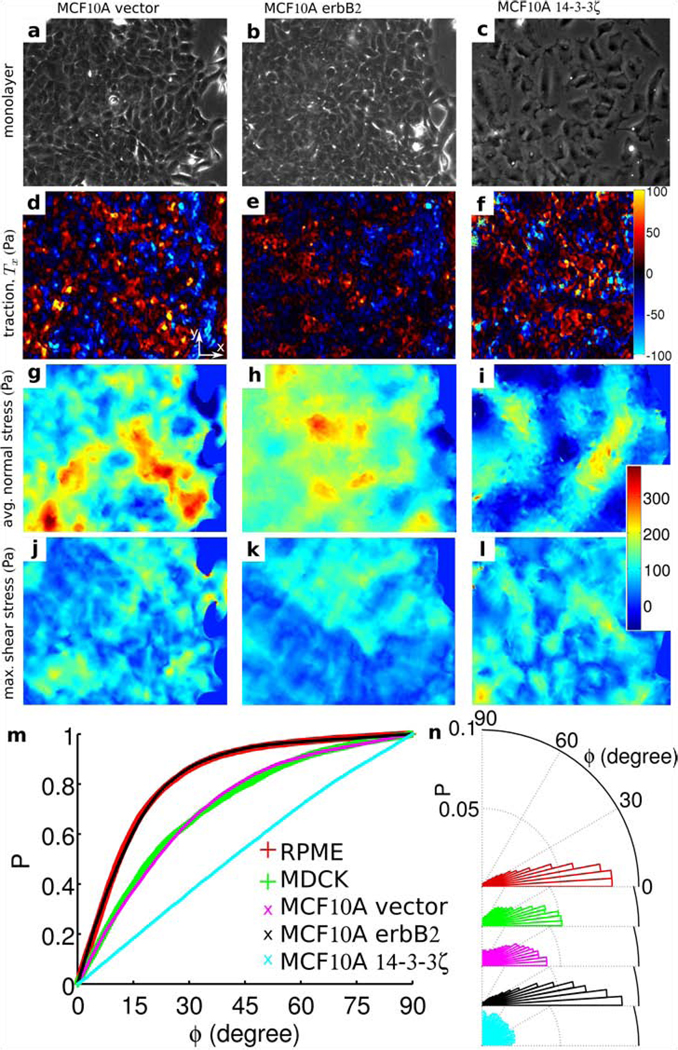
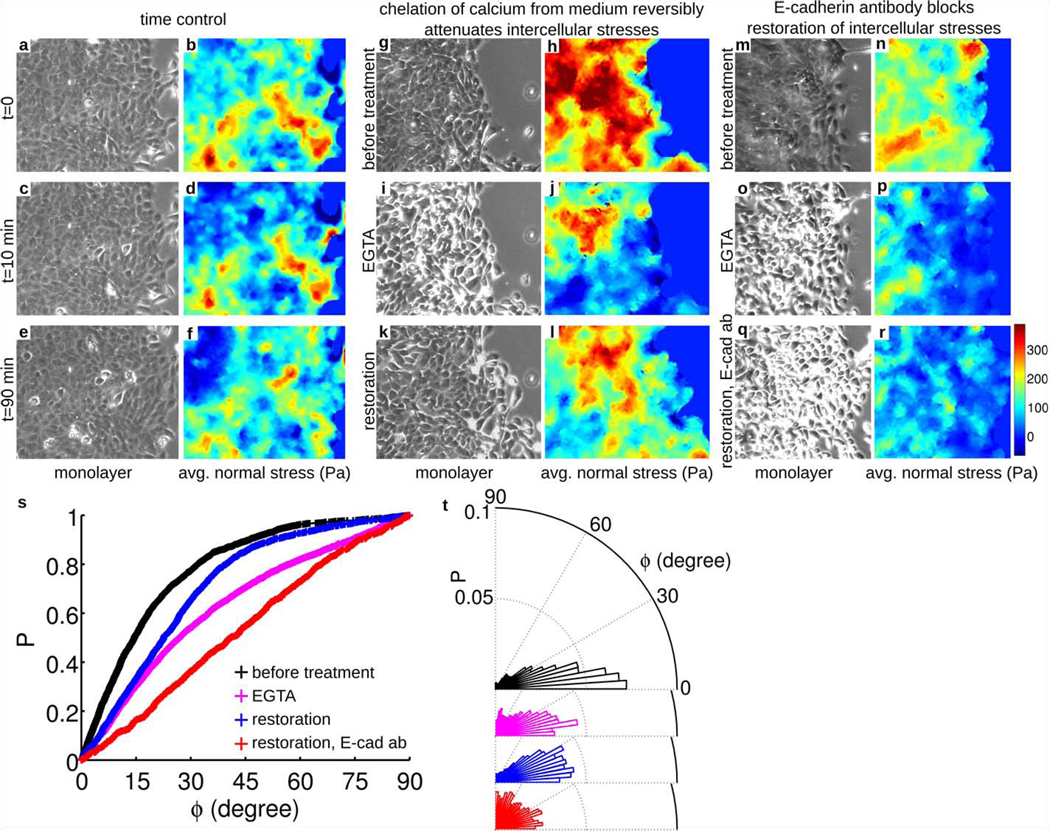
To assess the generality of this finding, we then examined monolayers comprising Madin-Darby canine kidney (MDCK) cells (Fig. 2 j), which were of particular interest because they are epithelial, not endothelial, and because they are rounded in the plane, not spindle-shaped as are RPME cells. Despite these differences in cell type and cell morphology, the stresses were dramatically heterogeneous (Fig. 2 k, l) and the local orientation of cellular migration was also found to follow the local orientation of maximal principal stress (Fig. 2 m, n). Remarkably, local cell motions tended to follow local principal stress orientations even when local cell geometry displayed no preferred orientation. To assess further the generality of this finding, we next examined the behavior of monolayers of well-established breast-cancer model systems: MCF10A cells (control or vector) (Fig. 3 a), MCF10A cells overexpressing ErbB2/HER-2/neu (Fig. 3 b), and MCF10A cells overexpressing 14-3-3ζ (Fig. 3 c). We chose these cell lines because each exhibits pronounced morphological differences as well as diverse levels of transforming potential, expression of cell-cell junction proteins, and cell proliferation.18, 19 Much as in the case of endothelial cells and control epithelial cells, ErbB2 cells moved in alignment with the direction of maximum principal stress (Fig. 3m). By contrast 14-3-3ζ cells, which have decreased expression of cell-cell junctional markers18, 19, were seen to move nearly