Abstract
Neuregulin 1 (NRG1) is a secreted trophic factor that activates the postsynaptic erbB4 receptor tyrosine kinase. Both NRG1 and erbB4 have been repeatedly associated with schizophrenia, but their downstream targets are not well characterized. ErbB4 is highly abundant in interneurons, and NRG1-mediated erbB4 activation has been shown to modulate interneuron function, but the role for NRG1-erbB4 signaling in regulating interneuron dendritic growth is not well understood. Here we show that NRG1/erbB4 promote the growth of dendrites in mature interneurons through kalirin, a major dendritic Rac1-GEF. Recent studies have shown associations of the KALRN gene with schizophrenia. Our data point to an essential role of phosphorylation in kalirin-7’s C-terminus as the critical site for these effects. As reduced interneuron dendrite length occurs in schizophrenia, understanding how NRG1-erbB4 signaling modulates interneuron dendritic morphogenesis might shed light on disease-related alterations in cortical circuits.
Introduction
While the mechanisms that govern the initial emergence of dendrites, mainly as they occur in pyramidal neurons, are relatively well understood, the molecular mechanisms underlying dendritic growth in inhibitory neurons are poorly understood. Knowledge of the regulators of interneuronal dendritic growth is important considering that a reduction in multipolar interneuronal dendritic length and reduced GAD67 levels occur in schizophrenia,1–3 and may underlie the dysfunction of inhibitory circuits in the disease.4 Contrary to cortical pyramidal neurons, cortical interneuron dendrites undergo considerable remodeling in adulthood, and even small changes in interneuron dendritic growth could influence the receptive properties of these cells.5
Neuregulin 1 (NRG1) is a trophic factor that can be released presynaptically in soluble form, and the postsynaptic erbB4 receptor tyrosine kinase is thought to be the predominant receptor for NRG1. NRG1 binds directly to erbB4, and this binding stimulates the intrinsic tyrosine kinase activity of the erbB4 receptor.6–7 The biological functions of NRG1 and erbB4 have received much recent attention due to several studies showing associations between these genes and schizophrenia.7–8 Nevertheless, the biological functions of NRG1 and erbB4 are incompletely understood. ErbB4 is highly abundant in interneurons, with lower expression in pyramidal neurons.9 NRG1-mediated erbB4 activation has been shown to modulate interneuron function;10–11 however, the role for NRG1-erbB4 signaling in regulating interneuron dendritic growth is not well understood. While NRG1 promotes dendritic growth in immature interneurons,12–13 the effects of NRG1/erbB4 on interneuronal growth have not been reported in mature interneurons.
In this study we show that NRG1/erbB4 regulate the growth of mature interneurons through the RacGEF kalirin. Moreover, we identify a residue in the kalirin-7 C-terminus as the critical mediator of the effects of NRG1 on interneuron growth, and reveal a new role for the src-family kinase member fyn in regulating kalirin-7 as well as interneuron growth. These findings are unexpected, as studies characterizing the roles of NRG1/erbB4 on neuronal structure have mainly focused on pyramidal neurons,14–15 and previous kalirin studies have centered almost exclusively on pyramidal neurons.16–18 Recent studies are suggestive of a potential role for kalirin dysfunction in schizophrenia as several missense mutations in the KALRN gene were recently reported in schizophrenia patients,19 and independent studies have found reductions in KALRN mRNA in the schizophrenia cortex.20–21 The identification of novel links between disease-associated genes and the regulation of neuronal morphology could provide new insight into the disease-related alterations in neuronal structure.
Materials and methods
Interneuron microscopy and imaging
Interneurons expressing GFP were imaged using on a Zeiss Axioplan2 upright microscope. Images were taken with a 10× objective (NA=0.17) and micrographs aquired using a Zeiss AxioCam MRm CCD camera. Confirmation that imaged interneurons expressed additional cDNA plasmids was easily discernable by examining cells at 63×. Interneuron dendrities were traced and measured using Image J and MetaMorph software (Universal Imaging) with the appropriate measurement correction factor for the Ziess microscope. All images were acquired and analyzed by a researcher blind to conditons. Analyses were done on sister cultures from at least two independent experiments. For interneuron analysis from cultures derived from erbB2/B4 KO mice and WT mice, 10 interneurons from each group were analyzed. For all other interneuron experiments, a minimum of 18 cells per condition were analyzed.
Confocal imaging
To assess the co-localization between kalirin-7 and erbB4 and to assess endogeous erbB4 immunostaining, confocal imaging was used. Confocal images of single- and double-stained neurons were obtained with a Ziess LSM5 Pascal confocal microscope. Images of neurons were taken using the 63× oil-immersion objective as a z-series of three to eight images, averaged four times, taken at 0.37 µm intervals, 1024 × 1024 pixel resolution at a scan speed of 8 seconds per section. Two-dimensional maximum projection reconstructions of images was done using MetaMorph software (Universal Imaging).
