Abstract
Background
Hemangiopericytomas and malignant solitary fibrous tumors (HPC/SFT) are rare, closely related sarcomas with unpredictable behavior that respond infrequently to chemotherapy. An optimal systemic treatment strategy for advanced HPC/SFT has not yet been identified.
Methods
We retrospectively analyzed the records of 14 patients with histopathologically confirmed HPC/SFT who were treated at The University of Texas MD Anderson Cancer Center from May 2005 to June 2007. All patients were treated with temozolomide 150 mg/m2 orally on days 1-7 and days 15-21 and bevacizumab 5 mg/kg intravenously on days 8 and 22, repeated at 28-day intervals. Computer tomographic assessment of tumor size and density (Choi criteria) was used to determine the best response to therapy. The Kaplan-Meier method was used to estimate progression-free survival.
Results
The median follow-up period was 34 months. Eleven patients (79%) achieved a Choi partial response, with a median time to response of 2.5 months. Two patients (14%) had stable disease as the best response, and one patient (7%) had Choi progressive disease as the best response. The estimated median progression-free survival was 9.7 months with a 6-month progression-free rate of 78.6%. The most frequently observed toxic effect was myelosuppression.
Conclusion
Combination therapy with temozolomide and bevacizumab is a generally well-tolerated and clinically beneficial regimen for HPC/SFT patients. Additional investigation in a controlled, prospective trial is warranted.
Keywords: Hemangiopericytoma, Solitary fibrous tumors, soft-tissue sarcoma, chemotherapy, antiangiogenesis inhibitors
Introduction
Hemangiopericytomas and solitary fibrous tumors (HPC/SFT) are closely related soft tissue sarcomas that appear to exhibit fibroblastic-type differentiation1, 2 and typically affect adults aged 20-70. Common sites of involvement include the lower extremities, retroperitoneum/pelvis, lung/pleura, and meninges, but these tumors may be found at virtually any body site.3-5 Histologic features that suggest aggressive behavior are not well defined in HPC/SFT. Surgery is typically the treatment of choice for localized disease, with reported 10-year overall survival rates of 54%-89% after complete surgical resection.6-8
For the approximately 20% of HPC/SFT patients who eventually develop local recurrences and/or distant metastases, additional resections should be considered but are not always feasible.6-9 Options for effectively treating unresectable tumors are limited. Radiotherapy can be used only in select cases, and to date, responses to systemic chemotherapy have been infrequent.10-15 Alternative therapeutic options are therefore needed for improved palliation and disease control.
Temozolomide is an oral cytotoxic alkylating agent whose active metabolite, monomethyltriazenoimidazole carboxamide is identical to that of dacarbazine, a drug with known antitumor activity against soft tissue sarcomas.16, 17 Bevacizumab is a recombinant monoclonal antibody that targets vascular endothelial growth factor (VEGF), a key mediator of a signaling pathway that affects many vital cellular processes, including angiogenesis and vascular permeability.18 Bevacizumab has shown antitumor activity when combined with a number of cytotoxic chemotherapeutic agents, such that its use in combination therapy has been approved for the treatment of metastatic colorectal, non-small cell lung, and HER2-negative breast cancers.19-21 The antitumor activity of temozolomide combined with bevacizumab is currently being studied in several phase II and III trials for glioblastoma and metastatic melanoma.22
In May 2005, a patient with a recurrent meningeal HPC that was refractory to multiple surgical resections, radiotherapy, and chemotherapy was empirically treated with temozolomide and bevacizumab at our institution. He subsequently achieved a radiologically evident reduction in tumor size as well as palliation of tumor-related symptoms. This anecdotal evidence led us to treat 13 subsequently consecutive patients with locally advanced, recurrent, or metastatic HPC/SFT with temozolomide plus bevacizumab. Here, we describe the responses and side effects of this therapeutic regimen.
Materials and Methods
Patients
We retrospectively reviewed the medical records of all patients with histologic diagnoses of HPC or SFT treated with temozolomide-bevacizumab combination therapy at The University of Texas MD Anderson Cancer Center through June 2007. Patients were identified through the institutional electronic medical records database. Patients for whom radiologic scans were unavailable for radiologic response assessment were excluded from the analysis. We collected data on patient characteristics, including age, sex, and ethnicity; disease characteristics, including primary tumor site and extent of disease; previous treatment and responses; toxic effects of temzolomide and bevacizumab; and survival. This study was approved by our institutional review board.
Treatment
All patients received temozolomide 150 mg/m2 orally on days 1-7 and days 15-21, and bevacizumab 5 mg/kg intravenously on day 8 and day 22 on a 28-day cycle. The median number of cycles given was 7.5 (range 2.5-27). Four patients required temozolomide dose modifications or treatment delay because of neutropenia (n=1) or thrombocytopenia (n=3), and one of them received granulocyte macrophage colony-stimulating factor support.
