Abstract
Recent studies have highlighted a connection between infection during pregnancy and increased risk for autism in the offspring. Parallel studies of cerebral spinal fluid, blood, and postmortem brains reveal an ongoing, hyper-responsive inflammatory-like state in many young as well as adult autism subjects. There are also indications of gastrointestinal problems in at least a subset of autistic children. Work with animal models of the maternal infection risk factor indicate that aspects of brain and peripheral immune dysregulation can be begin during fetal development and be maintained through adulthood. The offspring of infected, or immune-activated dams also display cardinal behavioral features of autism, as well as neuropathology consistent with that seen in human autism. These rodent models are proving useful for the study of pathogenesis and gene-environment interaction, as well as for the exploration of potential therapeutic strategies.
Maternal infection and autism
There is little public awareness that infection during pregnancy significantly increases the probability of the offspring becoming schizophrenic. In fact, it has been estimated that if viral (influenza, Herpes simplex virus, rubella), bacterial (urinary tract) and parasitic (toxoplasma) infections could be prevented in pregnant women, >30% of schizophrenia cases could be eliminated (1). The public health implications are enormous, but not widely recognized (2). Similarly, there is little public or scientific awareness that maternal infection also increases the risk for development of autism in the offspring. An extraordinary recent study of over 10,000 autism cases in the Danish Medical Register found a strong association with maternal viral infection in the first trimester and a less robust, but significant association with maternal bacterial infection in the second trimester (3). These new results greatly extend prior work on the connection between maternal infection and autism (4). Supporting the epidemiology, recent results with rodent models of the maternal infection risk factor reveal that the offspring display features of autism, as well as immune-related disruptions in the brain and periphery. Moreover, new work on human autism spectrum disorders (ASD) reinforces this immune connection.
Immune-related abnormalities in autism
A variety of organ systems exhibit inflammatory-like changes in autism. The evidence comes from quantifying immune-related proteins and RNAs, as well as immunohistochemistry. Findings from epidemiology are also relevant.
Brain and CSF
A groundbreaking paper by Carlos Pardo and colleagues (5) revealed an inflammatory-like state in post-mortem autism brains as indicated by elevated cytokines and activated microglia and astrocytes. Importantly, these changes were found in subjects ranging in age from 5 to 44 years old, indicating that this immune-activated state is established early and appears to be permanent. Moreover, cytokine elevation was also found in the cerebral spinal fluid (CSF) of living autistic children ages 3 to 10 years old. Recent results from studies of some of the same postmortem brains and new autism brain samples, as well as CSF, have supported these conclusions (6, 7). Consistent with these findings are results from a variety of microarray studies that show dysregulation of immune-related genes (e.g. cytokines and chemokines) in autistic brains (8). It is also clear that there is considerable heterogeneity among the autism samples, as might be expected from the extreme disparities in behavioral symptoms among ASD subjects.
Peripheral immune system and GI tract
Possibly related to the inflammatory-like state in the central nervous system are abnormalities in the peripheral immune system (9). Although there have been many papers on this topic over the years, recent reports from Judy Van de Water and Paul Ashwood have utilized blood samples from a well characterized, large cohort of ASD children. These authors report that, compared to controls and non-ASD children with developmental disabilities, several cytokines and chemokines, including interleukin-1β (IL-1β), IL-6, IL-8 and IL-12p40, are elevated in the ASD plasma of very young children (ages 2–5 years old), and that these increases are associated with more impaired communication and aberrant behaviors (8, 10, 11). In addition, peripheral blood mononuclear cells display altered cytokine responses to stimulation in vitro (12, 13).