Co-immunoprecipitation
For coimmunoprecipitation assays in hEK 293 cells, after transfection when cells reached approximately 95% conluencey, cells were harvested in RIPA lysis buffer (in mM: 150 NaCl, 10 Tris-HCl, pH 7.2, 5 EDTA, 0.1% SDS, 1% Triton X-100, 1% Deoxycholate, plus protease and phosphatase inhibitors). Lysates were then sonicated and cleared by centrifugation at 14,000 × g for 10 min. The supernatants were then tumbled with 5 µl of the appropriate antibody at 4°C. Finally, 60 µl of protein Sepharose A was added to the samples and tumbled for 2-hours at 4°C, after which time, samples were washed 3 times with RIPA buffer. Coimmunoprecipitation of cortical pyramidal neurons were carried essentially as described for hEK293 cells, however, cells were grown in 60 mm dishes. All samples were boiled for 5 min at 95°C after addition of Laemlli buffer, and stored at −80°C until they were resolved on SDS-PAGE. Quantification of bands was performed using Image J with normalization to the amount of kalirin-7 immunoprecipitated. A minimum of three independent experiments were performed.
Kalirin siRNA
The efficiency and specificity of the kalirin RNAi has been previously demonstrated.17 To deliver siRNA (small interfering RNA), we used the plasmid pGsuper, derived from “pSuper” which expresses siRNA and EGFP simultaneously.22–23 The siRNA is unique to the kalirin gene.
Statistical analysis
All statistical analyses were done using GraphPad Prism software (La Jolla, CA). Analysis between 2 groups was done using students unpaired t-tests. Analysis between 3 or more groups was done using a one-way ANOVA with Tukey post-hoc tests to determine differences between groups. For interaction analyses, a 2×2 ANOVA was used and the interaction term determined.
Results
NRG1 promotes dendritic growth in mature interneurons
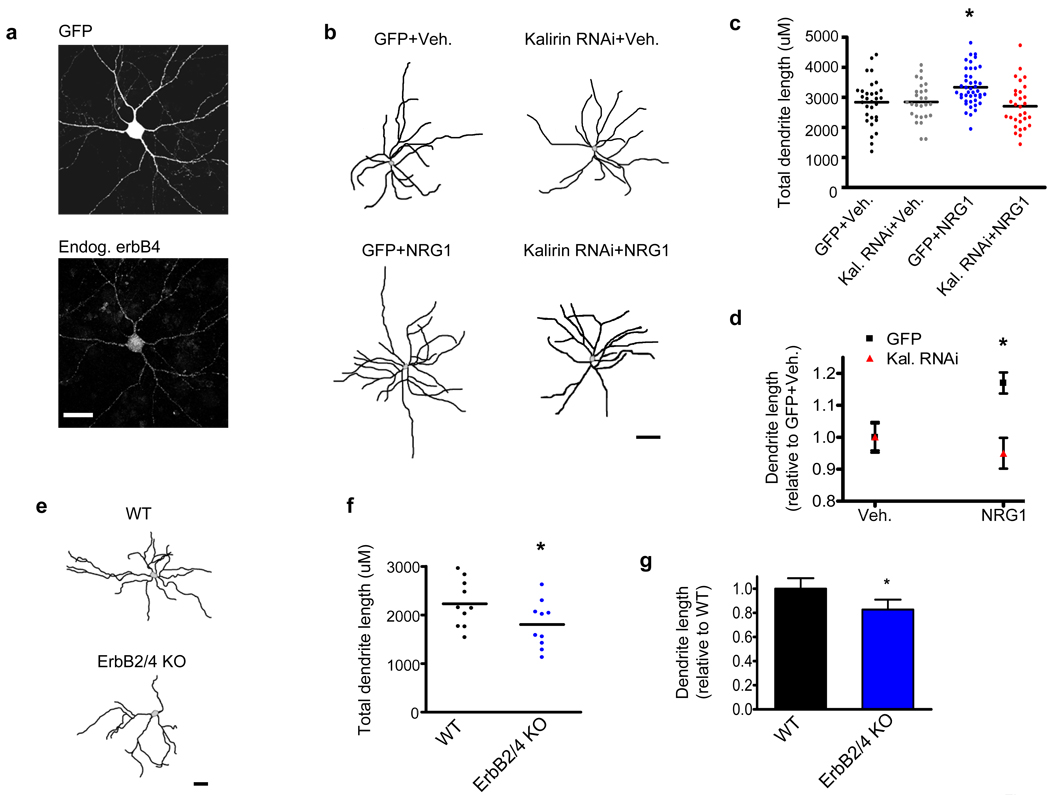
To assess the effects of NRG1 on mature interneurons, we treated GFP-expressing rat cultured cortical interneurons exhibiting endogenous erbB4 expression with NRG1β and examined dendrite morphology at DIV28. We specifically examined cortical multipolar interneurons as the vast majority of these cells (88±2.7%) exhibit erbB4 immunoreactivity (Figure 1a and Supplementary Figure 1a). Vehicle treated erbb4-positive cortical interneurons had a mean total dendritic length of 2840±137µm, which is similar to previous findings showing that the mean dendritic length of mature cortical interneurons is 2900µm.24 NRG1β treatment caused an increase in dendritic length in mature erbb4-positive multipolar interneurons (Vehicle: 2840±137µm; NRG1: 3335±94µm; p<0.05) (Figures 1b–d and Supplementary Figure 1b).
Figure 1. NRG1 and erbB4 promote interneuronal dendritic growth.
(a) ErbB4 is expressed in mature multipolar cultured cortical interneurons. Scale bar=35uM.
(b) Representative traces of DIV28 cortical multipolar interneuron dendritic trees. Axons were omitted for clarity. Neurons expressed either GFP or GFP-tagged kalirin RNAi for 3-days. 1-day posttransfection, neurons were treated with either NRG1β (5nM) or with vehicle for 2 days. Scale bar=100um.
