Abstract
Testosterone (T) is critical for the activation of aggressive behaviours. In many vertebrate species, circulating T levels rapidly increase after aggressive encounters during the early or mid- breeding season. During the late breeding season, circulating T concentrations did not change in wild male white-crowned sparrows after an aggressive encounter, and in these animals, changes in local neural metabolism of T might be more important than changes in systemic T levels. Local neural aromatization of T into 17β-œstradiol (E2) often mediates the actions of T, and we hypothesized that in the late breeding season, brain aromatase is rapidly modulated after aggressive interactions, leading to changes in local concentrations of E2. Here, wild male white-crowned sparrows in the late breeding season were exposed to simulated territorial intrusion (STI) (song playback and live decoy) or control (CON) for 30 min. STI significantly increased aggressive behaviours. Using Palkovits punch technique, 13 brain regions were collected. There was high aromatase activity in several nuclei, but enzymatic activity in the CON and STI groups did not differ in any region. E2 concentrations were much higher in the brain than the plasma. STI did not affect circulating levels of E2 but rapidly reduced E2 concentrations in the hippocampus, ventromedial nucleus of the hypothalamus, and bed nucleus of the stria terminalis. Surprisingly, there were no correlations between aromatase activity and E2 concentrations in the brain, nor were aromatase activity or brain E2 correlated with aggressive behaviour or plasma hormone levels. This is one of the first studies to measure E2 in microdissected brain regions, and the first study to do so in free-ranging animals. These data demonstrate that social interactions have rapid effects on local E2 concentrations in specific brain regions.
Keywords: estradiol, estrogens, songbird, aggression, hippocampus
INTRODUCTION
Numerous behavioural actions of testosterone, including the regulation of aggressive and reproductive behaviour, require the aromatization of testosterone into 17β-œstradiol (E2) within the brain (1–4). This conversion of testosterone into E2 is catalyzed by the enzyme cytochrome P450 aromatase (CYP19). Aromatase expression in the avian and mammalian brain is normally restricted to specific neuronal populations located mainly in the hypothalamic/preoptic and limbic system (5–7), as well as in the telencephalon in songbirds (8–10). Aromatase activity is modulated at the transcriptional level, a slow process (11–13). In addition, rapid modulation of aromatase activity has been demonstrated recently. In vitro, a rapid reduction of aromatase activity is observed in homogenate of the quail medial preoptic area incubated in calcium-rich and phosphorylating conditions (14, 15). Also, the activation of glutamatergic receptors in medial preoptic area explants rapidly reduces aromatase activity (16). In vivo, aromatase activity is rapidly reduced in specific brain regions following expression of sexual behaviour in quail (17).
Rapid, local changes in aromatase activity are likely to modulate the concentrations of strogens within specific locations (18, 19). Although mostly studied for its actions at the genomic level (20), E2 also affects brain and behaviour rapidly and independently of de novo transcription. For example, within seconds to minutes, E2 modulates neuronal firing rate (21), a variety of intracellular signalling pathways (22), and reproductive behaviours (23–25). E2 also rapidly influences agonistic behaviour. Indeed, aggressive behaviour in Beach mice and California mice rapidly (within 10 min) increases following E2 treatment (26, 27). In zebra finches, acute treatment with E2 increases the neuronal firing rate evoked by song playback within 5 min in the caudomedial nidopallium (NCM), a cortical-like auditory region involved in song recognition (28, 29).
Surprisingly, only one study has investigated local concentrations of E2 in the brain immediately following behavioural interactions (30). The quanti cation of E2 in discrete brain regions is technically challenging, so most work has focused on aromatase activity and assumed that E2 concentrations change in parallel with aromatase. To our knowledge, E2 quantification at the level of a single nucleus in wild animals has not been performed, nor has concurrent measurement of brain aromatase activity and E2 concentrations within individuals.
