Abstract
Background
Bortezomib, an inhibitor of the 26S proteasome and NF-κB, may have antitumor activity in adenoid cystic carcinoma (ACC). Preclinical studies have shown synergy between bortezomib and doxorubicin.
Methods
Eligibility criteria included incurable ACC, any number of prior therapies but without an anthracycline, unidimensionally measurable disease, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and ejection fraction within normal limits. Patients with stable disease for 9 months or more were excluded. Patients received bortezomib 1.3 mg/m2 IV push on days 1,4,8, and 11, every 21 days, until progression. Doxorubicin 20 mg/m2 IV on days 1 and 8 was added at the time of progression.
Results
25 patients were enrolled of whom 24 were eligible; the most common distant metastatic sites were the lung (n=22) and the liver (n=7). There was no objective response with single-agent bortezomib; best response was stable disease in 15 of 21 evaluable patients (71%). The median progression-free survival and overall survival were 6.4 months and 21 months, respectively. Of 10 evaluable patients who received bortezomib plus doxorubicin, 1 had a partial response and 6 stable disease. The most frequent toxicity with bortezomib was grade 3 sensory neuropathy (16%). With bortezomib plus doxorubicin serious toxicities seen more than once were grade 3–4 neutropenia (n=3) and grade 3 anorexia (n=2).
Conclusions
Bortezomib was well tolerated and resulted in disease stabilization in a high percentage of patients but no objective responses. The combination of bortezomib and doxorubicin was also well tolerated and may warrant further investigation in ACC.
Keywords: head and neck cancer, adenoid cystic carcinoma, bortezomib, doxorubicin
Introduction
Adenoid cystic carcinoma (ACC) comprises approximately 20% of all malignant salivary gland tumors and is the most common cancer type that arises from the minor salivary glands. ACC of the head and neck has a propensity for perineural invasion and demonstrates high local recurrence rates. However, these tumors may exhibit a protracted natural history with indolent growth of metastatic lung disease 1–2. Common sites of metastatic disease are the lung, liver, and bone. Traditional chemotherapeutics have mild to modest activity in salivary gland tumors 3. A handful of prospective studies have focused on patients with ACC. A phase II study of epirubicin showed a 10% response rate, whereas 29% of patients noted symptomatic improvement 4. Mitoxantrone as well as 5-FU have also been reported to have modest single-agent activity 5–6. Combinations that include doxorubicin, such as cyclophosphamide, doxorubicin, and cisplatin 7–8 as well as vinorelbine 9–10 have shown activity in salivary gland tumors, including ACC. The Eastern Cooperative Oncology Group (ECOG) conducted a phase II trial of paclitaxel (E1394) in patients with chemotherapy naïve, advanced or metastatic salivary gland malignancies. Although paclitaxel demonstrated modest activity in mucoepidermoid carcinoma (3 of 13 responded) and adenocarcinoma histologies (5/17), no responses were observed in 14 patients with ACC 11. Despite lack of response, the 5-year survival for patients with ACC was 32% and the median survival was 25 months 11. The evaluation of novel systemic agents in patients with ACC is highly warranted.
Bortezomib (PS-341) is highly selective inhibitor of the 26S proteasome which is central for the ubiquitin-proteasome that is involved in the degradation of multiple key regulatory proteins of the cell cycle. Bortezomib has demonstrated preclinical activity in a variety of malignant tumors and is synergistic with doxorubicin 12 13–14, one of the few drugs with modest activity in ACC. The combination of bortezomib and doxorubicin is well-tolerated and has shown promise for the treatment of multiple myeloma 15–16. NF-κB, a key target of bortezomib, is expressed in adenoid cystic carcinoma and relates to angiogensis and poor patient outcome 17–18. Inhibition of NF-κB activity can suppress the growth of salivary gland tumors 19. Moreover, the combination of chemotherapy and inhibition of NF-κB enhances the antitumor activity of chemotherapy in transformed salivary gland cells 20. Therefore, NF-κB inhibitors, such as bortezomib, may be a promising therapy for salivary gland tumors. We conducted a multicenter phase II trial of bortezomib followed by doxorubicin plus bortezomib at the time of progression in patients with incurable adenoid cystic carcinoma in order to evaluate the efficacy and toxicities of this regimen.