(c) Scatter plots showing that NRG1β significantly increases interneuronal dendritic length relative to control neurons treated with vehicle, while the knockdown of kalirin blocks the effect of NRG1β on dendrite length. Knockdown of kalirin by itself did not alter dendrite length. Each point represents the total dendritic length of a single cell. Significance determined using a one-way ANOVA with Tukey post-hoc. Black lines are the mean, n=27–43 cells per condition; *p<0.05
(d) Significant interaction of NRG1β treatment by kalirin expression with regard to total interneuron dendritic length. Significance determined using a 2×2 ANOVA and the interaction term determined. Data are the mean±SEM; *p<0.05
(e) Representative traces of mature cultured interneurons derived from wildtype (WT) mice or from erbB2/B4 conditional knockout (KO) mice. Axons were omitted for clarity. Scale bar=50um.
(f) Scatter plots showing that multipolar cultured interneurons from erbB2/B4 KO mice show a reduced total dendritic length relative to WT mice. Each point represents the total dendritic length of a single cell. Significance determined using an unpaired t-test. Black lines are the mean, n=10 cells per condition; *p<0.05
(g) Relative change in total dendritic length of erbB2/B4 KO interneurons as compared to WT interneurons. Significance determined using an unpaired t-test. Data are the mean±SEM; *p<0.05
In pyramidal neurons, RacGEFs are central regulators of pyramidal neuronal dendrite morphology, and the role of RacGEFs in neuronal structure have focused almost exclusively on pyramidal neurons rather than interneurons.16, 25–26 Emerging evidence indicates that RacGEFs are also expressed in interneurons.27 Kalirin-7 is the only known RacGEF with appreciable levels in mature cortical neurons,28 and is strongly expressed in the dendrites and shaft excitatory synapses of mature interneurons.27 Because kalirin has been shown to be necessary for trophic factor-mediated neurite growth,29 and because kalirin is a major regulator of dendritic remodeling in cortical pyramidal neurons,25 we hypothesized that kalirin is important for the trophic effects of NRG1 on cortical interneuron growth.
To test whether acute loss of kalirin prevented NRG1-dependent interneuronal dendrite growth, we used RNAi-mediated partial knockdown of kalirin. Although acute kalirin loss did not affect the basal dendritic length of interneurons, kalirin RNAi blocked NRG1-dependent increases in dendrite length (Vehicle: 2840±137µm; Kalirin RNAi+Vehicle: 2846±120µm; NRG1: 3335±94µm; Kalirin RNAi+NRG1: 2706±136µm) (Figures 1b–d and Supplementary Figure 1b). Importantly, interneurons expressing kalirin RNAi still exhibited strong erbB4 expression (Supplementary Figure 2a). Collectively, these data indicate that NRG1 signals through kalirin to promote dendrite growth in mature interneurons.
ErbB4 expression is strongest in interneurons, with reduced expression in pyramidal neurons.9 To determine if NRG1’s effect on dendritic growth are specific for interneurons, we examined the effects of NRG1 on GFP-expressing pyramidal neurons. Pyramidal neurons did not undergo dendritic remodeling following trophic NRG1 treatment (Supplementary Figures 2b–c). Pyramidal neurons from conditional central nervous system erbB2/B4 knockout (KO) mice show no overt changes in pyramidal neuronal gross morphology,15 which is consistent with our finding that NRG1 treatment does not alter the dendritic growth of pyramidal neurons. Nevertheless, the effect of reduced erbB2/B4 on interneuronal morhphology has not been reported. We thus examined mature forebrain interneuron dendritic length in neurons derived from conditional central nervous system erbB2/B4 KO mice as compared to WT mice. Interneurons with reduced erbB2/B4 show reduced dendritic length (WT: 2230±153µm; ErbB4/B4KO:1802±152µm; p<0.05) (Figures1e–g), consistent with a role for NRG1-erbB4 signaling in promoting interneuron growth.