Although an early report from Wakefield and colleagues muddied the waters considerably, there have been several subsequent papers providing evidence of inflammation in the gastrointestinal (GI) tract of at least a subset of ASD children (14, 15). These findings include immune cell infiltrates present in the colon, ileum and duodenum, as well as increased T cell activation in the intestinal mucosa. These inflammatory changes are associated with autoimmune responses that could contribute to the observations of decreased mucosal integrity, or “leaky gut” (16). Disruption of the mucosal barrier can also occur in the apparent absence of inflammation, however, as in irritable bowel syndrome. Thus, leaky gut symptoms do not necessarily connote inflammation. This issue needs to be clarified in ASD, as does the question of the frequency of GI symptoms in ASD compared to controls. These are difficult questions to answer because of the problems in obtaining GI samples from ASD and control children without overt GI symptoms. Perhaps related to GI symptoms is the finding of an abnormal gut microbiota composition in ASD (17). It is thus of particular interest that a small study of antibiotic treatment aimed at the gut found temporary improvement in some behavioral symptoms (9). This is potentially important, as it represents a possibly safe intervention and thus could be followed up with a large, blinded study. In addition, dietary modification is reported to provide behavioral improvements for some ASD children (14). There is considerable interest among parents of autistic children regarding the possibility of adverse reactions to certain dietary components. This could then lead to GI problems such as a leaky gut, which may or may not have an inflammatory basis.
The possibility of a connection between peripheral immune abnormalities and altered behavior in ASD is fascinating, but unsubstantiated, at this point. On the one hand, it could be that genetic susceptibility or an environmental insult biases the subject toward both brain and immune dysregulation simultaneously and that these symptoms occur independently. For example, several ASD candidate genes are known to regulate both brain and immune system development and/or function (9). On the other hand, it is possible that immune irregularities, such as in peripheral immune cells or the GI tract, interact with an abnormal brain to exacerbate behavioral symptoms. For instance, it is well known from animal and human studies that elevated peripheral cytokines can cause striking changes in behavior (4).
Autoimmune connections
Further support for immune system dysregulation comes from epidemiologic studies of ASD. The largest of these found that some autoimmune diseases (rheumatoid arthritis, celiac disease and type 1 diabetes) are more common in mothers of ASD children than in mothers of typically developing children (18). These results and those from prior studies (9) could mean that an abnormal immune system is genetically passed on to the offspring. It is also possible that maternal autoimmune reactions could have deleterious effects on fetal brain development. The latter hypothesis receives support from the finding that ~12% of mothers with children with ASD offspring have anti-fetal brain antibodies in their serum, a figure significantly higher than in mothers of typical children (9). Animal studies support the idea that such antibodies could be relevant for pathophysiology; when IgG from human mothers of children with ASD is injected into rhesus macaques or mice at midgestation, some of the offspring display abnormal behaviors that are not seen in offspring of animals injected with IgG from mothers of typically developing children (19, 20).
Animal models of the maternal infection risk factor
The maternal infection risk factor is currently being studied in mice, rats and monkeys. The experiments involve infecting the mother or simply activating her immune system in the absence of pathogens.
Rodent models
The epidemiologic evidence highlighting maternal infection as a risk factor for autism and schizophrenia has stimulated the development of several rodent models. These involve infection of pregnant mice or rats (nasal application of influenza virus) or mimicking such infections by activating the maternal immune system in the absence of pathogen. The latter approach has proven particularly popular, and involves maternal injection of the synthetic double stranded RNA, poly(I:C), to evoke an anti-viral inflammatory response, or maternal injection of lipopolysaccharide (LPS), to evoke an anti-bacterial inflammatory response. Although these three approaches undoubtedly yield somewhat different cascades of gene activation, analysis of the offspring has thus far revealed considerable overlap in behavioral abnormalities and neuropathology (4,21). Moreover, similar results have been obtained in both the mouse and rat models of maternal immune activation (MIA). Several cardinal symptoms of autism are observed in the offspring of immune-activated dams, including deficits in communication (assayed by ultrasonic vocalizations; 22) and social interaction (assayed in the 3 chamber paradigm; 23). Other behaviors in the offspring that are consistent with autism symptoms include elevated anxiety and a prepulse inhibition deficit (4, 21). There is also a Theory of Mind deficit in autism, in which the subject has difficulty intuiting the thoughts of another person, which can lead to social difficulties. Approaching this type of deficit in rodents is just beginning, using assays for empathy (24), for instance. Regarding neuropathology, the offspring of infected mothers, or mothers given poly(I:C), also exhibit several abnormalities commonly found in autism including a spatially-restricted deficit in Purkinje cells in the cerebellum (25).
