Abstract
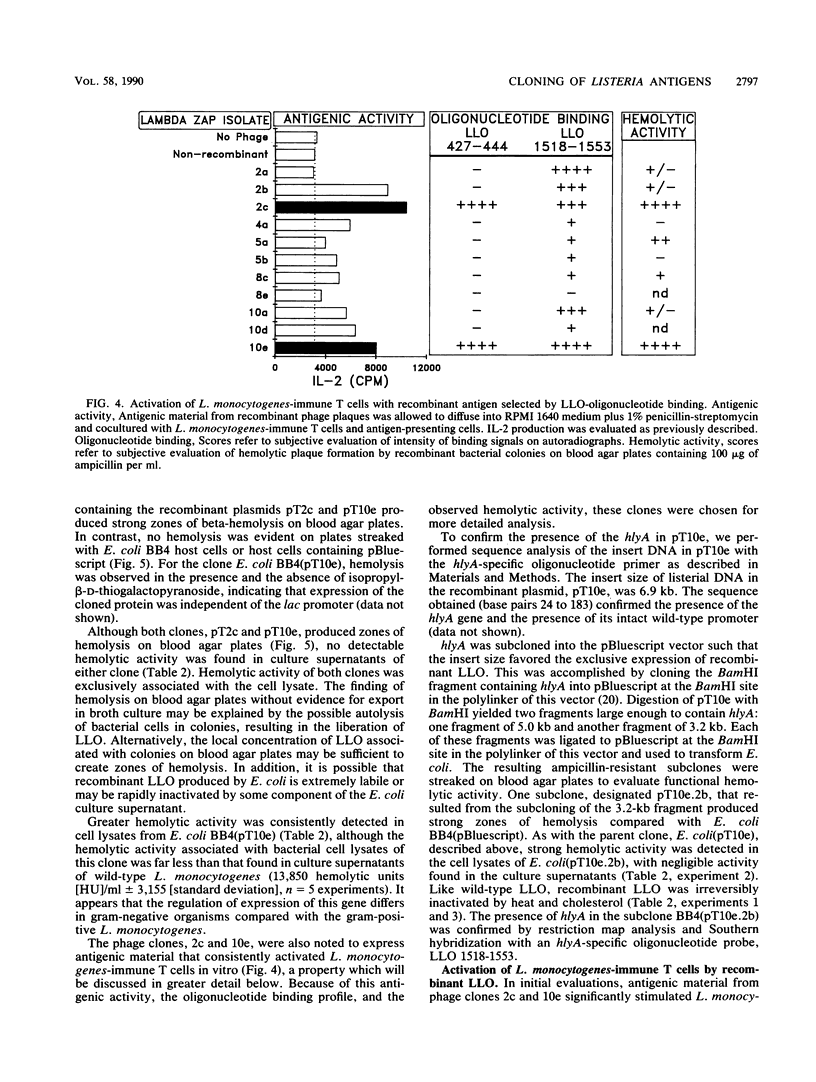
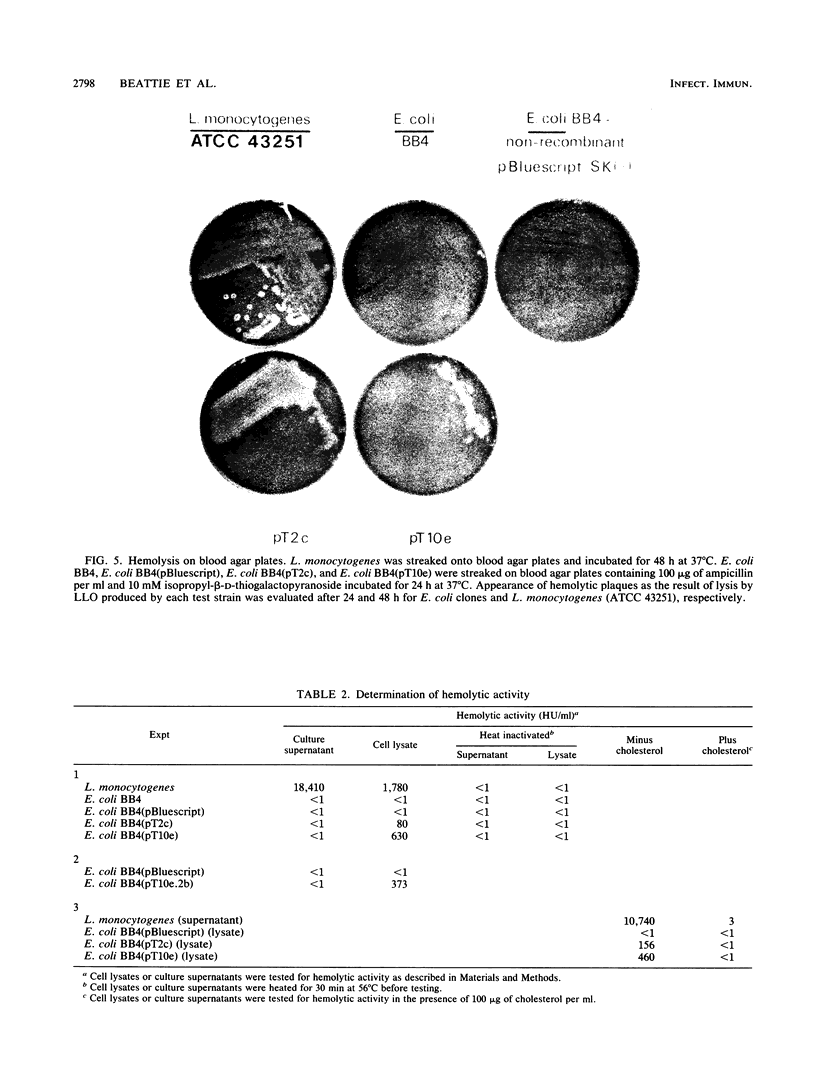
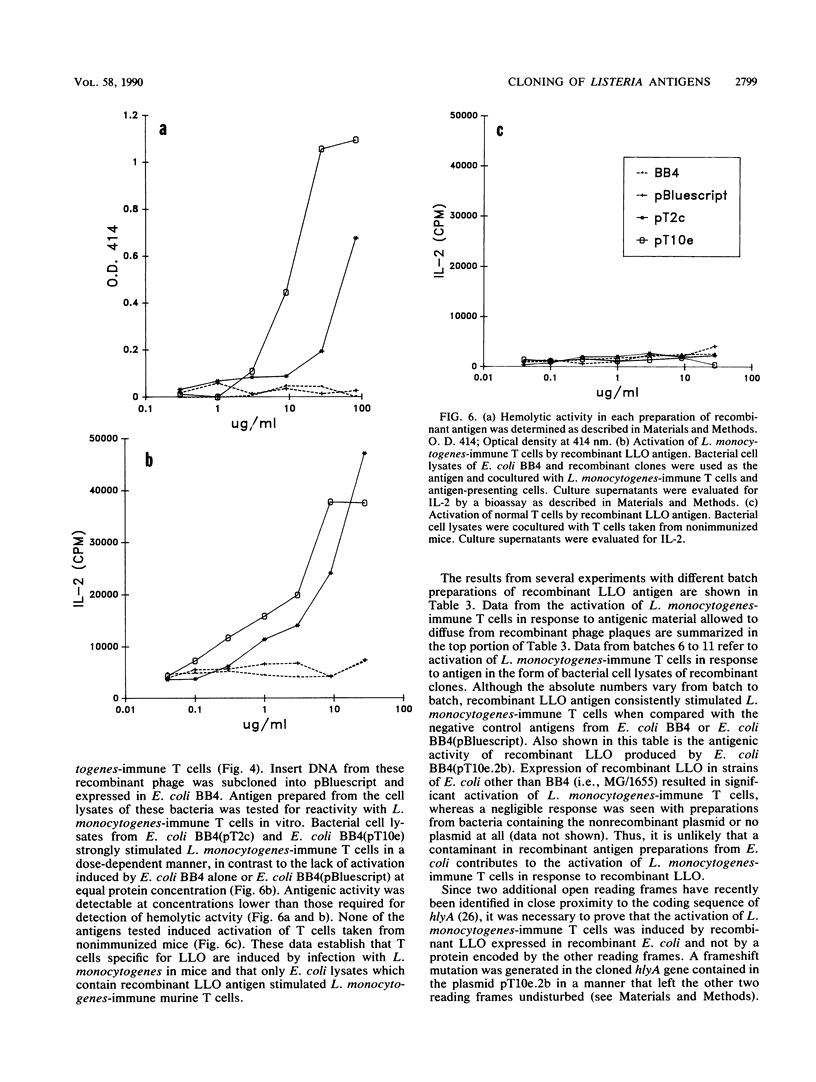
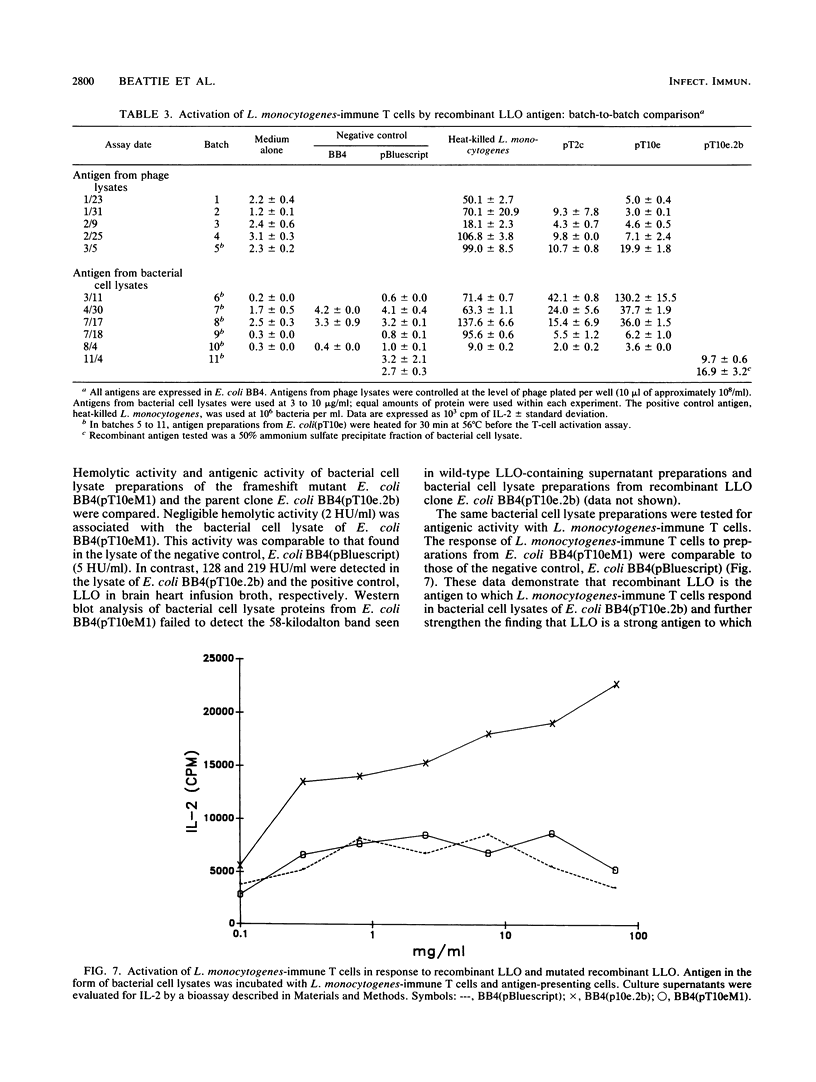
To explore the molecular basis of the T-cell-mediated immune response to Listeria monocytogenes, we cloned and expressed listerial antigens in Escherichia coli using the lambda-ZAP bacteriophage and Bluescript plasmid vectors. A two-stage screening strategy was implemented to identify T-cell-reactive antigens; the first stage involved antibodies or oligonucleotide probes and the second stage was based on assays for T-cell activation. A library of genomic DNA from L. monocytogenes was generated in lambda-ZAP, and then antigens, were detected in infected cells with a polyclonal rabbit anti-L. monocytogenes antiserum and an L. monocytogenes-specific monoclonal antibody. Also, synthetic oligonucleotide probes corresponding to the structural gene for listeriolysin O (LLO) were used to screen the recombinant DNA library. In each case, positive isolates were evaluated for T-cell antigenicity by measuring