ErbB4 and kalirin-7 interact
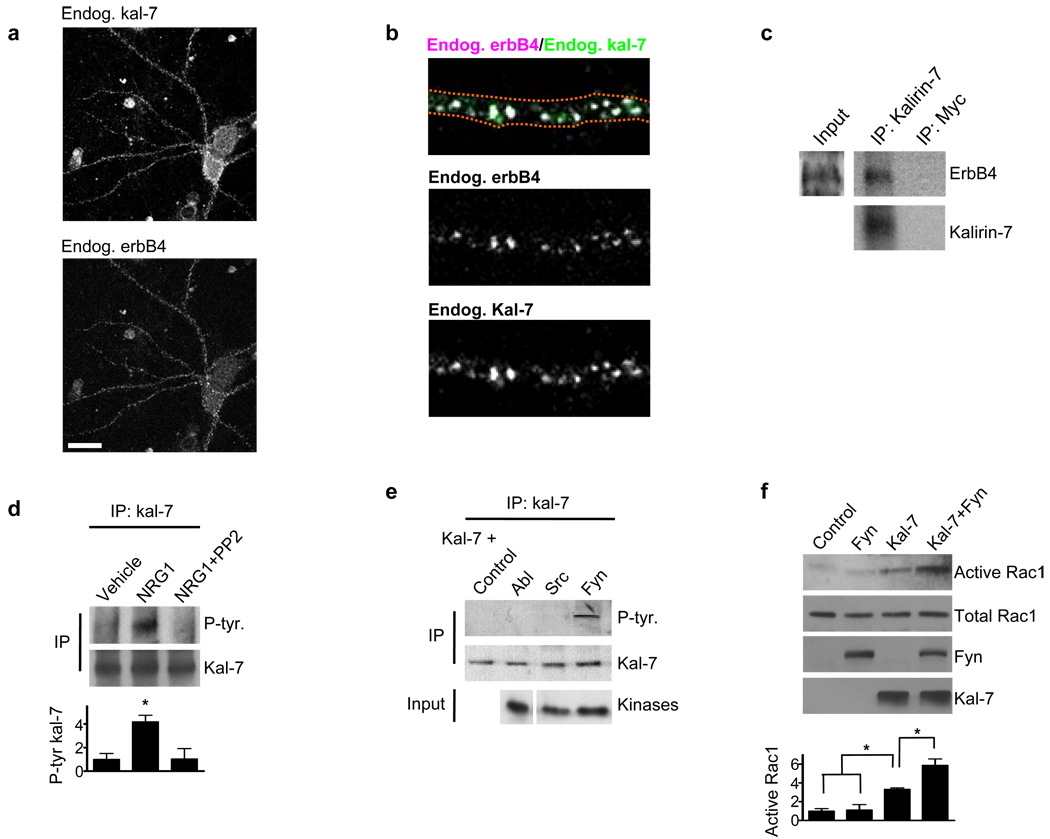
Independent evidence indicates that both kalirin-7 and erbB4 are present in the dendrites of interneurons.13, 27 Indeed, we found a strong colocalization between erbB4 and kalirin-7 in mature multipolar cultured cortical interneurons as 71±4.6% of erbB4 dendritic puncta colocalized with kalirin-7 (Figures 2a and b). Moreover, we found that kalirin-7 interacted with erbb4 in cultured cortical neuronal coimmunoprecipitates (Figure 2c).
Figure 2. Kalirin-7 and erbB4 interact and fyn phospho-activates kalirin-7.
(a) DIV28 cortical cultures were immunostained for endogenous erbB4 and endogenous kalirin-7. Immunostaining shows a high degree of colocalization between kalirin-7 and erbB4 in interneurons. Scale bar=35uM.
(b) 71±4.6% of erbb4 puncta in mature interneuronal dendrites colocalized with kalirin-7.
(c) Kalirin-7 was immunoprecipitated from mature cortical cultures and the immunoprecipitate probed for erbB4. Myc was used as an immunoprecipitation control. Kalirin-7 interacted with erbB4.
(d) Kalirin-7 was immunoprecipitated from mature cortical cultures and the immunoprecipitate probed with a phospho-tyrosine antibody. Whereas NRG1β (5nM, 3-days) treatment increased kalirin-7 tyrosine phosphorylation, co-treatment with PP2 (10uM) blocked this effect. Graph displays change relative to the vehicle condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=3 independent experiments; *p<0.05
(e) hEK293 cells overexpressing various kinases in combination with kalirin-7. Kalirin-7 was immunoprecipitated and the immunoprecipitate probed with a phospho-tyrosine antibody. Of the tested tyrosine kinases, only fyn phosphorylated kalirin-7.
(f) hEK293 cells overexpressing fyn and kalirin-7 individually or together, and active Rac1 levels assessed. Fyn in combination with kalirin-7 increased active Rac1 levels beyond that of kalirin-7 or fyn alone. Graph displays change relative to the control condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=3 independent experiments; *p<0.05
NRG1 phosphorylates kalirin-7 in a src-family kinase-dependent manner
The src-family tyrosine kinase member fyn is among the few known direct targets of erbB4 in neurons,30 and similar to previous findings,30 we found that erbb4 activates fyn/src (Supplementary Figure 2d). We thus wanted to determine if NRG1 altered the tyrosine phosphorylation status of kalirin-7, and if so, if this occurs in a fyn/src-dependent manner. To test this we treated cortical cultures with NRG1 in the presence or absence of the specific fyn/src activity inhibitor PP231 (PP2’s effect on active fyn/src shown in Supplementary Figure 2e). We then immunoprecipitated kalirin-7, and probed the immunoprecipitate with an anti-phosphotyrosine antibody. Indeed, NRG1 increased kalirin-7 tyrosine phosphorylation, and this effect was abolished by PP2 (Figure 2d). This suggested the fyn and/or src phsophorylate kalirin-7. To test this we expressed fyn and src in combination with kalirin-7 in hEK293 cells, immunoprecipitated kalirin-7, and probed for phosphotyrosine. We found that fyn, but not src or abl, increased kalirin-7 tyrosine phosphorylation (Figure 2e). Moreover, we found that fyn promotes kalirin-7 mediated Rac1 activation in hEK293 cells (Figure 2f).