independently of the orientation of the maximum principal stress (Fig. 3 m). To assess further the importance of cell-cell adhesion, we weakened cell-cell contacts of MCF10A vector cells by calcium chelation (Fig. 4 g, i). As expected, alignment between orientations of local stress and orientation of local cellular motions was lessened (Fig. 4 s, magenta), but was restored upon returning to normal growth medium (Fig. 4 i, s, blue). This reversibility was blocked in the presence of E-cadherin antibodies, however (Fig. 4 r, s, red). Together, these observations establish that transmission of mechanical stresses from cell-to-cell across many cells is necessary for plithotaxis, i.e., for each individual cell to follow the local orientation of the maximal principal stress.
Figure 3. Stress maps and migration in monolayers of breast-cancer model systems.
Phase contrast image of nontransformed human mammary epithelial cell line, MCF10A, control or vector (a), cells overexpressing ErbB2 (b), and 14-3-3ζ (c). Maps of cell-substrate tractions, Tx, (d, e, f), normal stress (g, h, i), and maximum shear stress (j, k, l) corresponding to each of these three mammary epithelial cell lines. (m) Cumulative probability distribution of ϕ for the regions corresponding to highest quintile of the shear stress for five different cell sheets. (t) Distributions corresponding to the curves in (m). Vertical size of the images of monolayer: 410 µm. Each curve in (m) has more than 8,000 observations.
Figure 4. Local cell guidance requires force transmission from cell-to-cell.