Patients and Methods
Patient selection
Eligible patients were age 18 years or older with locally advanced, recurrent, or metastatic adenoid cystic carcinoma of the head and neck which was considered incurable by known therapies. Any number of prior therapies but no prior anthracyclines (doxorubicin, epirubicin, daunorubicin, idarubicin) or mitoxantrone, or bortezomib were allowed. Other key eligibility criteria included unidimensionally measurable disease measured within 3 weeks prior to registration according to RECIST 21, ECOG performance status 0–2, left ventricular ejection fraction at or above the institutional lower limits of normal, serum creatinine within normal institutional limits or creatinine clearance ≥60 mL/min/1.73 m2 for patients with creatinine levels above institutional normal, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 times the institutional upper limit of normal, absolute neutrophil count 1500/µL or more, platelets of at least 100,000/µL, and total bilirubin within normal limits. Patients with stable disease at study entry for 9 months or more were excluded. Pregnant women as well as patients with pre-existing neuropathy of grade > 1, known brain metastases, human immunodeficiency virus positivity, history of congestive heart failure or uncontrolled intercurrent illness were excluded. The study was coordinated by the ECOG (protocol E1303) and was registered in clinicaltrials.gov (NCT00077428). The study protocol was approved by the Institutional Review Board of each ECOG institution and all patients signed written informed consent. Archival baseline tumor tissue was retrieved for biomarker studies.
Treatment plan
Bortezomib was administered at a dose of 1.3 mg/m2as an intravenous (IV) push over 3–5 seconds, twice weekly for 2 weeks, followed by one week off treatment (i.e. days 1, 4, 8, and 11, every 21 days) until disease progression at which time doxorubicin was added at a dose of 20 mg/m2, given as IV push over 2 to 5 minutes with extravasation precautions, on days 1 and 8, every 21 days (starting within 10–28 days from the last bortezomib administration) assuming patients had an ECOG performance status 0–2 and adequate organ and marrow function, and no evidence of congestive heart failure. Bortezomib was given first followed one hour later by doxorubicin; a minimum of 72 hours should have elapsed between bortezomib doses. Cardioprotection with dexrazoxane 200 mg/m2 IV (i.e. dexrazoxane/doxorubicin ratio of 10:1) was administered prior to doxorubicin and after bortezomib on days 1 and 8 starting with the 8th cycle (i.e. cumulative doxorubicin dose of 280 mg/m2) and all subsequent cycles. Treatment with bortezomib and doxorubicin continued until progression or to maximum of 14 cycles (i.e. cumulative doxorubicin dose of 560 mg/m2).
Study assessments and monitoring
Baseline evaluations included complete blood counts (CBC), chemistry studies, including liver function tests, Multigated Acquisition (MUGA) scan or echocardiogram to assess left ventricular ejection fraction. Scans and x-rays were used to assess all measurable or non-measurable sites of disease within 3 weeks of registration. Patients were reevaluated for tumor response after every two cycles or 6 weeks. Objective response was evaluated using the RECIST 21. When a patient was deemed to have an objective response, tumor measurements were to be repeated 6 weeks later to confirm the response. Toxicities were assessed using the NCI Common Terminology Criteria for Adverse Events (CTAE) Version 3.0. CBC, chemistry studies and liver function tests were performed in the beginning of each cycle and CBC on the day of each bortezomib or doxorubicin administration.
Statistical design and methods
The primary endpoint of the study was objective tumor response rate (including complete and partial overall responses) among patients receiving single-agent bortezomib. A two-stage design was employed. A true response rate of 20% was considered worthy of further study, whereas a true response rate of 5% was considered not worthy of further study. To allow for 10% ineligibility rate, a total of 23 patients were to be accrued in the first stage in order to obtain 21 eligible patients. If at least 2 responses were observed in the first stage, another 14 patients would have been accrued in the second stage in order to obtain 13 eligible patients. Otherwise, accrual would be terminated. If at least 4 responses were observed, the treatment would be considered promising and worthy of further study. Based on the accrual of previous ECOG studies in salivary gland tumors (e.g. E1394) the study was expected to take approximately 2.3 years to accrue a total of 37 patients.
The analysis of efficacy outcomes excluded ineligible patients; toxicity analysis excluded patients who never received treatment. Overall survival (OS) was defined as the time from registration to death from any cause or censored at the time of last contact. Time to progression (TTP) was defined as the time from registration to progression. Progression-free survival (PFS) was defined as the time from registration to documented progression or death without progression. Patients without documented progression or death were censored at the time of the last disease assessment. The survival data were analyzed using the Kaplan-Meier method 22. No comparison of study outcomes by patient characteristics, such as race and sex, was made since the study not powered to detect such differences.
Results
From June 2004 to June 2005, a total of 25 patients entered the study. Of these 25 patients, 1 patient was ineligible because not all areas of disease were assessed within 3 weeks of registration. Thus, 24 patients were classified as eligible for the study. Thirteen of these patients were registered to the second step of the study after progressing on single-agent bortezomib, one of whom was ineligible because of lack of documentation of progressive disease on bortezomib. Among the eligible patients, there were 20 deaths. Two patients died without documented progression by objective criteria.