Radiologic Assessment
Baseline radiologic studies had been performed up to 4 weeks prior to the initiation of chemotherapy and follow-up scans had been performed every 8 to 12 weeks. Contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was used at the discretion of the treating physician. Radiologic tumor response was determined as described below.
The longest cross-sectional dimension for each measurable lesion was measured at the start of therapy and on each follow-up study. The sum of the longest selected measurable lesions at each timepoint was computed for each patient. Radiologic response was then determined by calculating the absolute and percentage change from the baseline sum.
In patients whose responses were assessed with contrast-enhanced CT scans, the tumor density of each was measured in Hounsfield units by drawing a region of interest around the margin of the entire lesion. In patients who underwent CT with triphasic techniques, tumor density was measured on scans obtained in the portal venous phase. The mean baseline tumor density was compared with the mean tumor density on the subsequent studies.
Response Assessment
Using the Choi response criteria,23, 24 a complete response (CR) was defined as the disappearance of all lesions without the appearance of new lesions. A Choi partial response (PR) was defined as a ≥10% decrease in the sum of the target lesions or a ≥15% decrease in tumor density in the absence of new lesions or obvious progression of nonmeasurable disease. Choi progressive disease (PD) was defined as a ≥10% increase in tumor size in the absence of favorable tumor density change required to achieve Choi PR. Patients whose disease did not meet the criteria for Choi CR, PR, or PD and who did not have tumor-related symptomatic deterioration were classified as having stable disease (SD). Only the best response for each patient was used in determining response rates. Response was also assessed using Response Evaluation Criteria in Solid Tumors (RECIST)25 to compare to Choi responses.
Pathology Review
All tumor specimens had been reviewed by an MD Anderson sarcoma pathologist who established the diagnosis of HPC or SFT at the time of the patients' initial presentation to our institution. For the purpose of this study, two experienced sarcoma pathologists (AJL and WLW), who were blinded to the patients' outcome, re-reviewed all the available specimens to confirm the diagnoses. Histopathologic variables including size, number of mitoses, cellularity, pleomorphism, and presence of necrosis and/or hemorrhage, were noted during the re-review whenever possible. Tumors were sub-categorized as typical, malignant (based on hypercellularity, mitotic activity of > 4 per 10 HPF, moderate to severe cytologic atypia, tumor necrosis and/or distinctly infiltrative margins), or uncertain/unknown and classified according to the 2002 World Health Organization disease classification criteria for sarcomas.26 The criteria for malignancy in hemangiopericytoma are less well defined than for solitary fibrous tumor so these same criteria were used for hemangiopericytoma.
Statistical Analysis
Patient characteristics were summarized using medians and ranges for continuous variables and frequencies and percentages for categorical variables. The response rates and the 95% confidence interval (CI) were calculated from variance estimates. Fisher's exact test was used to assess the association between patient or tumor characteristics and best response. Time to best radiologic response was calculated from the initiation of temozolomide and bevacizumab therapy to development of Choi CR or Choi PR. Progression-free survival (PFS) was defined as the time interval between the start of temozolomide and bevacizumab therapy and radiologic evidence of PD, as defined by either the Choi response criteria or the RECIST criteria, or death from any cause. Survival data were updated on October 15, 2009, and the patients' data were censored at that point. The Kaplan-Meier method27 was used to estimate the PFS and overall survival (OS). All statistical analyses were carried out with S-plus 8.0 (TIBCO Software Inc., Somerville, MA).
Results
Patient and Disease Characteristics
Sixteen patients with advanced, recurrent, or metastatic HPC/SFT who received temozolomide-bevacizumab therapy between May 2005 and June 2007 were identified. Two patients for whom radiologic scans were missing were excluded from the analysis. The remaining 14 patients' characteristics are summarized in Table 1. All 14 patients (9 men, 5 women) were white, and the median age was 59 years (range, 44–75 years). Ten patients (71%) had HPC and 4 patients (29%) had SFT. The most common site of primary disease was the meninges (n=6). Seven patients had metastatic disease when they began temozolomide-bevacizumab therapy, while the remaining patients had either primary or locally recurrent disease deemed surgically unresectable.
Table 1. Patient and disease characteristics.