Because maternal infection is a risk factor for both autism and schizophrenia, it is not surprising that some of the features of the latter disorder have also been found in the offspring of immune-activated mothers. These include enlarged ventricles, changes in the serotonergic and dopaminergic pathways, as well as enhanced responses to a hallucinogen (4, 21, 27). At least some of these symptoms can be differentially expressed as a function of the timing of the maternal infection (28). It is also possible that the severity of MIA is a factor in which symptoms are expressed in the offspring. Genetic background also likely influences the outcome of maternal infection in terms of ASD versus schizophrenia. It should also be pointed out that, in addition to the obvious phenotypic differences, there are a number of striking similarities between schizophrenia and ASD phenotypes in humans. These include some shared behavioral abnormalities as well as neuropathological features (29). These overlaps in phenotypes make it difficult to firmly identify features that can be used to distinguish ASD and schizophrenia phenotypes in animal models. The response to hallucinogenic drugs, or even the presence of spontaneous hallucinogen-like activity in the brain, is a potentially fruitful area that can be explored in this context. Regarding features specific to autism, deficits in male neonate and adult communication are found in the MIA mouse model (22), and this can be further examined by analyzing the qualitative nature of these ultrasonic vocalizations (USVs), and as a function of the social settings in which they occur (24). That is, experiments can evaluate the types of syllables, their grouping and order, the consistency of usage in various social situations, how they change with development, and whether there is indeed “mouse song” that has the characteristics of bird song.
How does activation of the maternal immune response alter fetal brain development? The manipulation of cytokines has revealed that the elevation of the pro-inflammatory cytokine IL-6 (which is induced by MIA) is essential for development of the abnormal behaviors and changes in brain gene expression in the offspring (23). That is, injection of IL-6 alone is sufficient to yield the abnormal behaviors in the offspring seen with MIA. Conversely, blocking IL-6 during MIA prevents the development of these behaviors. Moreover, elevation of the anti-inflammatory cytokine IL-10 also protects against MIA (30). These results support the theory that the balance between pro-and anti-inflammatory influences is important in fetal brain development.
Where do these cytokines act? Several groups have found that MIA induces pro-inflammatory cytokines in the fetal brain itself. For example, IL-6 mRNA and protein are elevated in the fetal brain following maternal poly(I:C) administration. This finding is consistent with a feed-forward, self-reinforcing inflammatory cycle. This possibility needs to be further tested with assays in postnatal offspring to determine if parallels to the findings in autism brains can be found. It must also be determined if maternal IL-6 (or other signals) is directly responsible for evoking the cytokine responses observed in the fetal brain.
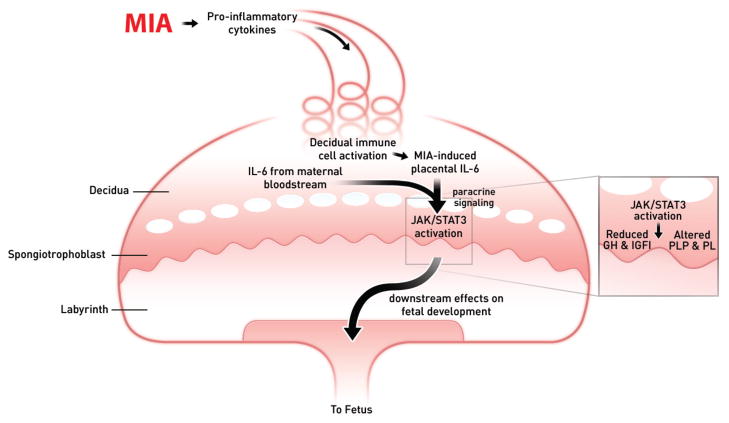
Other work has highlighted the placental response to MIA as an indirect pathway towards altering fetal brain development (31,32). Maternal injection of poly(I:C) increases IL-6 mRNA as well as maternally-derived IL-6 protein in the placenta. This activates the endogenous immune cells in the decidua, and maternally-derived IL-6 activates the Janus kinase (JAK)- signal transducer and activator of transcription 3 (STAT3) pathway specifically in the spongiotrophoblast layer, which results in expression of acute phase genes. Importantly, this parallels an IL-6-dependent disruption of the growth hormone-insulin-like growth factor axis in the placenta. Together, these IL-6-mediated effects of MIA represent an indirect mechanism by which MIA can alter fetal development (Fig. 1). There is also severe placental inflammation when pregnant rats are given a high dose of LPS, and this reaction can be blocked by administration of an IL-1 receptor antagonist (33). Interestingly, a greater occurrence of placental trophoblast inclusions is found in placental tissue from births of children who go on to develop ASD compared to non-ASD controls (34). Relevant in this context are findings that chorioamnionitis and other obstetric complications are significantly associated with socialization and communication deficits in autistic infants (35).