Time-controls of intercellular stress maps of MCF10A-vector cell monolayers (a–f). The stress patterns do not change appreciably over a period of 80 minutes. After 10 minutes in presence of the calcium chelator EGTA (4mM), however, cells lose contacts with their neighbors (g, i and m, o). These changes lead to attenuation of intercellular average normal stress (h, j and n, p). After returning to normal growth medium for 80 minutes, the stresses and the cell-cell contacts are largely restored (k, l), but if the growth medium is supplemented with E-cadherin antibody (7 µg/ml) recovery of stresses and cell-cell contact is blocked (q, r). EGTA treatment widens the distribution of angle (ϕ) between local cellular velocity and local maximum principal orientation corresponding to highest of the maximum shear stress quintiles (s, t). The distribution of ϕ is narrowed if calcium is restored (s and t, blue), but widened further if the restoration medium is supplemented with E-cadherin antibody (s and t, red). Together, these data show that local cell guidance along the orientation of maximal principal stress (plithotaxis) requires force transmission across cell-cell junctions. These preferred orientations correspond to those engendering minimal intercellular shear stresses. Increased intensity at cell boundaries in phase contrast images (panels i, o, and q) reveals disruption of cell-cell junctions. Vertical size of the images of monlayer: 410 µm. Each data set in (s and t) has more than 1,500 observations.
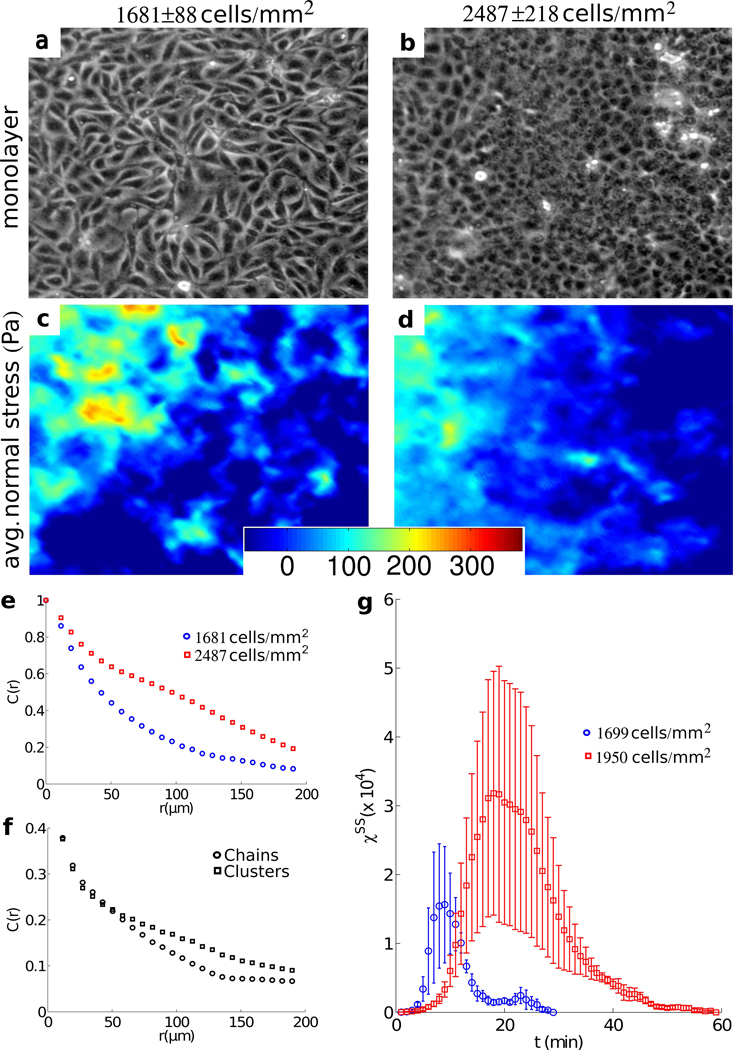
For collective migration to be coordinated across many cells, intercellular stresses might be expected to be cooperative over comparable distances; cooperativity of cell motions have been recently established20, 21, but cooperativity of cellular stresses have not. To quantify the spatial extent of any such stress cooperativity, we first examined the spatial autocorrelation function of the average normal stress:
where δσ̅i is the local departure of the average normal stress at position r⃗i from its spatial mean < σ̅i >, var(σ̅) is the variance of those departures, and the notation |r⃗i − r⃗j| = R means equality within a uniform bin width of 5 microns. Confining attention to regions many cell lengths from the leading edge of an MDCK monolayer (Fig. 5 a), fluctuations in normal stress (Fig. 5 c) were found to be correlated over a length scale of approximately 10–15 cell diameters (Fig. 5 e, blue). Cooperativity of normal stresses over 10–15 cell diameters might be attributable to alignment of principal stresses end-to-end, as in a tug-of war, or side-by-side, as police who lock arms during crowd control. To assess whether normal stresses are aligned according to either of these configurations, we decomposed the maximum principal stress into end-to-end and side-by-side contributions,
where ‖…‖ denotes L2 norm, Fi is the local maximal principal stress considered as a vector quantity (such that the angle between the maximal and minimal principle stress orientations is taken modulo π) and θij is the angle between adjacent vector pairs. The two components were found to contribute almost equally to force cooperativity, thus indicating the coexistence of both end-to-end and side-by-side force correlations (Fig. 5 f). Simply put, in order to move cooperatively neighboring cells join forces.