Patient characteristics and treatment
Table 1 provides demographics and disease characteristics at the time of the enrollment for eligible patients (n=24). The majority of patients were females and their performance status was 0–1. Most patients (n=22) had lung metastases. Ten patients had prior chemotherapy, of whom 7 patients had more than one chemotherapy regimen, 21 patients had prior surgery and 23 patients had prior radiation therapy. The median number of bortezomib cycles was 5 (range: 1–37) and of bortezomib and doxorubicin was 7.5 (range: 1–14). Disease progression was the most frequent reason for withdrawal from study treatment (Table 2). One patient completed the maximum 14 cycles of combined bortezomib and doxorubicin.
Table 1.
Patient characteristics (N=24)
| Na (%) | |
|---|---|
| Age (Years) | |
| Median (range) | 57 (22–73) |
| Gender | |
| Male | 7 (29) |
| Female | 17 (71) |
| Race | |
| White | 19 (83) |
| Black | 3 (13) |
| Asian | 1 (4) |
| Unknown | 1 (4) |
| Performance status | |
| 0 | 10 (42) |
| 1 | 13 (54) |
| 2 | 1 (4) |
| Weight loss in previous 6 months | |
| <5% of body weight | 22 (92) |
| 5–10% of body weight | 2 (8) |
| Primary site | |
| Oral tongue | 1 (4) |
| Floor of mouth | 1 (4) |
| Hard palate | 3 (13) |
| Nasopharynx, NOS | 2 (8) |
| Salivary glands, NOS | 1 (4) |
| Parotid | 4 (17) |
| Submaxillary | 1 (4) |
| Ethmoid | 1 (4) |
| Nasal cavity | 1 (4) |
| More than one site | 3 (12) |
| Other | 6 (25) |
| Primary site status at study entry | |
| Eradicated, no recurrence | 6 (25) |
| Eradicated, but recurred locally | 5 (21) |
| Residual disease after prior therapy | 12 (50) |
| Untreated | 1 (4) |
| Status of regional lymph nodes | |
| Never involved | 14 (64) |
| Never involved, but removed prophylactically | 3 (14) |
| Involved nodes eradicated | 2 (9) |
| Involved nodes eradicated, but new involvement | 1 (4) |
| Involved nodes not treated | 2 (9) |
| Unknown | 2 |
| Metastatic site involvement | |
| Lung | 22 (92) |
| Liver | 7 (31) |
| Bone | 1 (4) |
| Soft tissue | 2 (8) |
| Prior Treatment | |
| Chemotherapy | 10 (42) |
| Radiation therapy | 23 (96) |
| Surgery | 21 (86) |
except for age where actual age is shown.
NOS, not otherwise specified
Table 2.
Reasons for withdrawal from study
| Reasons | Step 1 (N=24) N (%) | Step 2 (N=12) N (%) |
|---|---|---|
| Treatment completed | - | 1(8.0%) |
| Disease progression | 13 (54 %) | 7 (54%) |
| Adverse events | 5 (21 %) | 0(0.0%) |
| Death on study a | 1 (4.0%) | 0(0.0%) |
| Withdrawal or refusal | 4 (17 %) | 2 (17%) |
| Alternative therapy | 0(0.0%) | 1(8.0%) |
| Other | 1 (4.0%)b | 1(8.0%)c |
Death was considered likely related to progression and unrelated to treatment
Progression could not be confirmed because cytology of new effusion was not obtained
Symptomatic deterioration with worsening performance status.
Objective response
Twenty-one patients were evaluable for response (Table 3). No complete or partial responses were observed with single agent bortezomib but 15 patients had stable disease (71%) and 6 patients had disease progression as their best response (Table 3). The median duration of stable disease was 4.2 months (range, 0–20.1). There was no difference in the percentage of patients with stable disease according to prior chemotherapy status: stable disease was observed in 9 out 12 (75%) chemotherapy naive patients and 6 out of 9 (67%) chemotherapy exposed patients. Ten patients who received bortezomib and doxorubicin were evaluable for response. One patient (10%), who had received no prior chemotherapy, achieved a partial response, 6 patients (60%) had stable disease, and 3 patients (30%) experienced disease progression as best response. The median duration of stable disease was 5.22 months (range, 0–10.09). Partial response or stable disease was observed in 5/7 (71%) patients without prior chemotherapy and 2/3 (67%) patients with a history of prior chemotherapy.
Table 3.
Best objective response
| Bortezomib alone | Bortezomib plus doxorubicin | |||||
|---|---|---|---|---|---|---|
| N | % evaluable |
% overall |
N | % evaluable |
% overall |
|
| Complete response | - | - | - | - | - | - |
| Partial response | - | - | - | 1 | 10 | 8 |
| Stable disease | 15 | 71 | 63 | 6 | 60 | 50 |
| Progressive disease | 6 | 29 | 25 | 3 | 30 | 25 |
| Non-evaluable | 3 a | - | 12 | 2 b | - | 17 |
- The only follow-up scan was done more than 10 weeks after registration and showed progression.