Figure 1. Summary of MIA-induced effects on the placenta.
Maternal injection of poly(I:C) activates the maternal immune system, elevating IL-6, which enters the spiral arteries that descend through the decidua and spongiotrophoblast layers, filling the maternal bloodspaces of the labyrinth. Resident immune cells in the decidua are activated to express CD69 and further propagate the inflammatory response. IL-6 produced by decidual cells acts on target cells in the spongiotrophoblast layer. Ligation of the IL-6Ra with gp130 causes JAK/STAT3 activation and increases in acute phase proteins, such as SOCS3, and down-regulation of placental growth hormone (GH) production. This leads to reduced insulin-like growth factor binding protein 3 (IGFBP3) and IGFI. Global changes in STAT3 activation in the spongiotrophoblast layer alter the production of placenta-specific pro-lactin protein (PLP) and other pro-lactin proteins. These various changes in endocrine factors very likely to lead to acute placental pathophysiology and subsequent effects on fetal development. (Reproduced from ref. 31, with permission.)
As noted above, a number of abnormalities have been found in peripheral immune cells in autism. Thus, it is of interest that T cells in mouse MIA offspring are in a hyper-responsive state for at least a year after birth [36]. The prolonged nature of this pathology lends further support to the hypothesis of a feed-forward, self-reinforcing cycle that begins in during gestation and continues through adulthood. This is also consistent with the findings of immune-related abnormalities in the brains of adult autism cases.
Non-human primate model
Thus far, there is a single report of a non-human primate model of maternal infection using nasal application of influenza virus in the 3rd trimester of rhesus monkey pregnancy. The choice of 3rd trimester does not, however, fit with what is known about the windows of vulnerability for development of schizophrenia or autism. In the offspring of the infected monkeys, widespread reduction in gray matter volume in the cortex, and reduced white matter volume in the parietal cortex is observed (26). The infants born to infected mothers appear to show signs of early autonomy from the mother, yet also exhibit increased distress. Given the striking similarities between human and non-human primate behaviors during normal early postnatal development (unlike rodents), much could be done with this type of model using infection, LPS or poly(I:C).
Gene-environment interactions
A variety of mental disorders have been attributed in part to genes that increase the susceptibility to environmental risk factors. To date, however, there is very little evidence for such gene-environment interactions. Therefore, it is of interest that mice heterozygous for the tuberous sclerosis 2 (Tsc2) gene display a social interaction deficit only when they are born to mothers treated with poly(I:C) (37). That is, this behavioral deficit is most severe when the MIA environmental risk factor is combined with a genetic defect that, in humans, also carries a high risk for ASD. In addition, there is an excess of TSC-ASD individuals born during the peak influenza season, an association that is not seen for TSC individuals not displaying ASD symptoms (37). Similar experiments have been done looking at the effects of poly(I:C) MIA in lines of DISC1 mice. DISC1 (disrupted in schizophrenia 1) is a gene associated with schizophrenia, bipolar and major depressive disorders, as well as autism (38). In an inducible transgenic mouse line expressing a human truncated DISC1 protein in the forebrain, the combination of MIA and prenatal transgene expression results in increased anxiety and depression-like symptoms and decreased social interaction compared to either insult alone (39). An unexpected finding is that the combined gene-environmental risk factors cause a decrease in ventricular enlargement compared to either alone. This combination of insults alters the levels of several cytokines; notably IL-1β and IL-6 are increased. Much remains to be done in this important line of experiments that combine environmental risk factors with ASD candidate genes, both in characterizing the phenotypes and in exploring the cellular and molecular sites of action of each factor. Results from combining these factors should illuminate the pathways for each of them.