Fyn promotes interneuron growth in a kalirin-dependent manner
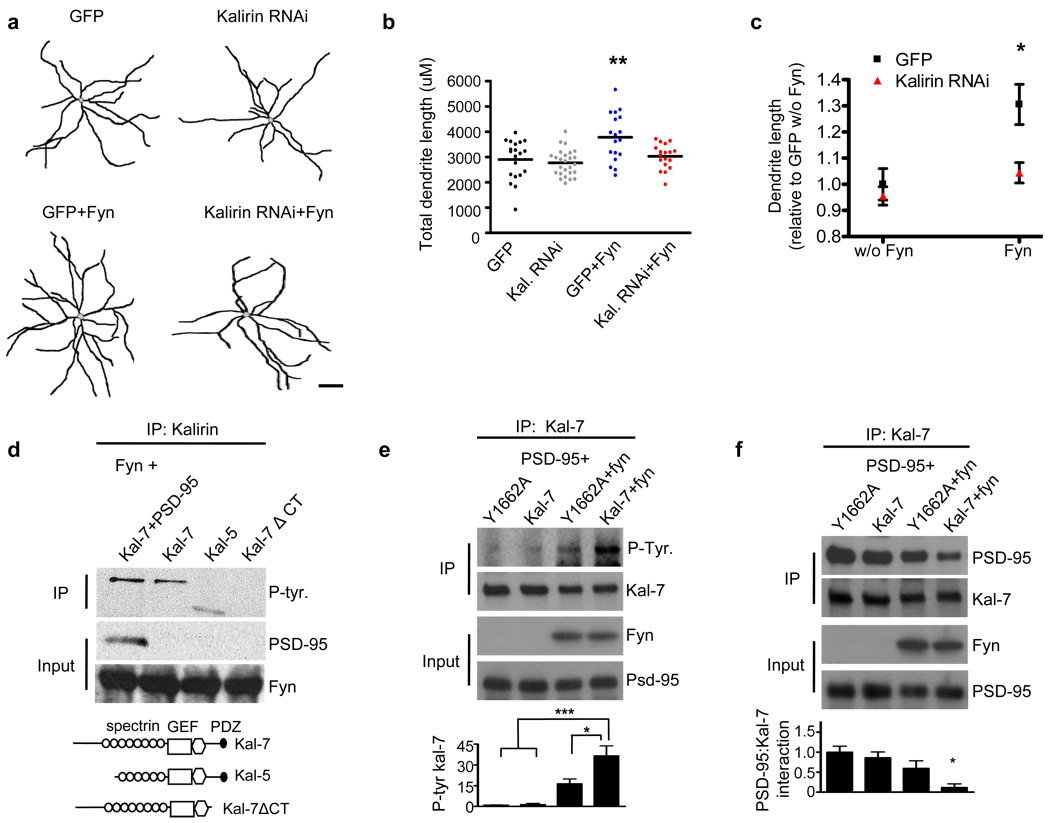
We found that endogenous fyn is enriched in the dendrites of multipolar interneurons (Supplementary Figure 3a), yet the role of fyn in interneuron structural morphology has not been previously reported. We found that fyn overexpression (Supplementary Figure 3b) increased the dendritic length of cortical interneurons relative to control interneurons (p<0.01), while the acute knockdown of kalirin blocked the effects of fyn on interneuronal dendrite length (Control: 2895±175µm; Kalirin RNAi: 2767±101µm; Fyn: 3780±223µm; Kalirin RNAi+Fyn: 3024.26±115µm) (Figures 3a–c and Supplementary Figure 3c). These data indicate that kalirin is required for the effects of fyn on interneuronal dendrites.
Figure 3. Fyn increases interneuronal dendritic growth through kalirin, and fyn phosphorylates kalirin-7 through its C-terminus.
(a) Representative traces of DIV28 cultured cortical multipolar interneuron dendritic trees. Axons were omitted for clarity. Neurons were transfected with either GFP, GFP-tagged kalirin RNAi, fyn, or GFP-tagged kalirin RNAi in combination with fyn. Scale bar=100um.
(b) Scatter plots showing that fyn overexpression increases interneuronal dendritic length. The knockdown of kalirin blocked the effects of fyn on interneuron dendritic length. Knockdown of kalirin by itself did not affect dendritic length. Each point represents the total dendritic length of a single cell. Significance determined using a one-way ANOVA with Tukey post-hoc. Black lines are the mean, n=18–26 cells per condition; **p<0.01
(c) Significant interaction of fyn expression by kalirin expression with regard to total interneuron dendritic length. Significance determined using 2×2 ANOVA and the interaction term determined. Data are the mean±SEM; *p<0.05
(d) Domain mapping in hEK293 cells overexpressing various kalirin constructs in combination with fyn. Kalirin was immunoprecipitated and the immunoprecipitate probed with a phospho-tyrosine antibody. Fyn phosphorylated kalirin-7 in the presence and absence of PSD-95, and fyn phosphorylated kalirin-5. Fyn failed to phosphorylate the kalirin-7 ΔCT construct. Schematics of kalirin constructs used are shown below.