Figure 5. Signatures of cooperativity and associated glassy dynamics.
Phase contrast images of a monolayer of Madin-Darby canine kidney (MDCK) cells well away from the leading edge at early (a, t=196 min, density=1681±88 cells/mm2) and late (b, t=3196 minutes, density=2487±218 cells/mm2) times. Also shown are corresponding maps of average normal stress (c, d). Note that any contribution to the stress field with a wavelength longer than the size of the field of view is not included in the calculation. Thus a stress build up extending over the entire monolayer as previously reported15 is absent from this analysis. (e) Time averaged spatial autocorrelation function, C(r), of average normal stress in low density (1681 cells/mm2, blue), and high density (2487 cells/mm2, red) regions. (f) C(r) of high density maximal principal stress resolved into components representing force chains (circles) and force clusters (squares). (g) Variance, χss, of the self-overlap parameter, qs, as a function of time, in early, low denisty (t=1–270 minutes, 1699 ±40 cells/mm2, blue) and late, high density (t= 1800–2070 minutes, 1950±156 cells/mm2, red) intervals. Each curve represents an average over three successive 90 minute windows of similar density. Error bars represent the standard deviation over the square root of the number of windows. Vertical size of the images of monolayer: 480 µm.
Cooperative motions emerge naturally in inert particulate systems that exhibit close-packing, structural disorder, and glassy dynamics, such as colloidal glasses.22 A central feature that identifies these systems as being glassy is the slowing of internal structural rearrangement as system density is increased; with increasing system density, each particle becomes increasingly trapped by its neighbors so that, in order to rearrange at all, many neighboring particles must rearrange cooperatively23. As such, the size of cooperative clusters increases as system density increases. Moreover, as size of the cluster grows the number of possible structural rearrangements decreases and, as such, the time needed for cooperative rearrangements increases precipitously until, eventually, the system becomes virtually frozen, or stuck.23 Cooperative cellular motions within the monolayer sheet exhibit these very signatures of glassy dynamics24, but to what extent might cellular stresses depict a complementary physical picture? To answer this question we analyzed motion of the MDCK monolayers as cellular density increased with the passage of time.15, 20 Consistent with an expectation of glassy dynamics, the spatial decay in C(r) was smaller when the density was greater (Fig. 5 e, red curve with corresponding monolayer and force map Fig. 5 b, d), indicating that force cooperativity extended to greater distances. As a direct measure of slowing of structural rearrangements we turned to metrics commonly used in soft condensed matter systems. We consider the average number of cells which change position between two points in time, which defines an overlap function qs :
where the weight function w is equal to 1 if the distance between cell positions at sequential times is less than half a cell diameter, and zero otherwise. The variance of qs is then a measure of the rate of overall structural rearrangement and is related to the so-called four-point susceptibility χss25. The peak in χss occurs at the overall structural relaxation time, and the height of that peak is related to the size of rearranging regions26, 27. If the system is glassy, the peak in χss is expected to shift towards longer times as system density is increased, and a clear shift of the peak in the more dense system confirms this expectation (Fig. 5 g). The peak height also increases in the more dense system, confirming the presence of growing velocity clusters. Moreover, these density-dependent shifts in the position and the peak height of χss, which are indicative of slowing of structural rearrangements, occur simultaneously with growth of force clusters as indicated by the slowing decay in the force autocorrelation function with increasing density (Fig. 5 e, red). Although a mechanistic link between inter-particle forces and spatially heterogeneous dynamics in glassy systems remains unclear28–30, the findings of Fig. 5 are consistent with approach to a glass transition (Online supplement 7).
