Abstract
The present study examined whether continued access to methamphetamine or food reinforcement changed economic demand for both. The relationship between demand elasticity and cue-induced reinstatement was also determined. Male Long-Evans rats lever-pressed under increasing fixed-ratio requirements for either food pellets or methamphetamine (20 μg/50 μl infusion). For two groups, demand curves were obtained before and after continued access (12 days, 2-hr sessions) to the reinforcer under a fixed-ratio 3 schedule. A third group was given continued access to methamphetamine between determinations of food demand and a fourth group abstained from methamphetamine between determinations. All groups underwent extinction sessions, followed by a cue-induced reinstatement test. Although food demand was less elastic than methamphetamine demand, continued access to methamphetamine shifted the methamphetamine demand curve upward and the food demand curve downward. In some rats, methamphetamine demand also became less elastic. Continued access to food had no effect on food demand. Reinstatement was higher after continued access to methamphetamine relative to food. For methamphetamine, elasticity and reinstatement measures were correlated. We conclude that continued access to methamphetamine – but not food – alters demand in ways suggestive of methamphetamine accruing reinforcing strength. Demand elasticity and reinstatement measures appear to be related indices of drug-seeking.
Keywords: food, methamphetamine, rat, reinstatement, behavioral economics, demand curve, self-administration
Introduction
Over the last two decades, methamphetamine addiction has increased to the point where illicit use of the drug worldwide is second only to cannabis (United Nations Office on Drugs and Crime, 2009). In the United States, methamphetamine abuse remains a significant public health problem (Gonzales et al., 2010), with approximately 850,000 people aged 12 and older reporting use of the drug in 2008 (Substance Abuse and Mental Health Services Administration, 2009). Methamphetamine addiction is associated with myriad physical and mental health problems, including impairments in executive functions underlying everyday activities (Henry et al., 2010; Cruickshank et al., 2009; Darke et al., 2008; Maxwell, 2005; Meredith et al., 2005).
The transition from substance use to dependence is in part characterized by allocating more time and resources to the procurement and use of drugs at the expense of occupational, social, or recreational activities (American Psychiatric Association, 2000). This pattern suggests that the relative reinforcing value of drugs increases with continued use. Ongoing work in the area of behavioral economics has shed light on the basic behavioral and economic processes governing such resource allocation (for a recent review, see Murphy et al., 2009). Fundamental to this field, a demand curve plots the consumption of a commodity as a function of its price (Pearce, 1992). In animal models, price can be operationalized as the prevailing response requirement (Hursh, 1980). To obtain a demand curve for a self-administered drug, the fixed-ratio (FR) response requirement needed to obtain a single drug infusion is manipulated across sessions, and total drug consumption (e.g., number of infusions per session) is measured at each ratio (Bickel et al., 1990). The rate at which consumption decreases with increases in price is termed elasticity of demand (Hursh, 1980). Commodities associated with less elastic demand (whereby consumption decreases more slowly with increases in price) are considered to be more effective reinforcers than commodities with greater demand elasticity. With respect to drug self-administration, it has been argued that elasticity of demand may correspond, in part, to abuse liability (Hursh et al., 2005; Hursh and Winger, 1995; Hursh, 2000, 1993, 1991).
Hursh and Silberberg (2008) proposed an exponential model to quantify economic demand. Christiansen et al. (2008a) subsequently demonstrated that the model adequately accounted for data variance in both food and cocaine consumption across increasing ratios. In this equation
Q represents the number of reinforcer deliveries at each FR, or price (P); Q0 is an estimate of consumption at zero-price and mathematically represents the y-intercept; k is a scaling parameter representing the range of the dependent variable in logarithmic units; and α provides an index of elasticity of demand. As α increases, demand becomes more elastic and consumption decreases more quickly with price increases. Conversely, smaller values of α reflect less elastic curves in which consumption decreases more slowly with increases in price. Hursh and Silberberg (2008) argued that α indexes reinforcing strength and represents the essential value of a commodity.
In order to compare qualitatively different commodities, such as food and drugs, and to adjust consumption for individual subject differences such as body weight, demand curves often are normalized according to a method described by Hursh and Winger (1995). A unit metric (d) representing one percent of the total consumption at the smallest FR is calculated for each subject. This unit metric is then used to calculate a normalized price (P/d) and normalized consumption (Q x d). These normalized terms then replace P and Q in the above equation.
Using a normalized demand analysis, Christensen et al. (2008a) demonstrated that demand for food was much less elastic than demand for cocaine, a result inconsistent with lay notions that the hedonic value of abused drugs is more powerful than that of so-called natural rewards, such as food or water. Similar results have been obtained in studies arranging choice between cocaine and sweetened water, in that most rats prefer sweetened water to cocaine (Cantin et al., 2010; Lenoir et al., 2007). Cantin et al. (2010) observed that the minority of rats that prefer cocaine to sweetened water corresponds to the epidemiology of human cocaine use in that only a minority of people who use the drug go on to develop a substance dependence disorder.
In addition to individual differences in the vulnerability to cocaine reinforcement, Christensen et al. (2008b) postulated that the reinforcing value of cocaine might increase as a function of extended exposure to the drug. To this end, they examined how rats’ food and cocaine (1 mg/kg/infusion) demand changed as a function of continued access to these commodities. Sessions were composed of eight 15-min components. Using block randomization, in half of these components, responding produced cocaine and in the other half, responding had no scheduled consequences. A stimulus light signaled cocaine components. In another group of rats, the same procedure was employed with food reinforcement. For both groups, the FR increased across sessions in order to measure demand. Subsequently, the reinforcer (cocaine or food) was made available under an FR 3 using the same block randomization procedure for seven consecutive sessions (continued access). Finally, the demand curves were redetermined. Normalized demand for cocaine was found to be less elastic (lower α values) after continued access, whereas demand for food was unchanged. These results suggest that continued access to cocaine self-administration increases the reinforcing strength of cocaine as indexed by demand elasticity, and that this result is specific to drug reinforcers.
Here, we focused on demand elasticity with methamphetamine or food as the reinforcers. We used 2-h daily self-administration sessions because, as discussed in Orio et al. (2010), psychostimulant self-administration sessions shorter than three hours usually constitute limited access and result in stable patterns of intake. We utilized a 20 μg/50 μl bolus infusion of methamphetamine because this dose has the most widely studied in rat methamphetamine self-administration experiments (Anggadiredja et al., 2004; Roth and Carroll, 2004; Xi and Kruzich, 2007; Rogers et al., 2008; Schwendt et al., 2009; Reichel and See, 2010; Parsegian et al., 2011; Reichel et al., 2011), allowing clearer comparisons to be made across studies.