antigen-induced interleukin-2 production by polyclonal T cells taken from L. monocytogenes-immune mice. Phage clones were subcloned and expressed in the Bluescript plasmid and tested further for antigenic activity and LLO expression. Using this screening strategy, we successfully identified bacterial clones producing recombinant listerial antigens which activate L. monocytogenes-immune T cells in vitro. Antigens operative in the T-cell response during infection with L. monocytogenes include LLO, 62- and 39-kilodalton proteins, and other poorly defined bacterial surface components. We also found that high concentrations of recombinant LLO inhibited macrophage-mediated antigen presentation. These results are discussed in terms of the multiple functions of LLO as a virulence factor, inhibitor of antigen presentation, and potent antigen in the T-cell response to L. monocytogenes. These studies represent the first step toward a genetic definition of the antigens recognized in immune defense to L. monocytogenes.
Full text
PDF


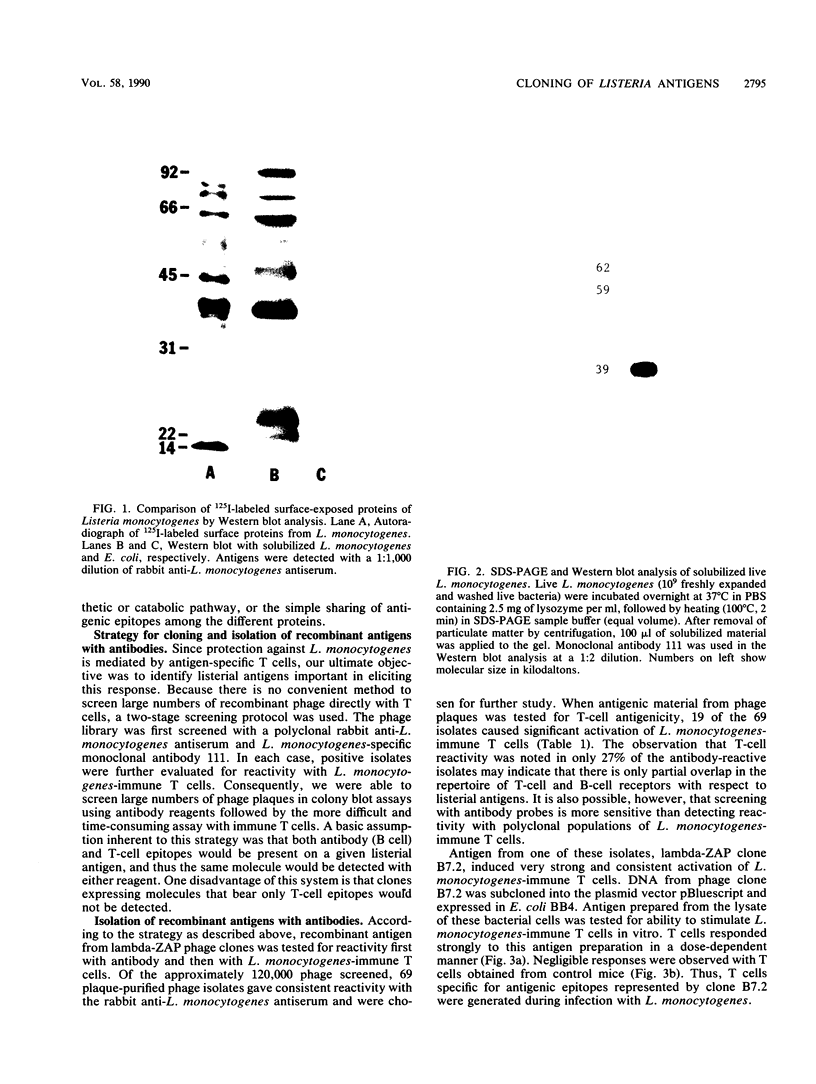