(e) hEK293 cells overexpressing Y1662A or WT kalirin-7 together with PSD-95, with or without fyn. Kalirin was immunoprecipitated and the immunoprecipitate probed for phosphotyrosine. Fyn strongly phosphorylated kalirin-7. Mutation of the Y1662 kalirin-7 residue to alanine (Y1662A) significantly reduced fyn-mediated phosphorylation. Graph displays change relative to the Y1662A condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=4 independent experiments; *p<0.05, ***p<0.001
(f) hEK293 cells overexpressing Y1662A or WT kalirin-7 together with PSD-95, with or without fyn. Kalirin was immunoprecipitated and the immunoprecipitate probed for PSD-95. WT kalirin-7 and the Y1662A mutant showed a similar magnitude of interaction with PSD-95 in the absence of fyn. However, fyn reduced the association of WT kalirin-7, but not Y1662A kalirin-7, with PSD-95. Graph displays change relative to the Y1662A condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=3 independent experiments; *p<0.05
Fyn phosphorylates the kalirin-7 C-terminal Y1662 residue
To identify the region of kalirin-7 phosphorylated by fyn, we performed domain mapping in which we coexpressed fyn with kalirin-7, with kalirin-5 (lacking the N-terminus of kalirin-7), or with a truncation mutant of kalirin-7 lacking the 25 amino acids comprising the C-terminus (kalirin-7ΔCT). While fyn phosphorylated both kalirin-7 and kalirin-5, it did not phosphorylate kalirin-7ΔCT (Figure 3d). This suggests that fyn phosphorylates kalirin-7 in the C-terminal region. Within the C-terminus of kalirin-7 there is a single tyrosine residue (Y1662), and this residue is located amongst the four residues comprising kalirin-7’s PDZ-binding domain (−STYV) (Supplementary Figure 4a). The Y1662 residue is the only residue in the kalirin-7 PDZ-biding domain that exceeds chance levels of being a phosphorylation substrate (Supplementary Figure 4b). Kalirin-7’s PDZ-binding motif enables it to bind to PSD-95,18 and thus the interaction of kalirin-7 with PSD-95 could block fyn’s access to kalirin-7. However, we found that fyn was still able to phosphorylate kalirin-7 in the presence of PSD-95 (Figure 3d).
Based on these experiments, we hypothesized that fyn phosphorylates the Y1662 residue of kalirin-7. To test this, we mutated the Y1662 site to alanine (Y1662A) to render it phospho-incompetent. We then compared the tyrosine phosphorylation status of Y1662A-kalirin-7 to WT kalirin-7 in the absence or presence of fyn in hEK293 cells. We found that while fyn increased kalirin-7 tyrosine phosphorylation levels relative to cells without fyn overexpression, mutation of the Y1662 residue of kalirin-7 to alanine blocked significant changes in fyn-mediated kalirin-7 tyrosine phosphorylation (Figure 3e). Next, we compared fyn’s ability to activate Rac1 in the presence of WT kalirin-7 and Y1662A-kalirin-7 and PSD-95 in hEK293 cells. In the presence of PSD-95 but absence of fyn, WT kalirin-7 and Y1662A-kalirin-7 produced similar levels of active Rac1 (Supplementary Figure 4c). This is expected given that PSD-95 has been previously shown to nearly fully inhibit kalirin-7’s intrinsic ability to activate Rac1.18 However, in the presence of PSD-95, fyn increased Rac1 activity in cells expressing WT kalirin-7 but not the Y1662A mutant (Supplementary Figure 4c).
As the interaction of kalirin-7 with PSD-95 is known to nearly fully inhibit kalirin-7’s ability to activate Rac1,18 we reasoned that in order to stimulate Rac1 activity in the presence of PSD-95, kalirin-7 might dissociate from PSD-95. This suggests that phosphorylation of the kalirin-7 PDZ-interacting motif by fyn might dissociate kalirin-7 from PSD-95. To test this, we coexpressed fyn together with PSD-95 and kalirin-7 in hEK293 cells, immunoprecipitated kalirin-7, and probed for PSD-95. Indeed, we found that in the absence of fyn, WT kalirin-7 and PSD-95 interacted strongly. In the presence of fyn, however, the interaction of WT kalirin-7 with PSD-95 was significantly reduced (Figure 3f). Conversely, fyn did not significantly reduce the interaction of the Y1662A mutant with PSD-95 (Figure 3f). These data indicate that the Y1662 residue of kalirin-7 is critical for fyn-mediated dissociation of kalirin-7 from PSD-95.
ErbB4 activation causes kalirin-7 tyrosine phosphorylation in the C-terminus