Therapeutic manipulations
The findings summarized in the prior sections demonstrate that the maternal infection and MIA models display face (similar symptoms) as well as construct (similar cause) value for both autism and schizophrenia. These models can have predictive value as well. For example, the manipulation of cytokines during pregnancy can prevent the development of abnormal behaviors in the offspring in the poly(I:C) and LPS models. In addition, pretreatment of pregnant rats with N-acetyl-cysteine, which increases calcium influx when binding to glutamate receptors in combination with the transmitter, and also suppresses fetal inflammatory responses to LPS, prevents many of the effects of maternal LPS administration (4). The ability of IL-10 to block the effects of MIA is an attractive intervention because endogenous IL-10 is essential for resistance to LPS-induced preterm labor and fetal loss. Thus, administration of this cytokine enhances a natural protective mechanism. However, increased IL-10 in the absence of MIA in pregnant mice can lead to behavioral abnormalities in the adult offspring (30), a finding consistent with the fact that normal human pregnancy involves increased inflammation. Therefore, postnatal cytokine perturbations would potentially be a safer therapeutic approach. It is also clear that postnatal cytokine manipulations can induce behavioral changes in the absence of MIA (40). Such manipulations in MIA models could be a fruitful area of research.
In fact, MIA models have proven valuable for testing other types of postnatal therapies. Whereas acute antipsychotic drug administration in adult influenza and poly(I:C) MIA offspring can ameliorate some of the behavioral deficits (4,21), administration of such medications in immature MIA offspring, before the onset of behavioral abnormalities and ventricular enlargement, is effective in preventing the onset of such symptoms (41,42). Treatment for a week during adolescence, many weeks before beginning behavioral testing, prevents the onset of abnormalities and the ventricular enlargement. Therefore, despite the fact that MIA has induced many changes in the brain during fetal development, postnatal behaviors can be subsequently altered. Although the classic action of these antipsychotic medications involves blockade of the D2 dopamine receptor in the brain, it is also worth noting in the present context that many of them have also been shown to influence cytokine expression in peripheral immune cells (4).
It is also important to note, in the context of potential postnatal treatments, that in mouse models of a number of rare genetic disorders with autistic symptoms such as fragile X, Rett syndrome and TSC, behavioral abnormalities can be at least partially reversed in adulthood. These findings have led to several clinical trials in these disorders (43).
Concluding remarks
A variety of techniques have been used to demonstrate the presence of a sub-clinical, inflammatory-like state in the brain, CSF and peripheral immune system in many ASD samples. There is also evidence for abnormalities in the GI tract, although the prevalence and the precise phenotype in that system remain to be determined. Mouse and rat models that mimic the autism maternal infection risk factor display face, construct, and predictive validity for ASD. Many of the symptoms in these rodent models are also similar to those expected for a schizophrenia model, which is consistent with the fact that maternal infection is a validated risk factor for the latter disorder as well. To aid in distinguishing ASD from schizophrenia behavioral symptoms in this and many other animal models, it will be of great interest to further develop assays for hallucination-like activity in the brain (27), and to explore the qualitative features of USVs (24). The application of electrophysiological tools to MIA models has only just begun (44, 45), and the same is true of combining MIA with ASD candidate genes. Some of the genes near the top of the list for future testing include CNTNAP2 (contactin associated protein-like 2) and MET receptor kinase, the former because of its connection with language (46), and the latter because of its roles in the nervous, immune and GI systems (47). Another area of great promise is extending MIA to non-human primates, where ASD-like behaviors can be assessed in much more human-like context than in rodents. The first report on the young offspring of influenza-infected rhesus mothers has recently appeared (26), and the development of non-human primate poly(I:C) and LPS models should prove useful.