| Characteristic | (N=14) | (%) | |
|---|---|---|---|
| Age | |||
| median | 59 years | ||
| range | 44 – 75 years | ||
| Gender | |||
| male | 9 | 64.3 | |
| female | 5 | 35.7 | |
| Ethnicity | |||
| white | 14 | 100 | |
| Diagnosis | |||
| HPC | 10 | 71.4 | |
| SFT | 4 | 28.6 | |
| Primary tumor site | |||
| meninges | 6 | 42.9 | |
| lung/pleura | 3 | 21.4 | |
| pelvis | 3 | 21.4 | |
| abdominal wall | 1 | 7.1 | |
| gluteal region | 1 | 7.1 | |
| Tumor classification at diagnosis | |||
| benign | 3 | 21.4 | |
| malignant | 5 | 35.7 | |
| unknown | 6 | 42.9 | |
| Metastatic disease | |||
| no | 7 | 50.0 | |
| yes | 7 | 50.0 | |
| Prior therapy | |||
| no | 2 | 14.3 | |
| yes | 12 | 85.7 | |
| Number of prior surgeries | |||
| 0 | 4 | 28.6 | |
| 1 | 3 | 21.4 | |
| 2 | 3 | 21.4 | |
| 3 | 2 | 14.3 | |
| 4 | 1 | 7.1 | |
| 6 | 1 | 7.1 | |
| Prior radiation therapy | |||
| no | 7 | 50.0 | |
| yes | 7 | 50.0 | |
| Number of prior systemic therapies | |||
| 0 | 9 | 64.3 | |
| 1 | 1 | 7.1 | |
| 2 | 1 | 7.1 | |
| 3 | 1 | 7.1 | |
| 4 | 1 | 7.1 | |
| 5 | 1 | 7.1 | |
Abbreviations: HPC, hemangiopericytoma; SFT, solitary fibrous tumor.
The majority of the patients (86%) had received prior therapy before starting treatment with temozolomide and bevacizumab (Table 1). Five patients had received prior systemic therapy (Table 2). Their best responses to each prior regimen were re-assessed using the Choi criteria. They had often achieved SD and improvement in their symptoms with the previous regimens, but none had achieved Choi PR. The main reasons for starting temozolomide-bevacizumab therapy included symptomatic disease, neoadjuvant treatment to potentially downstage the tumor and enable surgical resection, and disease progression after prior therapy.
Table 2. Patients' prior systemic therapy history.
| Tumor | Prior regimen(s) | Duration of therapy (months) | Best response (Choi) | Best response (RECIST) | Reason for stopping therapy |
|---|---|---|---|---|---|
| SFT | Gemcitabine-docetaxel | 2 | PD | PD | Disease progression |
| SFT | Gemcitabine-docetaxel | 1 | PD | PD | Disease progression |
| Doxorubicin-dacarbazine | 3 | SD | SD | Disease progression | |
| HPC | Imatinib | 5 | SD | SD | Disease progression |
| Imatinib-thalidomide | 1 | PD | PD | Disease progression | |
| Imatinib-thalidomide-etoposide | 1 | SD | SD | Toxicities | |
| Patient intolerance | |||||
| Imatinib-thalidomide-hydroxyurea | 7 | SD | SD | Disease progression | |
| Imatinib-hydroxyurea | 2.5 | SD | SD | Disease progression | |
| HPC | Celecoxib* | 14 | SD* | SD | Disease recurrence |
| Imatinib | 2 | PD | PD | Disease progression | |
| Paclitaxel* | 6 | SD* | SD | Physician decision | |
| Gemcitabine-docetaxel | 3 | SD | SD | Disease progression | |
| HPC | Endostatin | 7 | PD | PD | Disease progression |
| Toxicities | |||||
| Paclitaxel | 8 | SD | SD | Disease progression | |
| Gemcitabine | 8 | SD | SD | Disease progression | |
Abbreviations: HPC, hemangiopericytoma; SFT, solitary fibrous tumor; SD, stable disease; PD, progressive disease.
Received regimen as adjuvant therapy after R0 resection.
At the time of our analysis, all patients had discontinued therapy. Reasons for discontinuing therapy were disease progression (n=6); intolerable side effects such as thrombocytopenia (n=2), fungal infection (n=1), and decreased performance status (n=1); patient's preference to hold therapy (n=1); physician's decision to hold therapy after the patient achieved maximum clinical benefit (n=2); and death due to PD (n=1).
Clinical Outcome
The overall response rate was 79% (11 patients, 95% CI 49.2%-95.3%). All 11 patients who responded had Choi PR (Table 3). Two patients (14%) achieved Choi SD, and Choi PD was the best response in 1 patient (7%).
Table 3. Overall response to temozolomide and bevacizumab.
| Patient | Tumor | Maximum change in tumor size (%) | Maximum change in density (%) | Best response (Choi) | Best response (RECIST) | ||
|---|---|---|---|---|---|---|---|
| 1 | HPC | -56.2 | -41.3 | PR | ↓Size | ↓HU | PR |
| 2 | SFT | -42.1 | -67.6 | PR | ↓Size | ↓HU | PR |
| 3 | SFT | -26.7 | -16.2 | PR | ↓Size | ↓HU | SD |
| 4 | HPC | -19.5 | -19.1 | PR | ↓Size | ↓HU | SD |
| 5 | HPC | -18.5 | -39.4 | PR | ↓Size | ↓HU | SD |
| 6 | SFT | -13.7 | -83.1 | PR | ↓Size | ↓HU | SD |
| 7 | SFT | -6.5 | -23.7 | PR | ↓Size | ↓HU | SD |
| 8 | HPC | -26.9 | N/A* | PR | ↓Size | SD | |
| 9 | HPC | -6.1 | -28.7 | PR | ↓HU | SD | |
| 10 | HPC | -3.4 | -60.5 | PR | ↓HU | SD | |
| 11 | HPC | 4.9 | -15.5 | PR | ↓HU | SD | |
| 12 | HPC | 0 | N/A* | SD | SD | ||
| 13 | HPC | 4.6 | 4.4 | SD | SD | ||
| 14 | HPC | 15.5 | 5.4 | PD | SD | ||
| Median | -10.1 | -26.2 | |||||
Abbreviations: HPC, hemangiopericytoma; SFT, solitary fibrous tumor; PR, partial response; SD, stable disease; PD, progressive disease; HU, Hounsfield units.