In addition to studying food and methamphetamine demand both before and after continued access, we also determined if demand for food changes after continued access to methamphetamine. In order to rule out possible history effects not associated with continued access, methamphetamine demand was also assessed before and after a period of abstinence equal to the duration of the continued-access condition. Finally, we determined relationships between continued access, demand elasticity, and the degree of cue-induced reinstatement following extinction. Reinstatement procedures are commonly used as an animal model of drug- or food-seeking during relapse (Shaham et al., 2003; Nair et al., 2009). We predicted that the degree of reinstatement would negatively correlate with elasticity of demand. That is, demand that is less elastic should be associated with a greater degree of reinstatement following extinction. To date, this relationship has not been examined.
Methods
Subjects
Thirty-six adult male Long-Evans rats (Charles-River) were individually housed on a reversed 12:12 light-dark cycle in a temperature- and humidity-controlled vivarium. Rats received free access to water throughout the study and were maintained at 85% of their free-feeding weights through post-session feedings of standard rat chow (Harlan, Indianapolis, IN, USA) that occurred at least 30 min following sessions. The mean weight of the rats was 373 g with a range of 330 g to 437 g at the beginning of the experiment. Procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and approved by the IACUC of the Medical University of South Carolina.
Apparatus
Self-administration chambers (30×20×20 cm, Med Associates) were housed inside sound-attenuating cubicles fitted with a fan for airflow and masking noise. Each chamber contained two retractable levers, two stimulus lights, a speaker for tone delivery, and a house light to provide general illumination. Additionally, each chamber was equipped with a balanced metal arm and spring leash attached to a swivel (Instech). Tygon® tubing extended through the leash and was connected to a 10 ml syringe mounted on an infusion pump located outside the sound-attenuating cubicle.
Preliminary Training
All rats were trained to press the right lever for 45-mg food pellets using a procedure developed by Taylor et al. (2010) that gradually increased the response requirement within daily 2-hr sessions based on an interresponse time criterion. Training continued until responding was maintained under a FR 30 schedule.
Surgery
Following preliminary training, half of the rats underwent catheter implantation surgery, followed by a recovery period. Anesthesia consisted of injections (IP) of ketamine (66 mg/kg; Vedco Inc, St Joseph, MO, USA), xylazine (1.3 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA), and equithesin (0.5 ml/kg). Ketorolac (2.0 mg/kg, IP; Sigma, St. Louis, MO, USA) was given just prior to surgery as an analgesic. One end of a silastic catheter was inserted into the external right jugular and secured with 4.0 silk sutures. The other end ran subcutaneously and exited from a small incision just below the scapula. This end attached to an infusion harness (Instech Solomon, Plymouth Meeting, PA, USA) that provided access to an external port for IV drug delivery. An antibiotic solution of cefazolin (10 mg/0.1 ml; Schein Pharmaceuticals, Florham Park, NJ, USA) was given post surgery and during recovery along with 0.1 ml 70 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ, USA). Rats were allowed to recover for a minimum of 5 days following surgery. During the first 3 days of recovery, food was available ad libitum. Subsequently, food restriction was introduced to maintain weight at the 85% level.
Group Assignments
Rats were assigned to one of four experimental groups. Groups are labeled according to the reinforcer earned in each of the first three conditions of the experiment.
Food-Food-Food (FFF; n=9)
A demand curve for food was obtained before and after continued access (12 sessions) to food under an FR 3 schedule.
Methamphetamine-Methamphetamine-Methamphetamine (MMM; n=11)
A demand curve for methamphetamine hydrochloride (Sigma, St. Louis, MO, USA) was obtained before and after continued access (12 sessions) to the drug under an FR 3 schedule.
Food-Methamphetamine-Food (MMM; n=9)
A demand curve for food was obtained before and after continued access (12 sessions) to methamphetamine under an FR 3 schedule.
Methamphetamine-(Nothing)-Methamphetamine (M-M; n=7)
A demand curve for methamphetamine was obtained twice. Rats did not participate in daily experimental sessions during the second experimental condition, but were transported in their home cages to the hallway immediately outside of the experimental rooms, weighed, and had their catheters flushed during their normal session time. This was done to maintain as high a degree of similarity between experimental groups as possible.
Self-administration
Sessions lasted 2 h and began with the illumination of the house light and insertion of the two levers into the chamber. Only the right lever was active; presses on the left lever had no scheduled consequences. For rats responding for food, each completion of the ratio requirement resulted in the delivery of a 45-mg pellet. For rats self-administering methamphetamine, completion of the ratio requirement resulted in activation of the pump for a 2-s infusion (20 μg/50 μl bolus infusion). For all rats, reinforcer deliveries were accompanied by the presentation of a stimulus complex consisting of a 5-s tone (78 dB, 4.5 kHz) and a white stimulus light over the right lever, followed by a 20-s time out. Responses occurring during the time out were recorded, but had no scheduled consequences. The offset of the house light and retraction of the levers signaled the end of the session. Sessions took place during the dark cycle and were conducted 6 days/week.
Rats self-administering methamphetamine received an i.v. infusion (0.1 ml) of 10 U/ml heparinized saline before each session. After each session, catheters were flushed with cefazolin and 0.1 ml 70 U/ml heparinized saline. Catheter patency was periodically verified with methohexital sodium (10 mg/ml dissolved in 0.9% physiological saline; Eli Lilly, Indianapolis, IN, USA), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
Demand curves and continued access
A demand curve was first obtained for either food (FFF, FMF) or methamphetamine (MMM, M-M). Across sessions, the FR requirement increased every two sessions according to the following series: 3, 10, 18, 32, 56, 100, 178, 320, 560. If there was considerable variability in the number of reinforcers earned at a particular ratio, three or more sessions were conducted, and the most discrepant result was excluded from the data analysis. If there was not an easily identifiable discrepant result via visual analysis, all of the data were included. The ratio increased until zero reinforcers were earned for at least one of the two sessions, or until the FR 560 was reached.
Following the first demand curve determination, rats in the FMF group underwent jugular catheter surgery and at least 5 days of recovery as described above. Rats in the other groups did not participate in experimental sessions during this time. For catheterized rats, catheters were flushed daily with cefazolin and 0.1 ml 70 U/ml heparinized saline to maintain catheter patency.