Acknowledgments
Work cited from the author’s laboratory was supported by the National Institute of Mental Health, the California Institute of Regenerative Medicine, and the Autism Speaks and Binational Science foundations. Due to space limitations, reviews are cited rather than primary research articles wherever possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am J Psychiat. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AS, Patterson PH. Maternal infection and schizophrenia: Implications for prevention. Schiz Bull. 2011 doi: 10.1093/schbul/sbq146. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atladottir HO, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Devel Dis. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 4.Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Vargas DL, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 6.Chez MG, et al. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediat Neurol. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Morgan JT, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Lintas C, et al. Genome-wide expression studies in Autism spectrum disorder, Rett syndrome, and Down syndrome. Neurobiol Disease. 2011 doi: 10.1016/j.nbd2010.11.010. in press. [DOI] [PubMed] [Google Scholar]
- 9.Careaga M, et al. Immune dysfunction in autism: a pathway to treatment. J Amer Soc Exper NeuroTherap. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashwood P, et al. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.10.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwood P, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashwood P, et al. Altered T cell responses in children with autism. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.09.002. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enstrom AM, et al. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buie T, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 15.Ashwood P, et al. Autism, Gastrointestinal Disturbance, and Immune Dysfunction: What is the Link? In: Chauhan A, Chauhan V, BTW, editors. Autism: Oxidative Stress, Inflammation, and Immune Abnormalities. CRC Press; Boca Raton: 2010. p. 278. [Google Scholar]
- 16.deMagistris L, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their fist-degree relatives. J Pediatr Gastroenterol Nutrition. 2010;51:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 17.Feingold SM, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Atladotiir HO, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2010;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- 19.Martin LA, et al. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer HS, et al. Prenatal exposure of antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Progr Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Malkova N, et al. Maternal immune activation causes a deficit in social and communicative behavior in male mouse offspring. Program No. 561.29. Neurosci Mtg Planner, San Diego: Soc Neurosci. 2010 on line. [Google Scholar]
- 23.Smith SEP, et al. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman JL, et al. Behavioral phenotyping assays for mouse models of autism. Nature Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, et al. Activation of the maternal immune response alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short SJ, et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno JL, et al. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J Neurosci. 2011;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer U, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer U, et al. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric Res. 2011 doi: 10.1203/PDR.0b013e318212c196. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer U, et al. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10mediated anti-inflammatory signaling. Molec Psychiat. 2008;13:208–21. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- 31.Mandal M, et al. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2010.09.011. on line. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao E, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2010.12.017. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girard S, et al. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010;184:3997–4005. doi: 10.4049/jimmunol.0903349. [DOI] [PubMed] [Google Scholar]
- 34.Anderson GM, et al. Placental trophoblast inclusions in autism spectrum disorder. Biol Psychiatry. 2007;61:487–491. doi: 10.1016/j.biopsych.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 35.Limperopoulos C, et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics. 2008;121:758–765. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsiao E, et al. Modeling an autism risk factor in mice leads to permanent changes in the immune system. Internl Soc Autism Res. 2010;130:124. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehninger D, et al. Gestational immune activation and TSC2 haploinsufficiency cooperate to disrupt social behavior in mice. Molec Psychiatry. 2010 doi: 10.1038/mp.2010.115. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilpinen H, et al. Association of DISC1 with autism and Asperger syndrome. Molec Psychiatry. 2008;13:187–196. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- 39.Abazyan B, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe Y, et al. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64:217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 41.Piontkewitz Y, et al. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva A, Ehninger D. Adult reversal of cognitive phenotypes in neurodevelopmental disorders. J Neurodev Disorders. 2009;1:150–157. doi: 10.1007/s11689-009-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito HT, et al. Maternal immune activation alters nonspatial information processing in the hippocampus of the offspring. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickerson DD, et al. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alarcorn M, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Amer J Hum Gen. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell DB, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123:1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]