Recent advances have unraveled important features of stress transmission across specific molecular constituents of the focal adhesion and of the adherens junction, including vinculin, talin, and α-catenin for example14, 31–36, but the integrative context of these molecular events within integrated stress-bearing structures comprising highly redundant molecular pathways, or even across multi-cellular assemblies at larger scales of organization, have remained largely ambiguous. Logically, associated integrative principles have remained unstudied. Because distinct stress tensor components between contiguous cells in any complex living system have never before been measured, Monolayer Stress Microscopy now sets the study of underlying molecular events within an integrative mechanical context that is conceptually comprehensive and experimentally rigorous. The finding that each cell comprising a monolayer tends to migrate and remodel so as to maintain minimal local intercellular shear stress complements other integrative physiological principles (Online supplement 8).
A central question in morphogenesis and disease is how differentiated structures emerge from homogeneous cell populations37. Differentiation and pattern formation in multi-cellular systems is currently explained by the existence of morphogenetic gradients and by local variations in the composition, topology, and stiffness of the extracellular matrix38. In addition, once transduced by the sensory machinery of the individual cell39, the spontaneously emergent rugged stress landscape reported here would be expected to trigger non-uniform secretion of soluble or insoluble factors, thus altering the local cellular microenviroment, causing cytoskeletal reinforcement40 or cytoskeletal fluidization41, 42, as well as activating in a highly non-uniform fashion stress-dependent genetic programs that give rise to differentiated tissues. These emergent stress heterogeneities are severe and persistent but unanticipated. How they might become harnessed and regulated during morphogenesis or repair and, perhaps more importantly, how they might become unharnessed or dysregulated during disease or injury, we identify here as major open questions, but ones that are now accessible to direct experimental attack.
Supplementary Material
Acknowledgement
For their critical comments on the manuscript, we thank Rolf Hubmayr (Mayo Clinic), Rob Phillips(CalTech), Daniel Navajas (University of Barcelona), Lambert Ben Freund (Brown University), Daniel Tschumperlin (Harvard University), C. Forbes Dewey, Jr. (MIT), Vivek B. Shenoy (Brown University). We acknowledge the support of the European Research Council (Starting Grant), the Spanish Ministry of Science and Innovation, and the National Heart, Lung and Blood Institute of the National Institutes of Health.
Footnotes
Author contributions: DTT developed algorithms and performed stress measurements. CCH analyzed data pertaining to force chains and glassy dynamics. DTT, and TEA performed measurements of cell motions. KR, and CYP assisted in protocol design and optimization. CYP performed staining of actin cytoskeleton. XSP performed additional stress measurements on MDCK cells. MZ provided cancer cell lines and assisted with related data interpretation. DTT, and EZ made early conceptual contributions. JPB, DAW, JJF, and XT guided data interpretation and analysis. DTT, CCH, JPB, XT and JJF wrote the manuscript.