Subsequently, rats responded for food (FFF) or methamphetamine (MMM, FMF) according to an FR 3 schedule for 12 consecutive sessions (continued access). Rats in the M-M group were not studied during this condition, but continued to have their catheters flushed daily as described above. At the conclusion of this condition, demand curves for food (FFF, FMF) and methamphetamine (MMM, M-M) were redetermined.
Extinction and reinstatement
Extinction began immediately following the completion of the second demand curve. Rats were placed in the self-administration chamber for daily 2-h sessions and responding had no scheduled consequences. A minimum of 10 extinction sessions was conducted. Thereafter, the extinction condition ended when for two consecutive sessions, the number of responses on the right (previously active) lever was ≤ 25% of the average number of responses obtained under the FR 3 schedule during the first two sessions of the second demand curve. For those rats not meeting this criterion, a maximum of 25 extinction sessions were conducted.
Following extinction, animals were tested for cue-induced reinstatement, during which time active lever presses resulted in presentation of the light-tone stimulus complex according to an FR 3 schedule, followed by a 20-s timeout. Thus, the schedule of reinforcement was identical to the one described during continued access, except that the primary reinforcer (food or methamphetamine) was not delivered.
Data Analysis
For demand curve analyses, we analyzed the number of reinforcer deliveries per session as a function of the FR. Because reliable responding was not maintained in the majority of rats beyond FR 320 (food) and 100 (meth), these ratios were used as endpoints in the demand curve determinations. In cases where individual rats did not earn a reinforcer at a particular ratio, a value of 0.1 was assigned because the log of zero is undefined.
Best-fitting functions using the exponential demand model (Hursh and Silberberg, 2008) were fit to the normalized and nonnormalized averaged group data on log-log coordinates using GraphPad Prism 4 (GraphPad Software, La Jolla, CA). For all curve fits, the k parameter was set at a common value of 3, because this is the smallest integer power of 10 that results in an ordinate spanning the data range. For both normalized and nonnormalized analyses, we also fit the exponential equation to the individual subject data and obtained the best-fitting α and Q0 parameters for each rat in both the first and second demand curve determinations. We included the Q0 parameter in our analyses because it is possible that demand curves could shift vertically without elasticity changes. For each group, we determined if the α and Q0 parameters differed between the first and second demand curve determinations using Wilcoxon matched-pairs tests. Between-group food and methamphetamine comparisons of the α and Q0 parameters were accomplished using Mann-Whitney U tests.
The use of normalized demand eliminates the need to adjust consumption measures for body weight. For methamphetamine, however, intake during the continued-access condition was adjusted for body weight (mg/kg/day). The results from this condition, and subsequent results from extinction and reinstatement that used total active responses as the dependent variable, were analyzed with ANOVA. When statistical assumptions were violated, we conducted nonparametric tests when possible. If nonparametric tests did not exist for the analysis, we first performed a logarithmic transformation on the data to approximate a normal distribution before conducting ANOVA. All post-hoc analyses were conducted using Tukey multiple-comparison tests with the significance set at p < .05.
Results
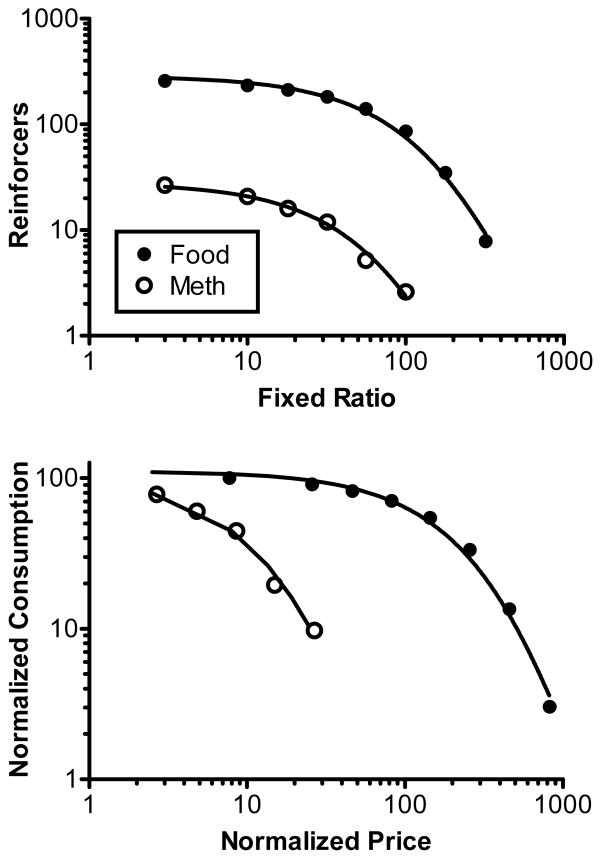
Fig. 1 shows nonnormalized (upper panel) and normalized (lower panel) demand curves for food and methamphetamine in the first determination of the demand curves. Food demand is based on the combined results from the FFF and FMF groups; methamphetamine demand is based on the combined results from the MMM and M-M groups. The Q0 and α values associated with these curves, along with goodness-of-fit (r2), are shown in Tables 1 (nonnormalized) and 2 (normalized). Subsequent fits of the equation to the results from each rat individually revealed that the food demand curve was associated with a higher Q0 parameter (Mann-Whitney, p < 0.001) and a lower α parameter (Mann-Whitney, p < 0.001). Thus, demand for food started at a higher level and was less elastic than demand for methamphetamine.
Fig. 1.
Upper panel: Mean number of food (closed circles) and methamphetamine (open circles) reinforcer deliveries plotted as a function of the prevailing response requirement on a log-log scale. Curved lines represent the best-fit functions using the exponential demand equation. Lower panel: Normalized demand curves for food and methamphetamine.
Table 1.
Estimated parameters and goodness-of-fit of the exponential demand model
| Group a | Determination b | Q0 | α | r2 |
|---|---|---|---|---|
| Food | First | 286.5 | 7.5E-6 | .99 |
| Meth | First | 28.1 † | 1.6E-3 † | .99 |
| FFF | First | 278.3 | 7.5E-6 | .99 |
| Second | 286.5 | 7.1E-6 | .99 | |
| MMM | First | 29.4 | 1.4E-4 | .99 |
| Second | 39.8 ** | 1.1E-4 | .99 | |
| FMF | First | 298.3 | 7.7E-6 | .97 |
| Second | 281.4 * | 8.9E-6 | .98 | |
| M-M | First | 26.1 | 1.8E-4 | .99 |
| Second | 27.5 | 1.5E-4 | .98 |
Note.