Response assessment done with MRI; unable to measure density changes.
Response was observed as a decrease in size (n=1), in density (n=3), or in both (n=7). Ten patients (71%) demonstrated some degree of tumor shrinkage; their median tumor size change was -10.1% (range -56.2%-15.5%). Ten patients (71%) demonstrated at least a 15% reduction in tumor density, and the median percent change in density was -26.2% (range -67.6%-5.4%). For the patients who demonstrated a Choi PR, response was seen early during treatment, with all patients achieving PR after 2 to 4 cycles; the median time to response was 2.5 months (range 1.6-4.7 months). For the seven patients who had symptomatic disease at the time of starting therapy, six achieved Choi PRs, which were seen with improvements in their symptoms.
The overall response rate was also calculated using the RECIST criteria. Two patients (14%) achieved a RECIST PR (95% CI 1.8%-42.8%). The remaining 12 patients all achieved RECIST SD, with 11 patients (79%) demonstrating RECIST SD for more than 4 months (95% CI 49.2%-95.3%). No statistically significant associations were found between response and any patient or tumor characteristics, including primary tumor location (meningeal vs. non-meningeal) and primary tumor histologic classification (benign vs. malignant vs. unknown).
At the time of analysis, the median follow-up was 34 months. The median Choi PFS was 9.67 months (95% CI 7.31 months-not estimable), and the proportion of patients who were progression-free at 6 months was 78.6% (Fig. 1). The median RECIST PFS was 10.8 months (95% CI 8.13 months-not estimable) (Fig. 2), and the 6-month PFS was 92.9%. To date, 5 patients are alive and 4 (28.6%) of them remain progression-free. Ten (71.4%) patients had ultimately had PD or had died. The median OS was estimated at 24.3 months.
Figure 1.
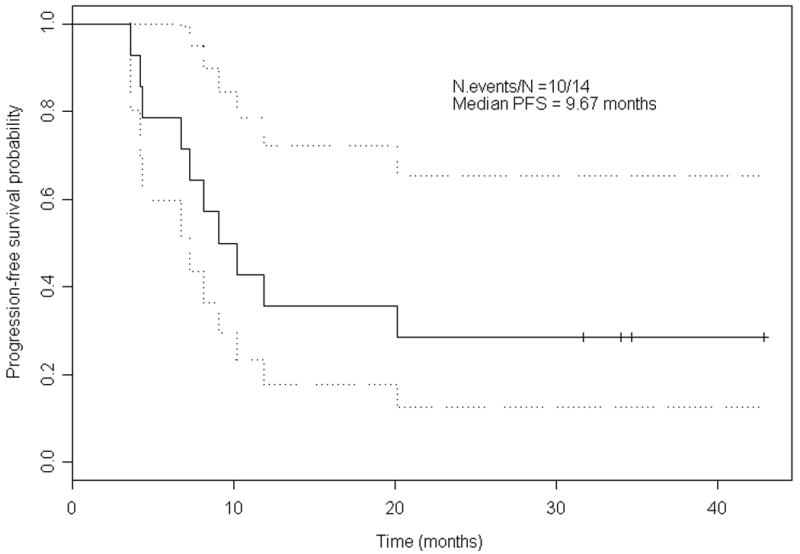
Kaplan-Meier estimates for progression-free surival (PFS) (Choi criteria).
Figure 2.
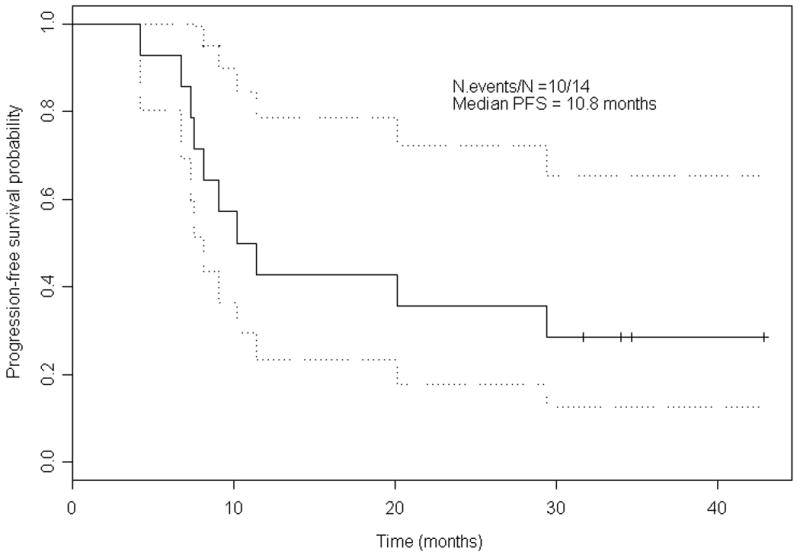
Kaplan-Meier estimates for progression-free survival (PFS) (RECIST).