List of References
- 1.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nature reviews. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Discher D, et al. Biomechanics: cell research and applications for the next decade. Ann Biomed Eng. 2009;37:847–859. doi: 10.1007/s10439-009-9661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. Journal of mammary gland biology and neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 6.Bianco A, et al. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- 7.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 8.Giampieri S, et al. Localized and reversible TGF[beta] signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montell D. Morphogenetic Cell Movements: Diversity from Modular Mechanical Properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- 10.Shaw TJ, Martin P. Wound repair at a glance. Journal of Cell Science. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson KJ, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 12.Vitorino P, Meyer T. Modular control of endothelial sheet migration. Genes and Development. 2008;22:3268–3281. doi: 10.1101/gad.1725808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bindschadler M, McGrath JL. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J Cell Sci. 2007;120:876–884. doi: 10.1242/jcs.03395. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trepat X, et al. Physical forces during collective cell migration. Nature Physics. 2009;5:426–430. [Google Scholar]
- 16.DePaola N, Gimbrone M, Jr, Davies P, Dewey C., Jr Vascular endothelium responds to fluid shear stress gradients. Arteriosclerosis and Thrombosis. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 17.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, et al. Breast Cancer Metastasis: Challenges and Opportunities. Cancer Research. 2009;69:4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 19.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelini TE, Hannezo E, Trepat X, Fredberg JJ, Weitz DA. Cell Migration Driven by Cooperative Substrate Deformation Patterns. Physical Review Letters. 2010;104:168104. doi: 10.1103/PhysRevLett.104.168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabó B, et al. Phase transition in the collective migration of tissue cells: Experiment and model. Physical Review E. 2006;74:061908. doi: 10.1103/PhysRevE.74.061908. [DOI] [PubMed] [Google Scholar]
- 22.Parisi G, Zamponi F. Mean-field theory of hard sphere glasses and jamming. Reviews of Modern Physics. 2010;82:789. [Google Scholar]
- 23.Weeks ER, Crocker JC, Levitt AC, Schofield A, Weitz DA. Three-dimensional direct imaging of structural relaxation near the colloidal glass transition. Science. 2000;287:627–631. doi: 10.1126/science.287.5453.627. [DOI] [PubMed] [Google Scholar]
- 24.Angelini TE, et al. Glass-like dynamics of collective cell migration. Proc Natl Acad Sci U S A. 2011;108:4714–4719. doi: 10.1073/pnas.1010059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthier L, et al. Direct experimental evidence of a growing length scale accompanying the glass transition. Science. 2005;310:1797–1800. doi: 10.1126/science.1120714. [DOI] [PubMed] [Google Scholar]
- 26.Keys A, Abate A, Glotzer SC, Durian DJ. Measurement of growing dynamical length scales and prediction of the jamming transition in granular material. Nature Physics. 2007;3:260–264. [Google Scholar]
- 27.Toninelli C, Wyart M, Berthier L, Biroli G, Bouchaud J-P. Dynamical susceptibility of glass formers: Contrasting the predictions of theoretical scenarios. Physical Review E. 2005;71:041505. doi: 10.1103/PhysRevE.71.041505. [DOI] [PubMed] [Google Scholar]
- 28.Hall RW, Wolynes PG. Intermolecular Forces and the Glass Transition. The Journal of Physical Chemistry B. 2007;112:301–312. doi: 10.1021/jp075017j. [DOI] [PubMed] [Google Scholar]
- 29.Mueth DM, Jaeger HM, Nagel SR. Force distribution in a granular medium. Physical Review E. 1998;57:3164. [Google Scholar]
- 30.Trappe V, Prasad V, Cipelletti L, Segre PN, Weitz DA. Jamming phase diagram for attractive particles. Nature. 2001;411:772–775. doi: 10.1038/35081021. [DOI] [PubMed] [Google Scholar]
- 31.del Rio A, et al. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 34.le Duc Q, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajfur Z, Roy P, Otey C, Romer L, Jacobson K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat Cell Biol. 2002;4:286–293. doi: 10.1038/ncb772. [DOI] [PubMed] [Google Scholar]
- 36.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. α-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 37.Engler AJ, Humbert PO, Wehrle-Haller B, Weaver VM. Multiscale modeling of form and function. Science. 2009;324:208–212. doi: 10.1126/science.1170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Goff L, Lecuit T. Gradient Scaling and Growth. Science. 2011;331:1141–1142. doi: 10.1126/science.1203270. [DOI] [PubMed] [Google Scholar]
- 39.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 40.Roca-Cusachs P, Gauthier NC, del Rio A, Sheetz MP. Clustering of α5β1 integrins determines adhesion strength whereas αvβ3 and talin enable mechanotransduction. Proc Natl Acad Sci U S A. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan R, et al. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS ONE. 2009;4:e5486. doi: 10.1371/journal.pone.0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trepat X, et al. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.