Food and Meth represent the first determination of food and methamphetamine demand shown in the top panel of Fig. 1. Food is based on the averaged results from the 18 rats in the FFF and FMF groups. Meth is based on the averaged results from the 18 rats in the MMM and M-M groups. The results from which individual group demand curves were derived are shown in Fig. 3.
The first determination refers to the demand curve obtained prior to continued access and the second determination refers to the demand curve obtained following continued access (Groups FFF, MMM, FMF) or abstinence (M-M). Asterisks represent differences between the first and second determinations that meet the p < .1 (*) or p < .01 (**), levels of significance.
The † symbol represents significant differences between the initial food and methamphetamine demand curves (p < .001).
The normalization process results in consumption at the smallest normalized price investigated equaling 100. Therefore, the Q0 parameter approximated this value and did not differ significantly between food and methamphetamine. Subsequent fits of the exponential equation to the normalized results from each rat individually revealed that the food demand curve was again associated with a lower α parameter (Mann-Whitney, p < 0.001).
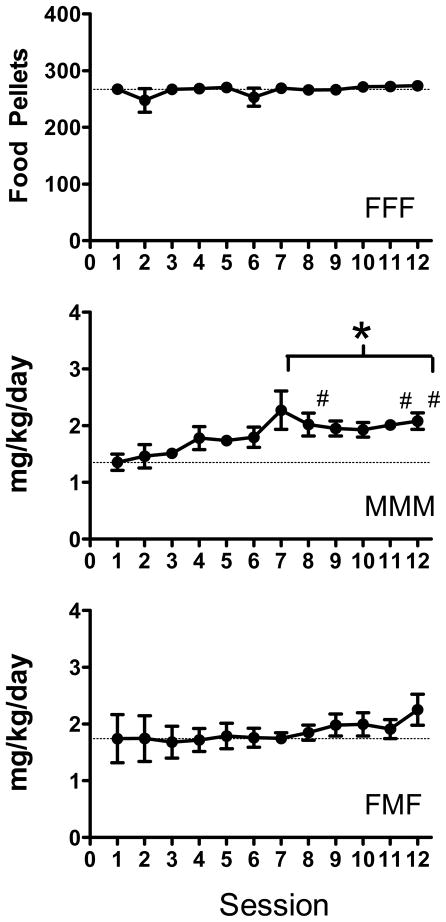
Fig. 2 shows the mean (± SEM) consumption of food and methamphetamine under the FR 3 schedule during the 12 sessions of continued access. Food consumption is expressed as the number of pellets earned per session, while methamphetamine consumption is expressed as drug intake (mg/kg/day). Statistical comparisons focused on each group separately to determine if intake increased across the 12 sessions. For each group, a Friedman repeated measures analysis of variance (ANOVA) on ranks was conducted. Consumption increased significantly only in the MMM group (χ2 11 = 55.59, p < 0.001). For this group, post-hoc comparisons on the ranks revealed that intake was significantly higher during Sessions 7–12 relative to Session 1 and also during Sessions 8, 11, and 12 relative to Sessions 2 and 3. Consumption in the other groups remained relatively unchanged across sessions; the increase in consumption during Session 12 for the FMF group is due to one rat approximately doubling its usual consumption on this day, and does not reflect a general pattern.
Fig. 2.
Mean (± SEM) consumption of food or methamphetamine during the 12 sessions of continued access. Symbols represent significant differences (p < .05) during Sessions 7–12 relative to Session 1 (*) and also Sessions 8, 11, and 12 relative to Sessions 2 and 3 (#).
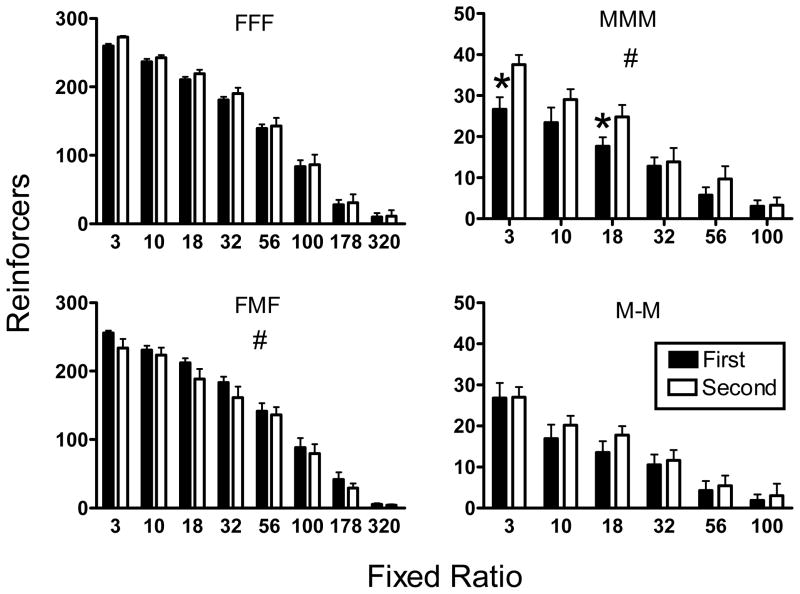
Fig. 3 shows the mean (± SEM) number of food or methamphetamine reinforcers earned as a function of the FR for each group in the first and second determinations of the demand curves. For statistical comparisons, each group was analyzed separately. A two-factor repeated measures ANOVA was conducted with both Determination and FR as repeated measures. For all groups, there was a signficant main effect (p < 0.001) of FR; reinforcer deliveries decreased as the ratio increased. For the MMM group, there was a significant main effect of Determination (F 1, 10 = 8.23, p < 0.05). Rats earned more methamphetamine infusions in the second determination than the first. Post-hoc comparisons revealed that this difference was significant at FR 3 and FR 18. In contrast, the number of reinforcer deliveries significantly decreased from the first to the second determination in the FMF group (F 1, 8 = 6.57, p < 0.05). These rats consumed fewer food pellets after a history of methamphetamine self-administration. While significant overall, post-hoc tests revealed that the decrease in intake was not significant at any individual FR values; however the difference at FR 3, 18, and 32 is suggestive of a trend (p < 0.1). Consumption in the other two groups generally increased slightly at most FR values, but this increase was not statistically significant.
Fig. 3.