Toxicity
Treatment was generally well tolerated, but because it was not administered in a clinical trial setting, toxicity data were not recorded systematically. The most notable toxic effect was myelosuppression, with neutropenia and thrombocytopenia requiring treatment modifications and/or delays in 4 patients. Fever, chills, fatigue, nausea, and headache were also noted.
One patient developed a pulmonary infiltrate after 20.5 cycles of temozolomide-bevacizumab therapy, at which point therapy was withheld. A follow-up chest CT study 6 weeks later showed a persistent lung nodule. A biopsy of the lesion showed inflammation and the cultures were positive for Cryptococcus. The patient was successfully treated with oral fluconazole, and the infection subsequently resolved.
One patient died during treatment. The 48-year-old woman with a recurrent HPC tumor adjacent to the cervical spine had undergone 3 prior surgeries and radiation therapy. On day 11 of cycle 4 of temozolomide-bevacizumab therapy, she was admitted to the hospital with Staphylococcus aureus bacteremia secondary to infected hardware in her cervical spine. She was treated with intravenous antibiotics and the bacteremia resolved. She received 2 additional cycles of treatment but was admitted again on day 7 of cycle 6 with renal failure, altered mental status, and hypotension. She died the following day.
Discussion
In patients with locally advanced, recurrent, or metastatic HPC/SFT who were treated with temozolomide and bevacizumab, reductions in tumor size and/or density consistent with PRs as assessed by the Choi criteria were evident in most patients. Several patients also demonstrated long periods of freedom from disease progression, with 5 patients having a time-to-progression period of ≥20 months.
Currently, the combination of doxorubicin and ifosfamide is the standard systemic chemotherapy regimen for many subtypes of soft tissue sarcoma. Gemcitabine combined with docetaxel has also emerged as a good therapeutic choice for these patients. Although cases of HPC/SFT responding to these chemotherapeutic agents have been sporadically reported,6, 10-13, 15 no systematic review or clinical trial to date has identified an effective systemic regimen for unresectable HPC/SFT.
Because of a lack of good historical data regarding response rates and disease progression-free survival with which we can readily compare our current findings, we turned to our existing patients' experiences with systemic chemotherapy. A review of our HPC/SFT patients' prior regimens showed that doxorubicin, gemcitabine-docetaxel, and paclitaxel did not produce a RECIST radiologoic response in any of the 5 patients. We then retrospectively re-assessed their responses using the Choi criteria, and concluded that none of the patients had achieved a Choi PR to prior therapy, but all 5 had a PR to temozolomide and bevacizumab. To further understand the activity of temozolomide and bevacizumab compared with standard chemotherapy regimens, we previously reported on a separate cohort of 5 advanced HPC/SFT patients who had received doxorubicin and ifosfamide, single-agent ifosfamide, or gemcitabine and docetaxel at our institution.14 Re-assessment of their radiologic scans using the Choi criteria showed that only 1 of 5 demonstrated a Choi PR, with median PFS of 6.1 months (range 1.6-9 months) further suggesting that standard chemotherapy regimens may only have limited efficacy in HPC/SFT.
The overall Choi response rate of 79% with temozolomide and bevacizumab observed in this retrospective review, therefore, seems to be much more favorable than that with standard chemotherapy regimens. Our study has the typical limitations of a retrospective analysis, including the possibilities of patient selection bias and observer bias, a small sample size, and the lack of a systematic, comprehensive recording of toxic effects. Nevertheless, the degree of Choi radiologic responses and the duration of PFS observed in our patients appear superior to those observed in historical studies with chemotherapy regimens.
The current evidenced-based method for response evaluation for soft tissue sarcomas is RECIST. However, several studies have demonstrated that RECIST may be insensitive for evaluating response in patients with gastrointestinal stromal tumors (GIST) treated with imatinib, and the Choi criteria have recently emerged as a more sensitive tool for assessing the degree of tumor necrosis in response to therapy in that setting.23, 24 Soft tissue sarcomas other than GIST, treated with cytotoxic or biologic therapies, display patterns of response similar to those of GISTs treated with imatinib, with patients exhibiting long time-to-progression periods despite a lack of significant reduction in their tumor size.28, 29 In fact, many invstigators who use RECIST to evaluate soft tissue sarcomas do not use response as an endpoint but only time to progression. Therefore, we chose to assess the activity of temozolomide and bevacizumab in HPC/SFT with the Choi criteria as well as RECIST. We believe that the Choi criteria, allows us to detect response--or lack of response--early in the course of treatment and thereby quickly identify potential non-responders who may benefit from switching to another therapy. In addition, because the Choi criteria for PD are more stringent than RECIST (≥10% increase in tumor size), failure of therapy may also be detected earlier. This earlier detection explains the shorter median PFS we found when using the Choi criteria than when using RECIST.