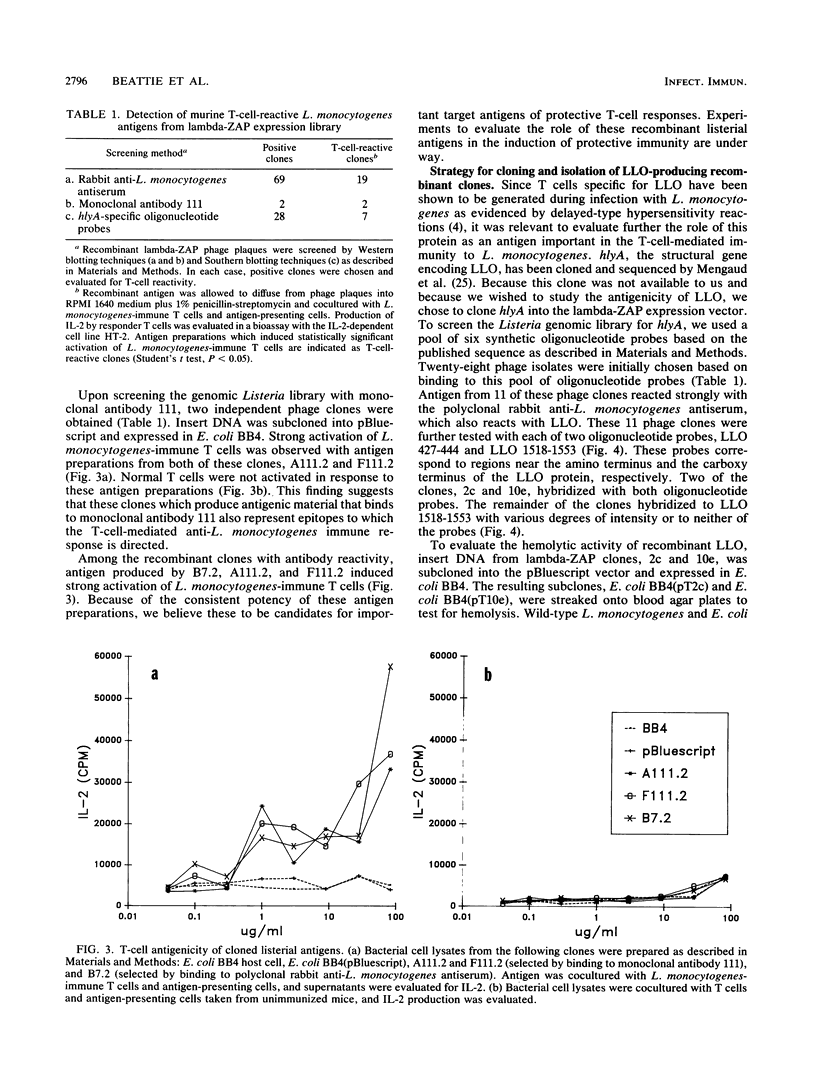


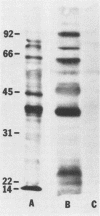
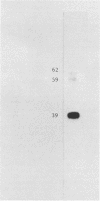
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG A. S., SWORD C. P. CELLULAR RESISTANCE IN LISTERIOSIS. J Infect Dis. 1964 Jun;114:258–264. doi: 10.1093/infdis/114.3.258. [DOI] [PubMed] [Google Scholar]
- Barza M. Listeriosis and milk. N Engl J Med. 1985 Feb 14;312(7):438–440. doi: 10.1056/NEJM198502143120710. [DOI] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Geoffroy C., Alouf J. E. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987 Dec 1;139(11):3813–3821. [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Bishop D. K., Hinrichs D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987 Sep 15;139(6):2005–2009. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cluff C. W., Ziegler H. K. Inhibition of macrophage-mediated antigen presentation by hemolysin-producing Listeria monocytogenes. J Immunol. 1987 Dec 1;139(11):3808–3812. [PubMed] [Google Scholar]