Mean (± SEM) number of food or methamphetamine reinforcers earned as a function of the prevailing response requirement for each group in the first (closed bars) and second (open bars) determinations of demand. The (#) symbol represents a significant (p < .001) main effect of determination. The (*) symbol represents significant differences (p < .05) between determinations at individual ratios.
The exponential demand model was fit to the individual subject data shown in Fig. 3. The Q0, α, and r2 values associated with the first and second determination of these curves as a function of group are shown in Table 1. For each group, we conducted two Wilcoxon matched-pairs tests, one comparing the change in the α parameter and the other comparing the change in the Q0 parameter. For all groups, there was no significant difference in the α parameter between determinations. For the FFF, MMM, and M-M groups, the Q0 parameter was higher in the second determination, but only in the MMM group was this difference significant (p < 0.01). For the FMF group, the Q0 parameter was lower in the second determination; while suggestive, this decrease did not meet statistical significance (p < 0.1).
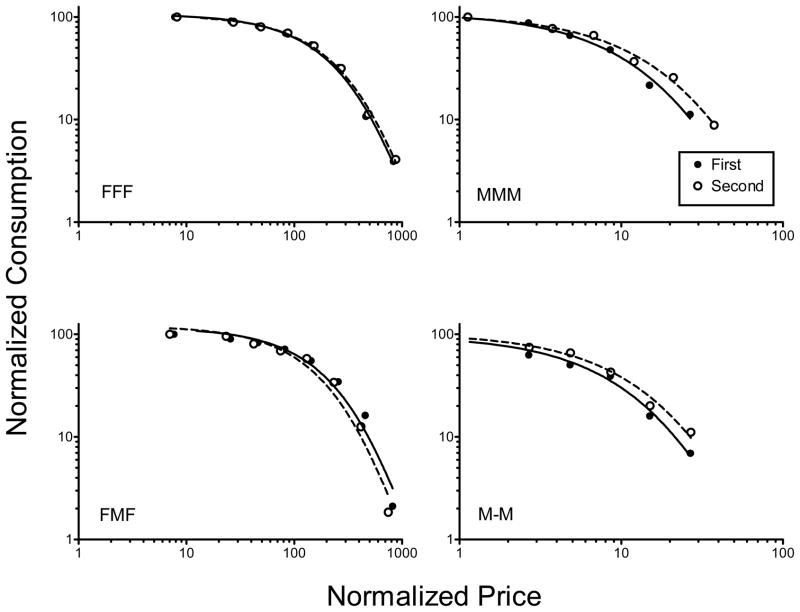
Fig. 4 shows normalized demand curves for each group. The Q0, α, and r2 values associated with the first and second determination of these curves as a function of group are shown in Table 2. For both the MMM and M-M groups, the second determination of the demand curve was slightly less elastic than the first. In addition, after continued access to methamphetamine, demand for food became slightly more elastic in the FMF group. However, Wilcoxon matched-pairs tests revealed that these small changes in elasticity were not statistically significant.
Fig. 4.
Normalized food and methamphetamine demand curves as a function of group and determination.
Table 2.
Estimated parameters and goodness-of-fit of the normalized exponential demand model
| Groupa | Determination | Q0 | α | r2 |
|---|---|---|---|---|
| Food | First | 111.1 | 7.5E-6 | .99 |
| Meth | First | 105.1 | 1.6E-4† | .99 |
| FFF | First | 107.3 | 7.5E-6 | .99 |
| Second | 105.0 | 7.1E-6 | .99 | |
| MMM | First | 110.3 | 1.4E-4 | .99 |
| Second | 106.4 | 1.1E-4 | .99 | |
| FMF | First | 116.6 | 7.7E-6 | .97 |
| Second | 120.4 | 8.9E-6 | .97 | |
| M-M | First | 97.9 | 1.9E-4 | .99 |
| Second | 102.4 | 1.5E-4 | .98 |
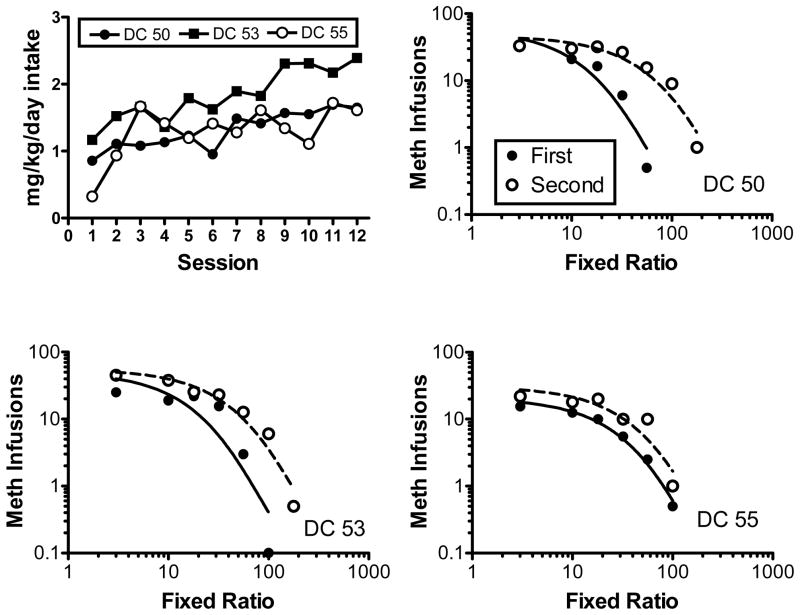
Elasticity of demand (α) did not change as a function of continued access to either food or methamphetamine. As a follow-up analysis, we investigated if the α parameter changed in individual rats that increased their intake during the continued-access condition. As shown in Fig. 2, only the MMM group significantly increased their intake during continued access. Of these 11 rats, only a subset demonstrated a consistent increase across sessions. We selected the three rats that increased their intake to the greatest extent for the additional analysis. For these rats, the upper-left panel of Fig. 5 shows methamphetamine consumption (mg/kg/day) across the continued-access condition. The remaining panels show the first and second determinations of the demand curve. The associated Q0, α, and r2 values are shown in Table 3. For each rat, the α parameter was lower in the second determination relative to the first. After continued access, demand for methamphetamine became less elastic, decreasing at a slower rate with increases in the response requirement.
Fig. 5.
Individual subject data from the three rats in the MMM group that demonstrated the greatest increase in methamphetamine intake during continued access. The upper left panel shows consumption during continued-access sessions. The remaining panels show demand curves for methamphetamine before (first; closed symbols – solid lines) and after (second; open symbols – dashed lines) continued access to methamphetamine.