Although toxicity data were not systematically gathered, all available clinic notes and laboratory values were thoroughly reviewed to capture as many side effects as possible. The majority of the patients did not exhibit significant complications while receiving treatment as scheduled. We did not note any serious side effects (e.g., thromboembolic events, cardiac toxicity, or gastrointestinal bleeding) associated with bevacizumab. Although one patient died during treatment, we found no definitive evidence that temozolomide and/or bevacizumab directly contributed to the immediate factors that resulted in her death. Rather, the patient's treating physician (RSB.) believed that her overall poor performance status most likely led to her death.
Our patients received a wide range of number of doses of therapy. Most patients received treatment until PD or intolerable side effects developed, but a few patients were empirically given treatment breaks because of patient or physician preferences. It is difficult to conclude, based on the sample size, how long temozolomide-bevacizumab therapy should be continued or whether it could be interrupted and resumed without significantly reducing the therapeutic benefit. The optimal dosage and the schedule of temozolomide and bevacizumab in HPC/SFT are yet to be defined. Currently, both temozolomide and bevacizumab are used in several different dosages and schedules, in other malignancies, with no one clearly proven strategy. We chose our particular regimen based on anecdotal experience from our first patient. A prospective study to address these questions and to establish the optimal temzolomide-bevacizumab therapy's safety and efficacy is currently under development.
Also unclear is the potential additional benefit of radiation therapy. Three patients with isolated sites of disease had received radiation therapy within 4 months before or after temozolomide and bevacizumab. All had achieved Choi PR after with their first treatment modality. Two patients were still responding when they initiated their second treatment modality, while one was exhibiting tumor re-growth. Despite having discontinued their last treatment 18-23 months prior to the time of our analysis, all 3 continue to show a long, durable maintenance of their responses, with PFS of 34-43 months to date. Further information regarding the potential synergistic effect of temozolomide-bevacizumab therapy and radiation is needed to confirm this encouraging finding.
As we stated earlier, the rationale for the temozolomide and bevacizumab was empirical, as this combination therapy has not been used in soft tissue sarcoma. We do not know if the success of this regimen is due to temozolomide, bevaziumab or their synergistic effect. Bevacizumab may enhance temozolomide's cytotoxic activity or may play an antiangiogenic role by modulating the VEGF signaling pathway. The VEGF-VEGFR pathway, which plays a key mediator role in angiogenesis, has recently emerged as a potential key therapeutic target in HPC/SFT. The anti-VEGF receptor tyrosine kinase inhibitors sorafenib and sunitinib have also demonstrated evidence of activity in HPC/SFT.30-33 Patients treated with sunitinib have also demonstrated Choi responses lasting greater than 6 months, similar to patients described in our report.33 Anecdotal experiences with sorafenib in HPC/SFT patients have demonstrated Choi responses and disease stabilization for up to 22 months.30, 32 It is not yet clear whether these anti-VEGFR tyrosine kinase inhibitors will be just as effective as temozolomide-bevacizumab in HPC/SFT, but these initial results are encouraging. Further studies will be needed to better elucidate the key therapeutic targets in HPC/SFT. Analysis of our patients' available tumor specimens for potential molecular correlative factors is ongoing.
To our knowledge, our report represents the largest published series of patients with advanced HPC/SFT treated with a single systemic regimen to date. We found that the combination of temozolomide and bevacizumab had a remarkably high rate of Choi overall response and a favorable duration of disease control. For these rare sarcoma subtypes that lack a well-established systemic therapeutic option, temozolomide-bevacizumab is a promising therapeutic regimen that warrants further investigation. Our results should be validated in a prospective trial, which would also allow additional insight into the efficacy, safety, and biologic mechanisms of temozolomide and bevacizumab in HPC/SFT.
Figure 3.