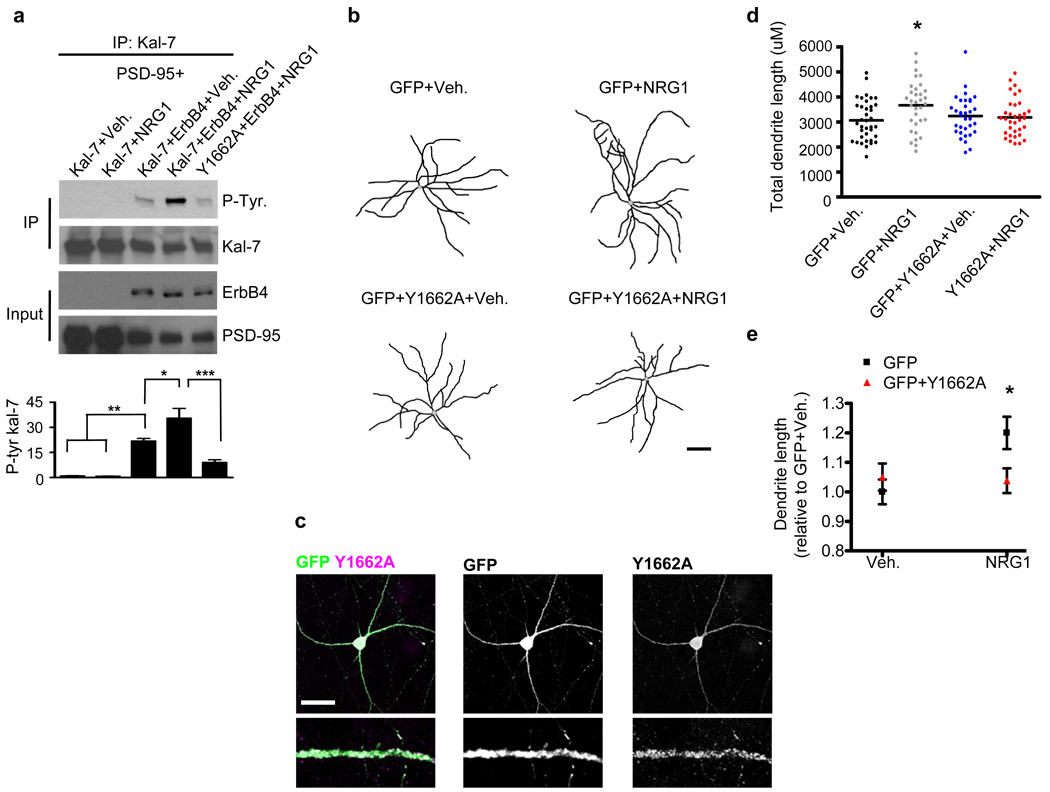
Next, we hypothesized that the kalirin-7-Y1662 residue is similarly the primary residue for NRG1-mediated phosphorylation on kalirin-7. To test this we overexpressed kalirin-7 in hEK293 cells with or without erbB4, in combination with NRG1 or vehicle treatment. We found that the overexpression of erbB4 in combination with vehicle treatment increased kalirin-7 tyrosine phosphorylation levels relative to cells expressing kalirin-7 without erbB4. This is likely due to the intrinsic kinase activity of the erbb4 receptor. The activation of erbB4 (erbB4+NRG1) further increased kalirin-7 tyrosine phosphorylation, an effect that was not detected in cells expressing the Y1662A mutant (Figure 4a). Although the full length erbB4 receptor can undergo intracellular cleavage,32–33 upon overexpression in hEK293 cells the vast majority of erbB4 remains in the full-length form with very little intracellular cleavage of the receptor occurring (Supplementary Figure 4d). This suggests that the erbB4 effects on kalirin-7 are likely mediated by the full length receptor.
Figure 4. The Y1662 kalirin-7 residue is important for NRG1-mediated interneuronal growth.
(a) hEK293 cells were transfected with PSD-95 in addition to WT kalirin-7 or Y1662A kalirin-7. ErbB4 was overexpressed in some conditions. Cells were treated with NRG1β or vehicle for 5-hours as indicated. Lysates were immunoprecipitated for kalirin-7 and subsequently probed for phospho-tyrosine. ErbB4 overexpression increased kalirin-7 tyrosine phosphorylation. Treatment of erbB4 overexpressing cells with NRG1β further increased kalirin-7 tyrosine phosphorylation, an effect not seen in cells overexpressing the Y1662A mutant. Graph displays change relative to the Kal-7+Veh. condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=3 independent experiments; *p<0.05, **p<0.01, ***p<0.001
(b) Representative traces of DIV28 cultured cortical multipolar interneuron dendritic trees. Axons were omitted for clarity. Neurons expressed either GFP alone or in combination with Y1662A kalirin-7 for 3-days. 1-day posttransfection, neurons were treated with either NRG1β (5nM) or with vehicle for 2 days. Scale bar=100um.
(c) Overexpressed Y1662A mutant kalirin-7 is strongly targeted to interneuronal dendrites. Scale bar=35um.
(d) Scatter plots. NRG1β treated interneurons overexpressing GFP show an increase in total dendritic length relative to vehicle treated cells. Inteneurons overexpressing Y1662A mutant kalirin-7 showed no significant changes in total dendritic length with either vehicle or NRG1β treatment. Each point represents the total dendritic length of a single cell. Significance determined using a one-way ANOVA with Tukey post-hoc. Black lines are the mean, n=33–37 cells per condition; *p<0.05
(e) Significant interaction of NRG1β treatment by Y1662A mutant expression with regard to total interneuronal dendritic length. Significance determined using a 2×2 ANOVA and the interaction term determined. Data are the mean±SEM; *p<0.05
The Y1662 kalirin-7 residue is crucial for NRG1-mediated interneuron growth
As kalirin RNAi blocks the effects of NRG1 on interneuronal dendritic growth, and because the Y1662 residue is critical for NRG1-mediated kalirin-7 tyrosine phosphorylation, we reasoned that Y1662A mutant kalirin-7 could act as a dominant negative for NRG1’s effects on interneuron growth. We thus examined the effects of Y1662A mutant kalirin-7 in affecting basal and NRG1-dependent dendrite growth in cultured cortical interneurons (Figure 4b). Overexpressed Y1662A kalirin-7 was targeted to the dendrites of multipolar interneurons (Figure 4c). We found that the overexpression of the Y1662A mutant with vehicle did not alter interneuronal dendrite growth relative to vehicle-treated control cells (Figures 4d and e), similar to our findings with kalirin RNAi. Whereas NRG1 increased dendritic growth relative to vehicle-treated control cells (p<0.05), the Y1662A mutant blocked NRG1-mediated interneuron growth (Vehicle: 3065±132µm; NRG1: 3666±171µm; Y1662A+Vehicle: 3232±141µm; Y1662A+NRG1: 3181±130µm) (Figures 4d and e and Supplementary Figure 4e). These data indicate that the Y1662 site of kalirin-7 is important for NRG1-mediated increases in interneuronal dendritic length.