Table 3.
Estimated parameters and goodness-of-fit of the exponential demand model for selected rats self-administering meth before (first) and after (second) the continued-access condition.
| Rat | Determination | Q0 | α | r2 |
|---|---|---|---|---|
| DC 50 | First | 59.2 | 2.7E-4 | .91 |
| Second | 46.4 | 7.9E-5 | .93 | |
| DC 53 | First | 51.1 | 2.4E-4 | .83 |
| Second | 55.6 | 9.1E-5 | .95 | |
| DC 55 | First | 20.6 | 3.5E-4 | .98 |
| Second | 30.7 | 1.8E-4 | .87 |
Note. Results are shown for the three rats selected for analysis, which increased their meth in take to the greatest extent over the continued-access period.
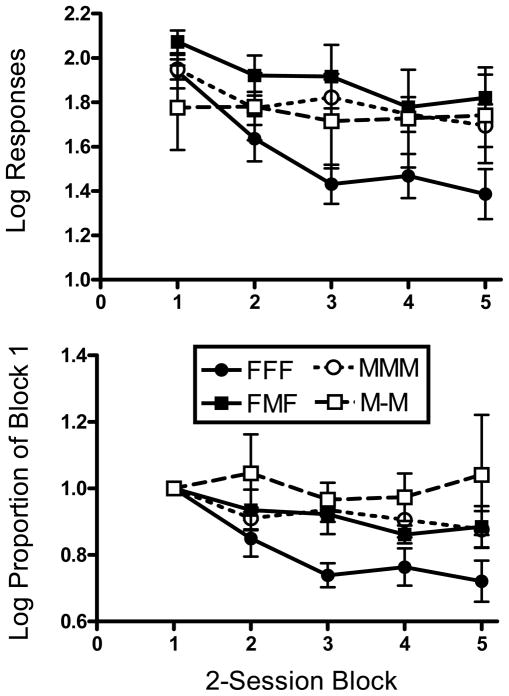
Fig. 6 shows responding during the first 10 sessions of extinction as a function of group. Only the first 10 sessions were included in this analysis, because some rats did not experience additional sessions. Due to highly variable and skewed extinction responding, we averaged the number of responses made during extinction into five 2-session blocks and then conducted a logarithmic transformation of these data. The log mean (± SEM) number of responses made in extinction as a function of group and block is shown in the upper panel of Fig. 6. The results were analyzed with a two-factor repeated measures ANOVA with Group as a between-subjects variable and Block as a repeated measure. There was a significant main effect of Block (F 4, 24 = 6.05, p < 0.001). Collapsed across Group, post-hoc tests revealed that extinction responses were significantly lower in Blocks 3, 4, and 5 relative to Block 1. No other differences between blocks were statistically significant. There also was a significant main effect of Group (F 3, 124 = 2.84, p = 0.05). Collapsed across Blocks, post-hoc tests revealed that the FFF group made fewer extinction responses than the MMM and the FMF groups. No other group differences were statistically significant.
Fig. 6.
Upper panel: Mean (± SEM) of the logarithms of the number of responses made during the first 10 sessions of extinction, sorted into 2-session blocks. Lower panel: The data in the upper panel are expressed as a proportion of Block 1 responding.
For each group, the lower panel in Fig. 6 expresses the logarithmic response data as a proportion of the log responses made in Block 1. Because the groups had different levels of responding for food and methamphetamine during the demand condition (food maintained considerably more responding), we wanted to express extinction responding for each group as a proportion of the respective baseline level of responding. However, we did not have a true baseline prior to extinction, as rats simply moved from the second demand condition to extinction. Because rats were exposed to different FR values and may or may not have earned reinforcers in the final session of the demand condition, this session could not be used as a suitable index of baseline responding. Therefore, we selected the first 2-session block of extinction as an arbitrary baseline and examined the proportional changes in responding thereafter. The results were analyzed with a two-factor repeated measures ANOVA, with Group as a between-subjects variable and Block as a repeated measure. Block 1 was excluded from the analysis. The main effect of Block and the Group × Block interaction were not significant. There was, however, a significant main effect of Group, (F 3, 93 = 3.78, p < 0.05). Collapsed across Blocks, post-hoc tests revealed that the FFF group had significantly lower proportional rates of extinction responding than each of the other groups.
The extinction condition lasted for a minimum of 10 sessions and until the number of responses emitted in a session was ≤ 25% of the responses emitted under the FR 3 schedule during the second determination of the demand curve. A maximum of 25 sessions was conducted if this criterion was not met. A one-way ANOVA on the number of extinction sessions conducted revealed a significant main effect of group (F 3, 40 = 18.70, p < 0.001). Post-hoc tests revealed that significantly more sessions were conducted in the MMM group relative to the FFF and FMF groups, and in the M-M group relative to the FFF and FMF groups.
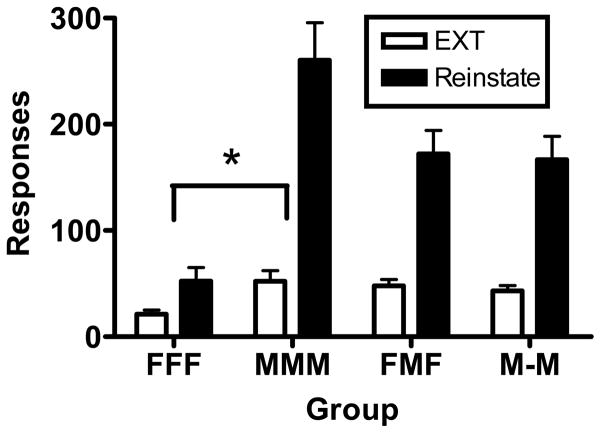
Fig. 7 shows the mean (± SEM) number of responses made during the final two sessions of extinction and the cue-induced reinstatement test as a function of group. Because the extinction data were variable for those rats that did not meet the stability criterion, for each rat we took the average number of responses made during the final two extinction sessions as representative of terminal performance. A two-factor repeated-measures ANOVA was conducted on a logarithmic transformation of these data with Group as a between-subjects variable and Condition as a repeated measure. The main effects of both Group (F 3, 31 = 6.92, p < 0.01) and Condition (F 1, 31 = 139.57, p < 0.001) were significant, as was the Group × Condition interaction (F 3, 31 = 4.09, p < 0.05). To explore this interaction, we conducted a series of follow-up ANOVAs comparing two groups per analysis. The Group × Condition interaction was significant when the FFF group was compared to the MMM group (F 1, 18 = 17.97, p < 0.001). Rats given continued access to methamphetamine showed a greater increase in responding from extinction to reinstatement than rats given continued access to food. While not significant in the other comparisons, results suggestive of an interaction were obtained in the FFF/FMF comparison (p = 0.09) and the MMM/M-M comparison (p = 0.07). Thus, continued access to methamphetamine appeared to enhance cue-induced reinstatement.