- Cossart P., Vicente M. F., Mengaud J., Baquero F., Perez-Diaz J. C., Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989 Nov;57(11):3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm R. K., Hinrichs D. J., Thomashow M. F. Introduction of pAM beta 1 into Listeria monocytogenes by conjugation and homology between native L. monocytogenes plasmids. Infect Immun. 1984 Apr;44(1):157–161. doi: 10.1128/iai.44.1.157-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Surface peptide mapping of protein I and protein III of four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):632–641. doi: 10.1128/iai.37.2.632-641.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Chakraborty T. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect Immun. 1989 Aug;57(8):2350–2357. doi: 10.1128/iai.57.8.2350-2357.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Cobian F., Unanue E. R. Intracellular interference with antigen presentation. J Immunol. 1988 Sep 1;141(5):1445–1450. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Shimonkevitz R., Hannum C., Haskins K., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. IV. An antiidiotypic antibody predicts both antigen and I-specificity. J Exp Med. 1983 Nov 1;158(5):1635–1646. doi: 10.1084/jem.158.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Chenevert J., Pereira J. M., Geoffroy C., Gicquel-Sanzey B., Baquero F., Perez-Diaz J. C., Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988 Apr;56(4):766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Cossart P. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect Immun. 1989 Dec;57(12):3695–3701. doi: 10.1128/iai.57.12.3695-3701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Oftung F., Deggerdal A., Gill H. K., Young R. A., Godal T. Gene isolation with human T lymphocyte probes. Isolation of a gene that expresses an epitope recognized by T cells specific for Mycobacterium bovis BCG and pathogenic mycobacteria. J Immunol. 1988 Oct 15;141(8):2729–2733. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Role of local immunosuppression in murine fetoplacental listeriosis. J Clin Invest. 1987 Apr;79(4):1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Morse S. A. Cleavage of the protein III and major iron-regulated protein of Neisseria gonorrhoeae by lysosomal cathepsin G. J Gen Microbiol. 1987 Jan;133(1):155–162. doi: 10.1099/00221287-133-1-155. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovic Z., Goebel W. Synthesis of listeriolysin in Listeria monocytogenes under heat shock conditions. Infect Immun. 1989 Jan;57(1):295–298. doi: 10.1128/iai.57.1.295-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth P. A., Ziegler H. K. Induction of macrophage Ia expression by lipopolysaccharide and Listeria monocytogenes in congenitally athymic nude mice. J Immunol. 1987 May 15;138(10):3167–3173. [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. K., Orlin C. A. Analysis of Listeria monocytogenes antigens with monoclonal antibodies. Clin Invest Med. 1984;7(4):239–242. [PubMed] [Google Scholar]
- Ziegler H. K., Orlin C. A., Cluff C. W. Differential requirements for the processing and presentation of soluble and particulate bacterial antigens by macrophages. Eur J Immunol. 1987 Sep;17(9):1287–1296. doi: 10.1002/eji.1830170911. [DOI] [PubMed] [Google Scholar]