- Baseline evaluation of target lesions not done within three weeks prior to registration.
- The patient developed a new pleural effusion after 2 cycles but no fluid cytology was obtained at that time. Not all target lesions were assessed.
- Inconsistent tumor measurements.
- alternative treatment (surgery).
Progression-free and overall survival
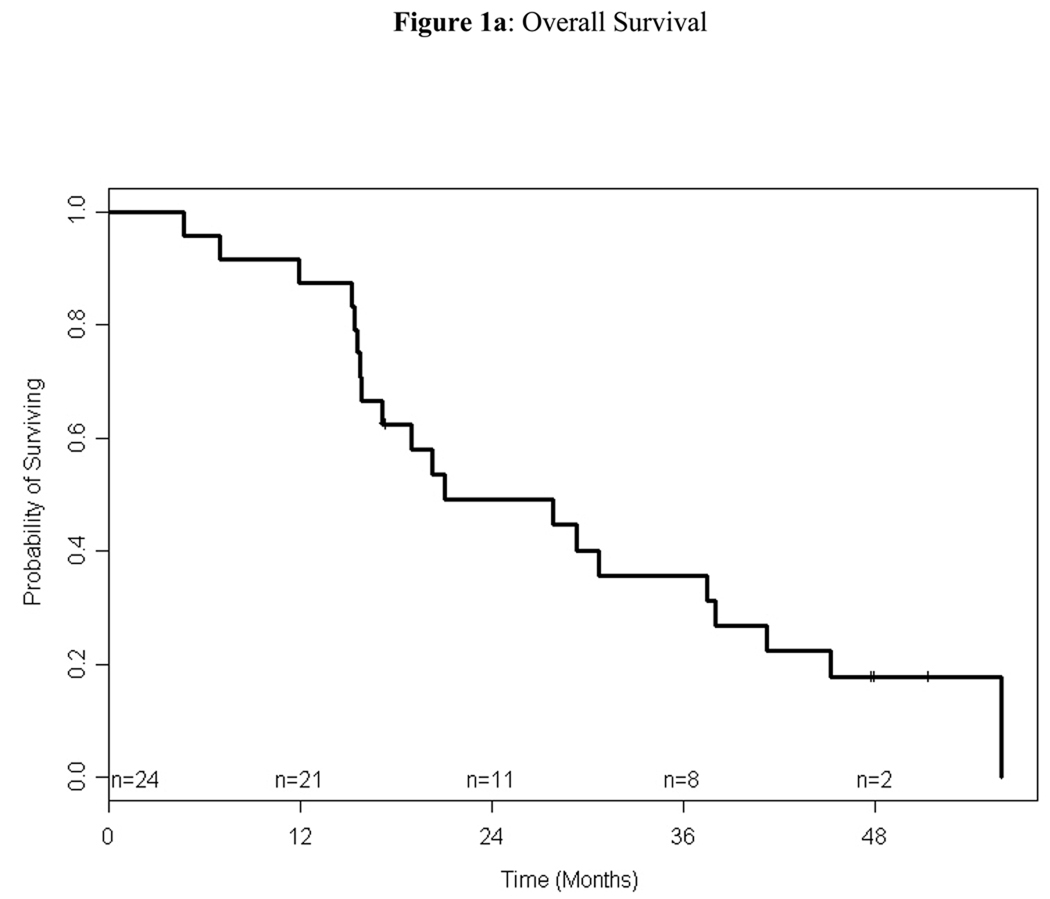
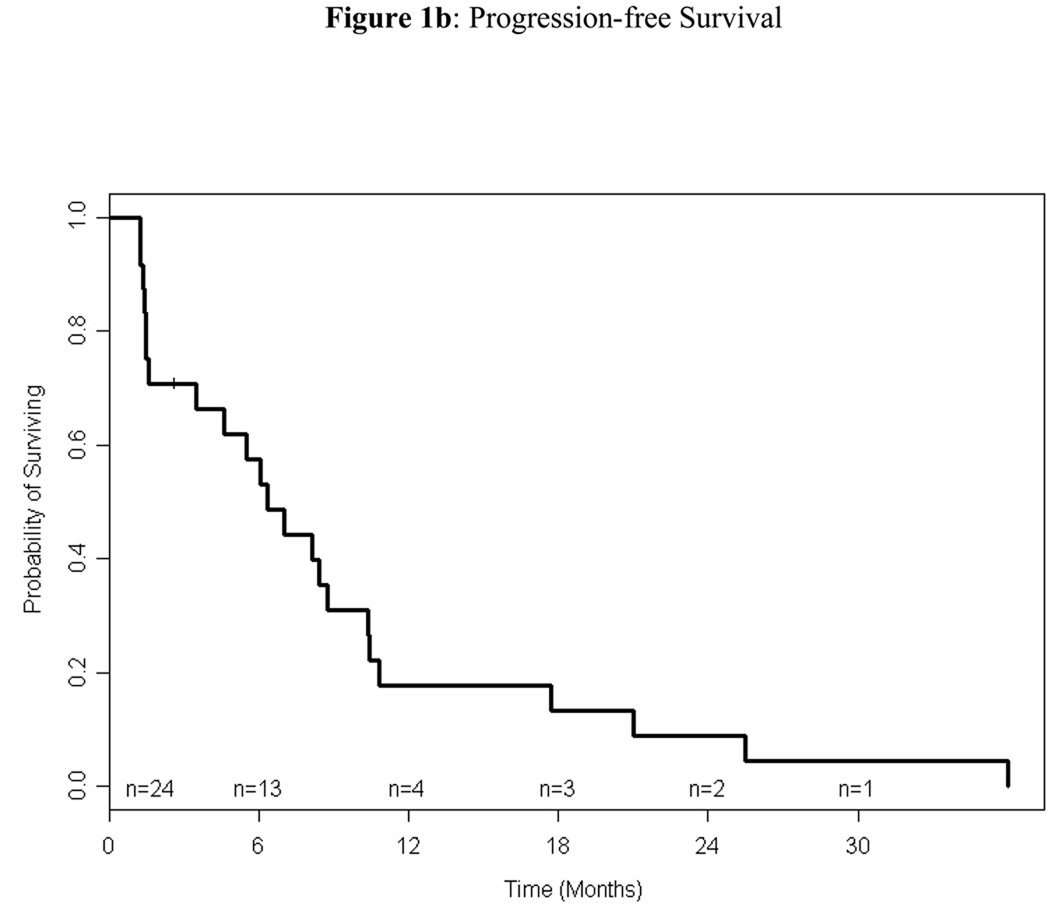
Among the 24 patients treated on study, 4 (17%) were alive at the time of analysis. For alive patients, the median follow-up time was 48 months (range: 17–51). The median and 1-year PFS were 6.37 months (95% CI: 1.61–8.74) and 18% (95% CI: 2%–33%), respectively, and the median and 1-year OS were 21 months (95% CI: 15.8–38.0) and 88% (95% CI: 74%–100%), respectively (Figures 1). Of the 12 eligible patients who received bortezomib and doxorubicin, 10 (83%) had documented progression. The median TTP of these patients was 5.63 months (95% CI: 1.05–8.51); the median TTP of the same patients on single-agent bortezomib was 2.49 months (95% CI: 1.28–8.44)
Figure 1.
Kaplan-Meier estimation of overall survival (1a) and progression-free survival (1b). The median overall survival was 21 months (95% CI, 15.8–38) and the median progression-free survival was 6.4 months (95% CI, 1.6–8.7).
Toxicity
Table 4 and 5 show grade 1 or higher toxicities related (possibly, probably or definitely) to treatment for all patients (eligible and ineligible) who received treatment in step 1 (bortezomib) and step 2 (bortezomib plus doxorubicin). Single-agent bortezomib was associated with rare grade 3 or 4 toxicities, all of which had a frequency of less than 10%, except sensory neuropathy which was noted in 16% of patients (Table 4). Among 12 patients treated with bortezomib plus doxorubicin, 1 patient had missing toxicity data; only 2 serious toxicities, neutropenia and anorexia, developed in more than 1 patient (Table 5). There was no treatment-related death. One patient died on study but the cause of death was reported as likely due to disease progression. The family reported that the patient died at home in his sleep; no autopsy was performed.
Table 4.