Discussion
The molecular mechanisms underlying the correct wiring of inhibitory circuits are incompletely understood yet have profound implications for normal brain function and for the pathogenesis of psychiatric disorders. Indeed, a reduction in multipolar interneuronal dendritic length has been reported in the schizophrenia cortex.1 The genes encoding NRG1 and erbB4 have been repeatedly associated with schizophrenia;8, 34 nevertheless, the biological functions of NRG1 and erbB4 are incompletely understood. Little is known about NRG1 regulation of interneuronal structure in mature cells, and the relevant downstream targets of soluble NRG1 in mature cortical neurons remained mostly unidentified.
Here we show that kalirin plays a key role in regulating interneuron dendrite growth downstream of NRG1 in mature cortical interneurons, and point to an important role of phosphorylation in kalirin’s C-terminus as the critical site for these effects. Most studies characterizing the roles of NRG1, erbB4, and kalirin in neuronal structure have focused on pyramidal neurons.14–18, 35 Together this is suggestive of common intracellular mechanisms of dendrite morphology in interneurons and in pyramidal neurons. However, because erbB4 shows robust expression in multipolar interneurons with reduced expression in pyramidal neurons,9–10 the NRG1-erbB4-kalirin signal transduction pathway revealed here is likely of particular importance in regulating interneuron growth. This is consistent with our finding that NRG1 fails to alter the dendritic length of mature pyramidal neurons.
In this study we identify novel links of disease susceptibility molecules to regulators of neuronal morphology. Of potential importance is that a recent study uncovered several missense mutations in the KALRN gene in schizophrenia patients that are theorized to have detrimental consequences on the gene’s function.19 Moreover, kalirin shows altered levels in schizophrenia,20–21 and interacts with the product of the DISC1 schizophrenia susceptibility gene.36 Interestingly, KALRN-null mice, and mice hypomorphic for NRG1 and erbB4 genes show similar behavioral phenotypes.37–39 Whether interactions between NRG1/erbB4 and kalirin are relevant to schizophrenia pathogenesis in humans remains an intriguing question for the future.
Supplementary Material
Acknowledgements
This work was supported by grants from NIH-NIMH (R01MH071316), National Alliance for Research on Schizophrenia and Depression (NARSAD) to P.P., NIH (MH078833) to U.M., Ruth L. Kirschstein National Research Service Awards 1F31AG031621-01A2 to M.E.C and 1F31MH085362 to K.A.J., Christopher Reeve Foundation to C.S.B. All experiments involving animals were done according to the Institutional Animal Care and Use Committee of Northwestern University.
Footnotes
Conflict of interest
None of the authors have completing financial interests in relation to the work described.
References
- 1.Kalus P, Bondzio J, Federspiel A, Muller TJ, Zuschratter W. Cell-type specific alterations of cortical interneurons in schizophrenic patients. Neuroreport. 2002;13:713–717. doi: 10.1097/00001756-200204160-00035. [DOI] [PubMed] [Google Scholar]
- 2.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain research reviews. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 5.Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT, et al. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS biology. 2006;4:e29. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 7.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nature reviews. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 11.Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Molecular and cellular neurosciences. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Krivosheya D, Tapia L, Levinson JN, Huang K, Kang Y, Hines R, et al. ErbB4-neuregulin signaling modulates synapse development and dendritic arborization through distinct mechanisms. The Journal of biological chemistry. 2008;283:32944–32956. doi: 10.1074/jbc.M800073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma XM, Huang J, Wang Y, Eipper BA, Mains RE. Kalirin, a multifunctional Rho guanine nucleotide exchange factor, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci. 2003;23:10593–10603. doi: 10.1523/JNEUROSCI.23-33-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, et al. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 19.Kushima I, Nakamura Y, Aleksic B, Ikeda M, Ito Y, Shiino T, et al. Resequencing and Association Analysis of the KALRN and EPHB1 Genes And Their Contribution to Schizophrenia Susceptibility. Schizophrenia bulletin. doi: 10.1093/schbul/sbq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 21.Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008;1239:235–248. doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 23.Kojima S, Vignjevic D, Borisy GG. Improved silencing vector co-expressing GFP and small hairpin RNA. BioTechniques. 2004;36:74–79. doi: 10.2144/04361ST02. [DOI] [PubMed] [Google Scholar]
- 24.Helmstaedter M, Sakmann B, Feldmeyer D. L2/3 interneuron groups defined by multiparameter analysis of axonal projection, dendritic geometry, and electrical excitability. Cereb Cortex. 2009;19:951–962. doi: 10.1093/cercor/bhn130. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Cahill ME, Penzes P. Kalirin loss results in cortical morphological alterations. Molecular and cellular neurosciences. 2010;43:81–89. doi: 10.1016/j.mcn.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J Neurosci. 2008;28:711–724. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penzes P, Cahill ME, Jones KA, Srivastava DP. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008;18:405–413. doi: 10.1016/j.tcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Chakrabarti K, Lin R, Schiller NI, Wang Y, Koubi D, Fan YX, et al. Critical role for Kalirin in nerve growth factor signaling through TrkA. Molecular and cellular biology. 2005;25:5106–5118. doi: 10.1128/MCB.25.12.5106-5118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. The Journal of biological chemistry. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 32.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. The Journal of biological chemistry. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 33.Vecchi M, Carpenter G. Constitutive proteolysis of the ErbB-4 receptor tyrosine kinase by a unique, sequential mechanism. The Journal of cell biology. 1997;139:995–1003. doi: 10.1083/jcb.139.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 35.Penzes P, Jones KA. Dendritic spine dynamics--a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- 38.Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.