We recently developed a specific and sensitive assay to measure local E2 concentrations (31) and the goals of the present study were to 1) measure the E2 content in discrete brain regions in wild male white-crowned sparrows (Zonotrichia leucophrys pugetensis), 2) correlate aromatase activity and E2 concentration within individuals and 3) test whether exposure to a 30-min simulated territorial intrusion (STI) affects aromatase activity and/or E2. We hypothesized that an aggressive interaction would rapidly affect aromatase activity, leading to a subsequent change in E2 concentrations.
MATERIALS AND METHODS
All animal protocols were approved by the University of British Columbia Committee on Animal Care and followed the guidelines of the Canadian Council on Animal Care, and all necessary permits were obtained.
Behavioural recording and blood sampling
Experiment 1
Wild adult male white-crowned sparrows from Vancouver, B.C. Canada were studied in the late breeding season, just before moulting (July 4–27, 2006 and July 8–16, 2007). Data on aggressive behaviour and plasma levels of testosterone, progesterone, corticosterone and corticosteroid binding globulin were previously presented (32), and here we focus on a randomly selected subset (n=20) of these subjects and provide important new data on aromatase activity and œstradiol levels in 13 brain regions (approximately 520 data points).
Briefly, behavioural tests and blood sampling were performed between 06:00 and 12:00. A mist net was quickly set up, furled, and placed near the ground within a subject’s territory. Subjects were exposed for 30 min to either (i) a simulated territorial intrusions using conspecific song playback and live decoy (STI; n=11) or (ii) an empty cage without playback (CON; n=9). During the challenge, we recorded the time it took for the subject to fly toward the decoy (latency), and the numbers of songs, flights, trills and wing waves. We also recorded the amount of time spent within 1 meter and 5 meters of the decoy. Songs and flights toward an intruder are recognized as aggressive in this context (33, 34) and trills and wing waves are also observed during territorial disputes (33, 35). A composite aggression score (PC1) was obtained from a principal components analysis and loaded heavily on the number of songs, number of flights, and time spent near the decoy and loaded only moderately on latency to approach (32). Here, the behavioural data for only the present subset of subjects was analyzed.
After the CON or STI, the mist net was quickly unfurled and playback was used to capture subjects within 7 min (CON: 3.97 ± 0.69 min; STI: 3.33 ± 0.69 min, df=18, t=0.63, p=0.54). Blood from the jugular and brachial veins was collected, and subjects were immediately sacrificed by rapid decapitation (time of sacrifice after capture, CON: 3.58 ± 0.36 min, STI: 3.81 ± 0.27 min, df=18, t=0.52, p=0.61). The brain was quickly removed from the skull and frozen on powdered dry ice. The testes were also collected and frozen on powdered dry ice. The brains and testes were kept at −80°C.
Several endocrine variables were measured in these plasma samples by techniques that are fully detailed in (32). Briefly, circulating levels of CBG were determined using a radioligand binding assay. Corticosterone levels in plasma were measured using a corticosterone radioimmunoassay (MP Biomedicals, Cat# 07-120103) that was validated for use with songbird plasma. Testosterone was extracted from plasma with dichloromethane and measured using a testosterone radioimmunoassay (DSL-4100) that was modified to increase sensitivity. Progesterone was extracted from plasma with diethyl ether and measured using a progesterone radioimmunoassay (DSL-3400) that was modified to increase sensitivity. The endocrine data obtained from only the brachial vein for the present subset of subjects was analyzed.
Experiment 2
To assess possible changes in systemic E2 levels, another group of wild adult male white-crowned sparrows was studied again in the late breeding season, just before moulting (July 14–26, 2010). Subjects were exposed to a simulated territorial intrusion using conspecific song playback and live decoy for 15 min (STI 15min: n=7) or 30 min (STI 30min: n=7). Controls were exposed to an empty cage without playback for 30 min (CON; n=7). After the CON or STI, the mist net was quickly unfurled and playback was used to capture subjects within 7 min. Upon capture, a blood sample (~150 μl) was immediately collected from the brachial vein.