Grade 1–4 toxicities in patients treated with single agent bortezomib (N=25)
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Anemia | 13 | 3 | - | - |
| Lymphopenia | - | - | 1 | - |
| Neutropenia | 3 | 1 | 2 | - |
| Thrombocytopenia | 10 | 1 | 2 | - |
| Hypotension | - | 2 | 1 | - |
| Fatigue | 8 | 7 | 2 | - |
| Fever without neutropenia | - | 1 | - | - |
| Insomnia | 2 | 1 | - | - |
| Rigors/chills | 1 | 1 | - | - |
| Sweating | 2 | - | - | - |
| Weight gain | 1 | - | 1 | - |
| Weight loss | 5 | 1 | - | - |
| Flushing | 1 | - | - | - |
| Alopecia | 1 | - | - | - |
| Injection site reaction | 2 | - | - | - |
| Pruritus/itching | 1 | - | - | - |
| Rash/desquamation | 4 | - | - | - |
| Anorexia | 5 | 1 | 2 | - |
| Constipation | 5 | 1 | - | - |
| Dehydration | 1 | 1 | 2 | - |
| Diarrhea without prior colostomy | 5 | 3 | 2 | - |
| Dry mouth | 1 | - | - | - |
| Gastritis | 1 | - | - | - |
| Dyspepsia | - | 1 | - | - |
| Stomatitis by exam, | 3 | 1 | - | - |
| Stomatitis (symptom) | - | 1 | - | - |
| Nausea | 7 | 3 | 2 | - |
| Obstruction, ileum | 1 | - | - | - |
| Taste disturbance | 1 | - | - | - |
| Vomiting | 1 | 1 | 1 | - |
| Gastrointestinal-other | - | 1 | - | - |
| Urinary tract infection with grade 0–2 neutropenia | - | 1 | - | 1 |
| Edema, limb | 1 | - | - | - |
| Edema, trunk/genital | 1 | - | - | - |
| Alkaline phosphatase | 3 | 1 | - | - |
| Elevated ALT | 3 | 1 | - | - |
| Elevated AST | 3 | - | - | - |
| Hypercalcemia | 1 | - | - | - |
| Elevated creatinine | 3 | - | - | - |
| Hyperkalemia | 1 | - | - | - |
| Hypokalemia | 3 | - | - | - |
| Hypernatremia | 1 | - | - | - |
| Hyponatremia | 5 | - | 2 | - |
| Nonneuropathic generalized weakness | - | - | 2 | - |
| Dizziness | 3 | - | - | - |
| Depression | 1 | - | - | - |
| Neuropathy-motor | - | 1 | - | - |
| Neuropathy-sensory | 3 | 3 | 4 | - |
| Dry eye syndrome | 1 | - | - | - |
| Tearing | 1 | 1 | - | - |
| Abdomen, pain | 2 | 5 | - | - |
| Back, pain | 1 | - | - | - |
| Chest pain | 4 | - | - | - |
| Extremity-limb, pain | - | 1 | - | - |
| Headache | 2 | 3 | 1 | - |
| Joint pain | - | 1 | - | - |
| Muscle pain | 1 | 2 | - | - |
| Neuropathic pain | 1 | - | - | - |
| Pain, not otherwise specified | 1 | - | - | - |
| Tumor pain | 1 | - | - | - |
| Cough | 2 | - | - | - |
| Dyspnea | 3 | - | 1 | - |
| Urinary retention | 1 | - | - | - |
Table 5.
Grade 1–4 toxicities in patients treated with bortezomib plus doxorubicin (N=12†)
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Anemia | 9 | 1 | - | - |
| Lymphopenia | - | 1 | 1 | - |
| Neutropenia | 1 | 2 | 2 | 1 |
| Thrombocytopenia | 7 | - | - | - |
| Fatigue | 4 | 6 | - | - |
| Fever without neutropenia | 1 | - | - | - |
| Rigors/chills | 1 | 1 | - | - |
| Weight loss | 1 | 2 | - | - |
| Alopecia | 6 | 1 | - | - |
| Injection site reaction | 1 | - | - | - |
| Rash/desquamation | 3 | 1 | - | - |
| Anorexia | 2 | 2 | 2 | - |
| Constipation | 4 | 1 | - | - |
| Diarrhea without prior colostomy | 3 | 1 | 1 | - |
| Dry mouth | 1 | - | - | - |
| Gastritis | 1 | - | - | - |
| Dyspepsia | 1 | - | - | - |
| Stomatitis by exam | 3 | 1 | - | - |
| Stomatitis (symptom) | 4 | 1 | - | - |
| Nausea | 7 | 2 | - | - |
| Obstruction, ileum | 1 | - | - | - |
| Vomiting | 2 | 1 | - | - |
| Stoma, upper respiratory hemorrhage | 1 | - | - | - |
| Infection with grade 0–2 neutropenia, upper airway | - | 1 | - | - |
| Edema, head and neck | 1 | - | - | - |
| Elevated alkaline phosphatase | 3 | 1 | - | - |
| Elevated ALT | 1 | - | 1 | - |
| Elevated AST | - | - | - | 1 |
| Elevated bilirubin | - | - | 1 | - |
| Elevated gamma-glutamyl transferase | - | 1 | - | - |
| Hypokalemia | 1 | - | - | - |
| Hyponatremia | 2 | - | - | - |
| Neuropathy, cranial nerve VII | 1 | - | - | - |
| Neuropathy, cranial nerve XII | 1 | - | - | - |
| Neuropathy-sensory | 3 | 1 | - | - |
| Dry eye syndrome | 1 | - | - | - |
| Vision-blurred | 1 | - | - | - |
| Tearing | 1 | - | - | - |
| Abdominal pain | 1 | - | 1 | - |
| Chest pain | 1 | - | - | - |
| Esophageal pain | 1 | - | - | - |
| Facial pain | 1 | - | - | - |
| Headache | 1 | - | - | - |
| Joint pain | 1 | - | - | - |
| Neck pain | 1 | - | - | - |
| Oral cavity pain | 1 | - | - | - |
| Pain, not otherwise specified | 1 | - | - | - |
| Cough | 1 | - | - | - |
| Dyspnea | 1 | - | - | - |