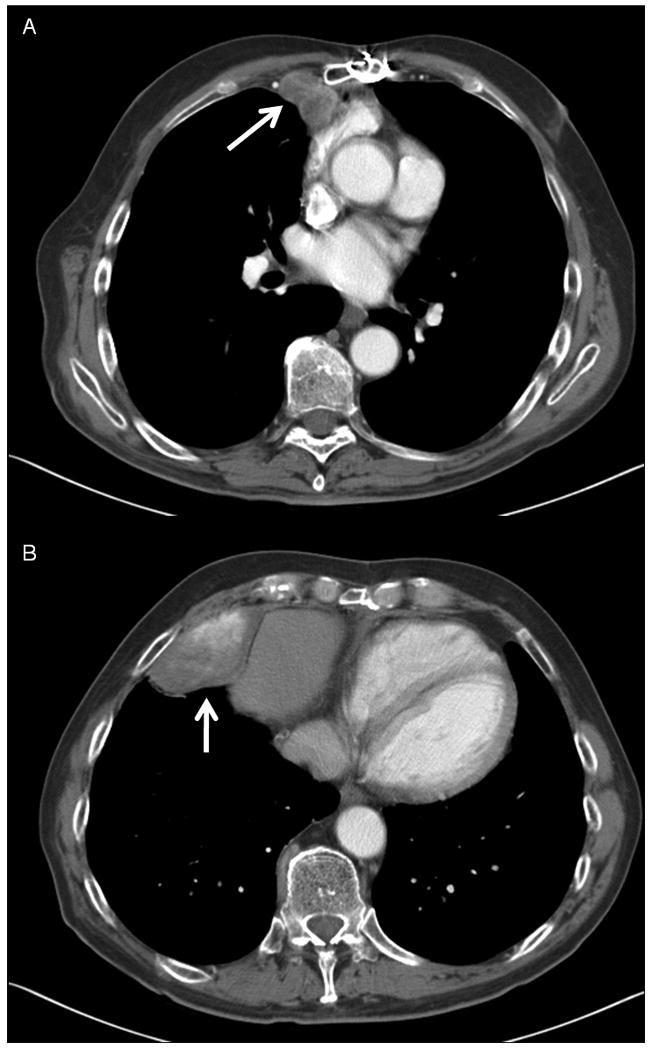
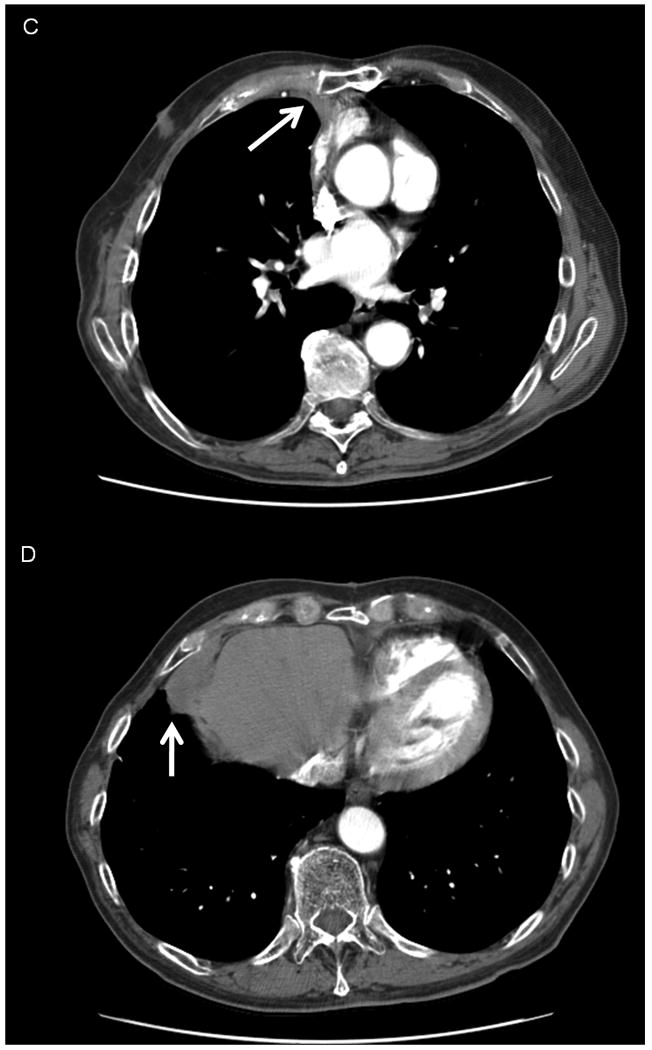
CT images demonstrating a Choi PR to temozolomide and bevacizumab in a patient with recurrent, unresectable SFT of pleura. Left, images show baseline disease at the start of therapy (3A, an anterior mediastinal mass measuring 3.3 cm, 63.5 HU and 3B, a lower anterior mediastinal mass measuring 7.2 cm, 100.0 HU). Right, images show the decrease in size and density of disease after 27 cycles of treatment. (3C, the mass now measuring 2.3 cm, 58.6 HU, and 3D, now measuring 4.1 cm, 44.0 HU).
Acknowledgments
Research support received for the study: This research is supported in part by the National Institutes of Health through MD Anderson's cancer Center Support Grant CA016672.