Fig. 7.
Mean (± SEM) number of responses made during the final two sessions of extinction (averaged in one column) and the cue-induced reinstatement test as a function of group. The relative increase in responding from extinction to reinstatement was significantly less (p < .05) for the FFF group (*) relative to the MMM group.
Table 4 shows the relationship between the degree of cue-induced reinstatement and the Q0 and α parameters from the nonnormalized exponential demand model for both food and methamphetamine using the second determination of demand. For methamphetamine, the results from the MMM and M-M groups were combined. For food, we analyzed only the results from the FFF group because the FMF group had a history of the same cues being paired with both food and methamphetamine. Spearman’s rank-order correlations revealed a significant negative correlation between the α ranks and the reinstatement ranks for the methamphetamine groups (ρ = −.68, p < 0.01). Rats exhibiting relatively inelastic demand (low α) for meth, but not food, had a greater degree of cue-induced reinstatement. No other correlations were significant.
Table 4.
Spearman rho correlations on ranks (and corresponding p-values) between the number of responses in reinstatement and the estimated Q0 and α parameters from the nonnormalized exponential demand model.
| Food (n = 9)
|
Meth (n = 18)
|
|||
|---|---|---|---|---|
| Q0 | α | Q0 | α | |
| Reinstatement | .06 (.88) | .10 (.81) | .20 (.42) | −.68(.002)** |
Note. Estimated parameters from the second determination of the demand curves were used in these calculations. The food results are based on the FFF group. The methamphetamine results are based on both the MMM and M-M groups. Spearman’s rank-order correlations revealed a significant negative correlation between the α ranks and the reinstatement ranks only in the meth groups (**p < .01).
Discussion
Demand for methamphetamine was more elastic than demand for food, consistent with previous results comparing cocaine and food (Christensen et al., 2008a,b) and demonstrating that rats better defend their food – relative to drug – consumption against increasing prices by increasing their response output. This result is probably due to the fact that food is a biological necessity, although rats also have a much longer history of consuming food than drugs and the protocol in this study maintained rats in a state of mild food deprivation. The fact that food is actually a less elastic commodity than drugs is not as obvious in human drug addicts, because the price of food usually is relatively cheap and varies little. Drug users might spend the majority of their income on drugs since it requires a relatively smaller percentage of their income to satisfy basic needs such as food.
Continued access to food did not change the food demand function. Unlike Christensen et al. (2008b), however, continued access to methamphetamine did not significantly lower the normalized α parameter. Although the normalized demand functions for methamphetamine became less elastic in the second determination, this difference was not significant and not necessarily a result of continued access because a similar elasticity shift was obtained in the M-M group. One possible reason why we failed to observe a consistent shift in drug elasticity with continued access is the considerably longer duration of action for methamphetamine as compared to cocaine. These direct effects of the drug on responding might have masked elasticity changes. Moreover, the study of Christensen et al. (2008b) incorporated timeout periods within their experimental sessions. Thus, possible response suppression due to direct drug effects may have been more prevalent in the current study.
Continued access to methamphetamine in the MMM group produced a significant upward shift in the methamphetamine demand curve as indexed by the Q0 parameter. Christensen et al. (2008b) reported similar upward shifts in nonnormalized cocaine demand after continued access, an effect that could reflect tolerance. Using a rhesus monkey drug self-administration procedure, Hursh and Winger (1995) demonstrated that when the number of cocaine infusions was expressed as a function of the response requirement, decreasing the dose resulted in an increased number of infusions earned at lower prices and a decreased number of infusions earned at higher prices. Based on these results, tolerance to methamphetamine would be indicated by an increase in both the Q0 and α parameters. That is, rats would consume more methamphetamine at lower prices but not defend this consumption at higher prices because the dose is functionally smaller. While increases in the Q0 parameter were obtained, we actually found slight decreases in the α parameter. In the second determination of demand, rats usually earned more methamphetamine infusions at all response requirements. As a result, the entire demand curve – not just consumption at the lowest price – shifted upward, an effect not predicted by a tolerance account.
In a review of the demand curve literature, Bickel et al. (2000) argued that the non-normalized level of demand for a commodity at any particular price strongly predicts choice behavior. For example, Bickel and Madden (1999) obtained demand curves for money and cigarettes in human smokers when both were available separately. Subsequently, the participants chose between money and cigarettes across a range of prices. At any price comparison, the commodity associated with the higher level of demand when it was available separately was the one chosen when both were available concurrently. This suggests that, in our study, an upward shift in the entire methamphetamine demand curve might be associated with a general increase in the relative reinforcing effectiveness of methamphetamine relative to other reinforcers in the rats’ environment. Consistent with this account, the level of demand for food significantly decreased after continued access to methamphetamine in the FMF group, although this decrease was slight. Christensen et al. (2008a) reported that demand for food decreased if cocaine was available within the same session, an effect that could be attributed to the anorectic effects of the drug. In this study, however, determinations of food demand were made in the absence of methamphetamine with body weight held constant.
Although the decrease in demand elasticity after continued access to methamphetamine was not significant, individual rats that increased their intake across the continued-access condition subsequently exhibited considerably less elastic methamphetamine demand curves. This suggests that intake escalation during continued access might predict subsequent changes in demand elasticity. To this end, Christensen et al. (2008b) reanalyzed results from Ahmed and Koob (1998), who first demonstrated that rats’ cocaine intake escalated when provided extended access (6 hr) to cocaine self-administration relative to limited access (1 h). Because Ahmed and Koob (1998) did not manipulate the FR requirement, but instead examined self-administration of multiple doses of cocaine before and after extended access, in the reanalysis a unit-price of the drug was calculated as the FR divided by the dose. Consumption at each price was then used to construct demand curves. Christensen et al. (2008b) reported that cocaine demand was less elastic in rats previously given extended access to cocaine self-administration. These results, along with our results showing less elastic demand in rats that escalated their methamphetamine intake, suggest that lengthening the duration of the continued-access sessions might engender greater intake escalation (e.g., Kitamura et al., 2006) and subsequently produce more reliable changes in demand elasticity.