One patient is missing toxicity data in step 2
Discussion
Adenoid cystic carcinoma responds poorly to traditional chemotherapies. Attempts at palliation with systemic therapy are usually reserved for symptomatic or rapidly progressive disease. As several molecular abnormalities have been described in adenoid cystic carcinoma, the study of novel targeted agents has attracted significant interest. Bortezomib is an agent approved for hematologic malignancies that inhibits NF-κB which may be critical to the growth of salivary gland tumors. We conducted a phase II trial of bortezomib in patients with incurable adenoid cystic carcinoma that accrued rapidly in the cooperative group setting which confirmed the major unmet need for new therapies in salivary gland cancers. The toxicity profile of bortezomib was expected with sensory neuropathy being the most frequent serious toxicity. Although we did not observe an objective response with single-agent bortezomib, the median PFS and OS were 6.37 months (95% CI: 1.61–8.74) and 21 months (95% CI: 15.8–38.0), respectively. Moreover, the addition of doxorubicin to bortezomib at the time of progression resulted in 1 partial response among 10 evaluable patients and the median TTP was longer with combination therapy versus monotherapy in the patients who received combination therapy. It is unclear whether this reflects the activity of doxorubicin or the effect of combined therapy.
It is desirable to study adenoid cystic carcinoma separately from other salivary gland cancer histologies given their differential responses to chemotherapy 11. Only a few prospective clinical trials have been conducted exclusively in patients with adenoid cystic carcinoma of the head and neck. Despite high levels of expression of c-kit (90% or more) and EGFR/HER-2 there have been no objective response with imatinib 23–24 or lapatinib 25. In the imatinib study by Hotte at al 9 out of 15 patients had stable disease as best response 24, whereas in the lapatinib study by Agulnik et al among 19 assessable patients, 15 patients (79%) had stable disease (SD), and 9 patients (47%) had stable disease of 6 months or more 25. Other studies evaluated trastuzumab 26, gefitinib 27, and cetuximab 28 in salivary gland cancers, including patients with adenoid cystic carcinoma, but did not report objective responses. A phase II trial with cetuximab enrolled 30 patients, 23 with adenoid cystic carcinoma 28. Although there were no objective responses, 50% of patients achieved stable disease that lasted 6 or more months and the median time-to-progression was 6 months. In general, it has been difficult to compare results across studies because the natural history of adenoid cystic carcinoma is variable and sometimes very protracted. Stable disease, even prolonged, may not be the effect of treatment but rather represent indolent disease. In this study, we elected to exclude patients with very slow growing tumors that were stable for the previous 9 or more months by RECIST. Ultimately, randomized, placebo-controlled studies will be important to assess the impact of treatment on the natural history of disease. In the ECOG experience, neither paclitaxel in a previous study 11 nor bortezomib in the present trial resulted in objective responses. The median survival we reported in this trial is comparable to the median survival seen in the previous ECOG trial with paclitaxel (21 versus 25 months). A median PFS of 6 months or stable disease of 6 or more months in 50% of patients may be an appropriate null hypothesis for future trials in advanced adenoid cystic carcinoma. Nevertheless, the addition of doxorubicin to bortezomib at the time of disease progression resulted in one objective response and promising TTP and is a potentially active combination. Upfront treatment with bortezomib and doxorubicin may be worthwhile investigating and is the subject of a phase II clinical trial conducted at the University of Pittsburgh 29. Finally, although it was originally planned to evaluate the expression of biomarkers which may be affected by the ubiquitin-proteasome degradation pathway (e.g. NF-κB) on tumor tissue, only 8 tumor samples could be retrieved and this analysis was not performed. Molecular characterization of adenoid cystic carcinoma should be attempted in future studies.