Footnotes
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL, and at the 14th Annual Meeting of the Connective Tissue Oncology Society, November 13-15, 2008, London, UK
Conflicts of interest: None
References
- 1.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48(1):3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 2.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48(1):63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger FM, Smith BH. Hemangiopericytoma. An analysis of 106 cases. Hum Pathol. 1976;7(1):61–82. doi: 10.1016/s0046-8177(76)80006-8. [DOI] [PubMed] [Google Scholar]
- 4.Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE. Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol. 1991;22(1):84–91. doi: 10.1016/0046-8177(91)90067-y. [DOI] [PubMed] [Google Scholar]
- 5.Park MS, Araujo DM. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol. 2009;21(4):327–31. doi: 10.1097/CCO.0b013e32832c9532. [DOI] [PubMed] [Google Scholar]
- 6.Spitz FR, Bouvet M, Pisters PW, Pollock RE, Feig BW. Hemangiopericytoma: a 20-year single-institution experience. Ann Surg Oncol. 1998;5(4):350–5. doi: 10.1007/BF02303499. [DOI] [PubMed] [Google Scholar]
- 7.Espat NJ, Lewis JJ, Leung D, Woodruff JM, Antonescu CR, Shia J, et al. Conventional hemangiopericytoma: modern analysis of outcome. Cancer. 2002;95(8):1746–51. doi: 10.1002/cncr.10867. [DOI] [PubMed] [Google Scholar]
- 8.Magdeleinat P, Alifano M, Petino A, Le Rochais JP, Dulmet E, Galateau F, et al. Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome. Eur J Cardiothorac Surg. 2002;21(6):1087–93. doi: 10.1016/s1010-7940(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 9.Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25(4):514–22. [PubMed] [Google Scholar]
- 10.Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004;100(7):1491–7. doi: 10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 11.Beadle GF, Hillcoat BL. Treatment of advanced malignant hemangiopericytoma with combination adriamycin and DTIC: a report of four cases. J Surg Oncol. 1983;22(3):167–70. doi: 10.1002/jso.2930220306. [DOI] [PubMed] [Google Scholar]
- 12.Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG. Management of recurrent meningeal hemangiopericytoma. Cancer. 1998;82(10):1915–20. [PubMed] [Google Scholar]
- 13.Chamberlain MC, Glantz MJ. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery. 2008;63(4):720–6. doi: 10.1227/01.NEU.0000325494.69836.51. author reply 26-7. [DOI] [PubMed] [Google Scholar]
- 14.Park MS, Lazar AJ, Trent JC, Conrad CA, Ludwig JA, Wang WL, et al. Combination therapy with temozolomide and bevacizumab in the treatment of hemangiopericytoma/solitary fibrous tumor: an updated analysis. 14th Connective Tissue Oncologic Society Annual Meeting; 2008. abstract #35064. [Google Scholar]
- 15.Constantinidou A, Jones RL, Scurr M, Al-Muderis O, Judson I. Systemic therapy in solitary fibrous tumour [abstract #39407]. 15th Connective Tissue Oncologic Society Annual Meeting; 2009. [Google Scholar]
- 16.Gottlieb JA, Benjamin RS, Baker LH, O'Bryan RM, Sinkovics JG, Hoogstraten B, et al. Role of DTIC (NSC-45388) in the chemotherapy of sarcomas. Cancer Treat Rep. 1976;60(2):199–203. [PubMed] [Google Scholar]
- 17.Trent JC, Beach J, Burgess MA, Papadopolous N, Chen LL, Benjamin RS, et al. A two-arm phase II study of temozolomide in patients with advanced gastrointestinal stromal tumors and other soft tissue sarcomas. Cancer. 2003;98(12):2693–9. doi: 10.1002/cncr.11875. [DOI] [PubMed] [Google Scholar]
- 18.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 20.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 21.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. http://www.clinicaltrials.gov/, September 1, 2010.
- 23.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25(13):1760–4. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher CDM, Unni KK, Mertens F. Tumours of Soft Tissue and Bone. Lyon: IARCPress; 2002. World Health Organization Classification of Tumours. Pathology and Genetics. [Google Scholar]
- 27.Kaplan E, Meier P. Nonparametric estimator from incomplete observations. J American Statistical Association. 1958;53:457–81. [Google Scholar]
- 28.Grosso F, Jones RL, Demetri GD, Judson IR, Blay JY, Le Cesne A, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8(7):595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 29.Casali PG, Messina A, Stacchiotti S, Tamborini E, Crippa F, Gronchi A, et al. Imatinib mesylate in chordoma. Cancer. 2004;101(9):2086–97. doi: 10.1002/cncr.20618. [DOI] [PubMed] [Google Scholar]
- 30.Ryan CW, von Mehren M, Rankin CJ, Goldbum JR, Demetri GD, Bramwell VH, et al. Phase II intergroup study of sorafenib (S) in advanced soft tissue sarcomas (STS): SWOG 0505. J Clin Oncol. 2008;26(May 20 suppl) abstr 10532. [Google Scholar]
- 31.Casali PG, Stacchiotti S, Palassini E, Marrari A, Morosi C, Messina A, et al. Evaluation of the antitumor activity of sunitinib malate (SM) in solitary fibrous tumor (SFT) J Clin Oncol. 2009;27(15S) doi: 10.1093/annonc/mds143. suppl; abstr 10571. [DOI] [PubMed] [Google Scholar]
- 32.Maki RG, D'Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27(19):3133–40. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]