Consistent with previous reports examining the relative reinforcing value of food and cocaine in the context of economic demand (Christensen et al., 2008a,b) or choice (Cantin et al., 2010; Lenoir et al., 2007), we found food to be a more effective reinforcer than methamphetamine. Unlike food, however, there was an upward shift in the methamphetamine demand curve after continued access to the drug. Moreover, on behavioral measures associated with drug-seeking, such as extinction responding and cue-induced reinstatement, drug-seeking was greater than food-seeking. Previous studies have shown greater cue-induced reinstatement using a cocaine cue than an appetitive cue (Martin-Fardon et al., 2007; Gal and Gyertyan, 2006; Baptista et al., 2004). Stimulus-reinforcer (S-R) relations have long been implicated in response persistence under extinction (e.g., Nevin, 1974) and cue-induced reinstatement (e.g., Davis and Smith, 1976). S-R relations may simply be stronger with psychostimulants due to more profound neuronal alterations in multiple corticostriatal pathways that may underlie cue-induced reinstatement (Belin et al., 2009; Nair et al., 2009). Interestingly, in our study, a comparison of the FFF and FMF groups suggests that a prior history of cue-methamphetamine pairings is sufficient to enhance extinction responding and reinstatement in rats most recently studied on a food baseline, perhaps due to long-term alterations in these neuronal pathways.
For the MMM group, continued access to methamphetamine did not significantly increase extinction responding and cue-induced reinstatement relative to the M-M group that did not receive continued access, although the differences observed in the reinstatement test approached significance. The lack of a difference between these groups on drug-seeking measures could be due to the fact that, although not provided continued access, the M-M group received considerable access to methamphetamine self-administration during the two determinations of demand. In addition, the daily duration of the continued-access sessions was relatively brief (2 h). Longer drug self-administration sessions (e.g., 6 h) have been shown to subsequently increase cue-induced reinstatement (Kippin et al., 2006), although this effect has not been consistently obtained (Rogers et al., 2008).
Hursh and colleagues have argued that drug demand elasticity in animal self-administration assays provides an estimate of abuse liability in humans (Hursh et al., 2005; Hursh and Winger, 1995; Hursh, 2000, 1993, 1991). Reinstatement procedures in animal models are thought to resemble drug-seeking and relapse in humans (deWit and Stewart, 1981; Epstein and Preston, 2003). To our knowledge, this is the first study that has shown a relationship between economic demand and reinstatement of drug-seeking. For groups self-administering methamphetamine, the α parameter was inversely related to the degree of cue-induced reinstatement. Rats with less elastic demand for methamphetamine exhibited greater reinstatement than rats with more elastic demand. To the extent that defending consumption against increasing prices by increasing response output falls along the same continuum as response persistence during extinction, perhaps similar neural mechanisms are activated. Demand elasticity then, not only provides a metric to rank-order drug of abuse in terms of abuse liability, but also may correspond to the propensity to relapse.
Acknowledgments
This research was supported by NIDA grants DA022658 (RES). Portions of this research were presented in poster format at the 2010 annual convention of the Association for Behavior Analysis, International (San Antonio, TX). The authors would like to thank Paul Gonzalez, Bernard Smalls, and Sara Wade Boatwright for their technical contributions to this project and Dr. Stacia DeSantis for statistical advice.
References
- Ahmed SH, Koob GR. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–27. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–10. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Madden GJ. A comparison of measures of relative reinforcing efficacy and behavioral economics: Cigarettes and money in smokers. Behav Pharmacol. 1999;10:627–37. doi: 10.1097/00008877-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. Plos One. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Huntsberry ME, Riley AL. Essential value of cocaine and food in rats: Tests of the exponential model of demand. Psychopharmacology. 2008a;198:221–9. doi: 10.1007/s00213-008-1120-0. [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Roma PG, Riley AL. Demand for cocaine and food over time. Pharmacol Biochem Behav. 2008b;91:209–16. doi: 10.1016/j.pbb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–99. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, DuFlou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:257–62. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlovian J Biol Sci. 1976;11:222–36. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–98. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35:593–8. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. J Exp Anal Behav. 1980;34:219–38. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–93. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration: An introduction. Drug and Alcohol Depend. 1993;33:165–72. doi: 10.1016/0376-8716(93)90058-x. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economic concepts and methods for studying health behavior. In: Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavioral economics. Mahwah, NJ US: Lawrence Erlbaum Associates Publishers; 2000. pp. 27–60. [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–84. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: A quantitative assessment of drug demand. Mol Interv. 2005;5:20–8. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–7. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: A dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. Plos One. 2007;2(1):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–73. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18:235–2. doi: 10.1097/01.yco.0000165592.52811.84. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: A literature review. Harv Rev Psychiatry. 2005;13:141–54. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Corriea CJ, Vuchinich RE. Behavioral economics of substance abuse. In: Cohen LM, Collins FL, Young AM, McChargue DE, Leffingwell TR, Kook KL, editors. Pharmacology and treatment of substance abuse: Evidence- and outcome-based perspectives. New York: Routledge/Taylor & Francis Group; 2009. pp. 505–28. [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Response strength in multiple schedules. J Exp Anal Behav. 1974;21:389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addict Biol. 2010;15:312–23. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, Lavin A, Glen WB, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biological Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce DW, editor. The MIT dictionary of modern economics. 4. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine-seeking in an animal model of relapse. Psychopharmacology. 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Extended access to methamphetamine self-administration blocks object recognition memory: relationship with metabotropic glutamate receptor 5 expression in rat cortex. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199:615–24. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Taylor TG, Galuska CM, Banna K, Yahyavi-Firouz-Abadi N, See RE. Response acquisition and fixed-ratio escalation based on interresponse times in rats. J Exp Anal Behav. 2010;93:261–7. doi: 10.1901/jeab.2010.93-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 national survey on drug use and health: National findings. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2009. [Google Scholar]
- United Nations Office on Drugs and Crime. World drug report. Vienna: United Nations Office on Drugs and Crime; 2009. [Google Scholar]
- Xi J, Kruzich PJ. Black agouti (ACI) rats show greater drug- and cue-induced reinstatement of methamphetamine-seeking behavior than Fischer 344 and Lewis rats. Pharmacol Biochem Behav. 2007;87:90–97. doi: 10.1016/j.pbb.2007.04.003. [DOI] [PubMed] [Google Scholar]