Acknowledgments
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA39229, CA17145, CA27525, CA13650, CA16116 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Preliminary results from this study were reported at the 2006 meeting of the American Society of Clinical Oncology
Financial disclosures: Dr. Argiris has received research support from Millennium Pharmaceuticals, Inc
References
- 1.Jones AS, Hamilton JW, Rowley H, Husband D, Helliwell TR. Adenoid cystic carcinoma of the head and neck. Clin Otolaryngol Allied Sci. 1997;22:434–443. doi: 10.1046/j.1365-2273.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 2.Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495–498. doi: 10.1016/s0002-9610(97)00153-0. [DOI] [PubMed] [Google Scholar]
- 3.Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. doi: 10.1200/JCO.2005.05.3025. [DOI] [PubMed] [Google Scholar]
- 4.Vermorken JB, Verweij J, de Mulder PH, et al. Epirubicin in patients with advanced or recurrent adenoid cystic carcinoma of the head and neck: a phase II study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1993;4:785–788. doi: 10.1093/oxfordjournals.annonc.a058665. [DOI] [PubMed] [Google Scholar]
- 5.Verweij J, de Mulder PH, de Graeff A, et al. Phase II study on mitoxantrone in adenoid cystic carcinomas of the head and neck. EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1996;7:867–869. doi: 10.1093/oxfordjournals.annonc.a010770. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, Sutherland DJ. Chemotherapy for adenocystic carcinoma. Cancer. 1980;46:452–454. doi: 10.1002/1097-0142(19800801)46:3<452::aid-cncr2820460305>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Dreyfuss AI, Clark JR, Fallon BG, Posner MR, Norris CM, Jr, Miller D. Cyclophosphamide, doxorubicin, and cisplatin combination chemotherapy for advanced carcinomas of salivary gland origin. Cancer. 1987;60:2869–2872. doi: 10.1002/1097-0142(19871215)60:12<2869::aid-cncr2820601203>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Licitra L, Cavina R, Grandi C, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients. Ann Oncol. 1996;7:640–642. doi: 10.1093/oxfordjournals.annonc.a010684. [DOI] [PubMed] [Google Scholar]
- 9.Airoldi M, Bumma C, Bertetto O, Gabriele P, Succo G, Pedani F. Vinorelbine treatment of recurrent salivary gland carcinomas. Bull Cancer. 1998;85:892–894. [PubMed] [Google Scholar]
- 10.Airoldi M, Pedani F, Succo G, et al. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer. 2001;91:541–547. doi: 10.1002/1097-0142(20010201)91:3<541::aid-cncr1032>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert J, Li Y, Pinto HA, et al. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2006;28:197–204. doi: 10.1002/hed.20327. [DOI] [PubMed] [Google Scholar]
- 12.Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5:2638–2645. [PubMed] [Google Scholar]
- 13.Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–1144. [PubMed] [Google Scholar]
- 14.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 15.LoConte NK, Thomas JP, Alberti D, et al. A phase I pharmacodynamic trial of bortezomib in combination with doxorubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2008;63:109–115. doi: 10.1007/s00280-008-0719-5. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008;19:1160–1165. doi: 10.1093/annonc/mdn018. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Peng B. NF-kappaB promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell Prolif. 2009;42:150–161. doi: 10.1111/j.1365-2184.2009.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Peng B, Chen X. Expressions of nuclear factor kappaB, inducible nitric oxide synthase, and vascular endothelial growth factor in adenoid cystic carcinoma of salivary glands: correlations with the angiogenesis and clinical outcome. Clin Cancer Res. 2005;11:7334–7343. doi: 10.1158/1078-0432.CCR-05-0241. [DOI] [PubMed] [Google Scholar]
- 19.Azuma M, Motegi K, Aota K, Yamashita T, Yoshida H, Sato M. TGF-beta1 inhibits NF-kappaB activity through induction of IkappaB-alpha expression in human salivary gland cells: a possible mechanism of growth suppression by TGF-beta1. Exp Cell Res. 1999;250:213–222. doi: 10.1006/excr.1999.4503. [DOI] [PubMed] [Google Scholar]
- 20.Motegi K, Azuma M, Aota K, et al. Effect of a mutant form of IkappaB-alpha on 5-fluorouracil-induced apoptosis in transformed human salivary gland cells. Oral Oncol. 2001;37:185–192. doi: 10.1016/s1368-8375(00)00071-3. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Statis Assoc. 1958;53:457–481. [Google Scholar]
- 23.Pfeffer MR, Talmi Y, Catane R, Symon Z, Yosepovitch A, Levitt M. A phase II study of Imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007;43:33–36. doi: 10.1016/j.oraloncology.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585–590. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- 25.Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978–3984. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 26.Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39:724–727. doi: 10.1016/s1368-8375(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 27.Glisson BS, Blumenschein G, Francisco M, Erasmus J, Zinner R, Kies M. Phase II trial of gefitinib in patients with incurable salivary gland cancer. Journal of Clinical Oncology, 2005 ASCO Annual Meeting Proceedings. Vol 23, No. 16S Part I of II, (June 1 Supplement), 2005: 5532 2005. [Google Scholar]
- 28.Locati LD, Bossi P, Perrone F, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral Oncol. 2009;45:574–578. doi: 10.1016/j.oraloncology.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Kotsakis T, Gooding WE, Argiris A. Phase II trial of doxorubicin (D) and bortezomib (B) in patients with incurable adenoid cystic carcinoma of the head and neck. ASCO. 2010 [Google Scholar]