Palkovits punch technique
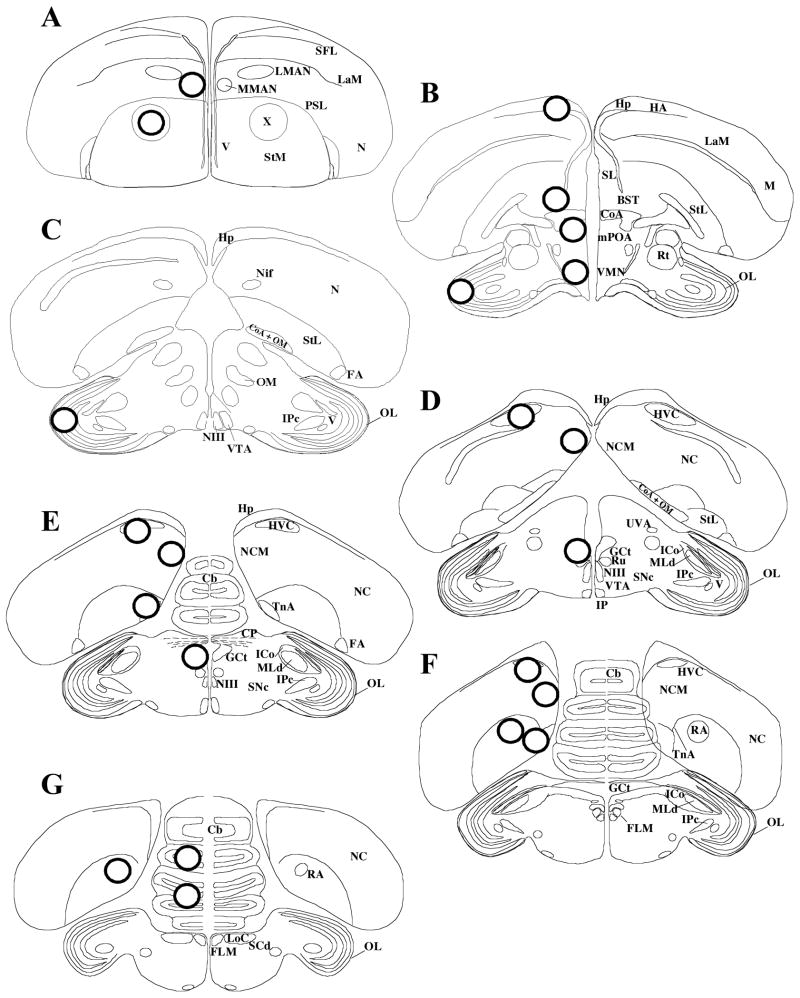
The technique originally developed by Palkovits for rat brain (36) and validated for zebra finch brain (31, 37) was used here with only minor modifications. Coronal sections (300 micrometers) were made on a cryostat (Microm HM505E) at −12°C and were collected starting from the caudal part of the brain. The plane of the sections was adjusted to match as closely as possible the plane of the zebra finch brain atlas (38). From these sections, individual nuclei were isolated from 1 to 6 adjacent sections, depending on the size of the region of interest, by punching them out with a stainless steel cannula (Brain Punch Set, #57401, Stoelting Co, IL). Punches (0.94 mm diameter) were obtained from Area X (X), medial magnocellular nucleus of anterior nidopallium (MMAN), medial preoptic area (mPOA), rostral hippocampus (Hp), ventromedial nucleus of the hypothalamus (VMN), bed nucleus of the stria terminalis (BST), HVC (used as a proper name), caudal medial nidopallium (NCM), nucleus taeniae of the amygdala (TnA), nucleus robustus of the arcopallium (RA), mesencephalic central grey (GCt), optic lobes (OL) and cerebellum (Cb) (Figure 1). Punches from the left and right sides were collected into separate “Pellet Pestle” 0.5 ml microcentrifuge tubes (Kimble/Kontes, NJ, USA) and stored at −80°C. The punches from the left side of the brain were used for the aromatase assay, while punches from the right side of the brain were used for E2 quantification. For each region within a subject, the number of punches taken from the left and right sides were always identical (Table 1).
Figure 1.
Schematic representation of the location of the punches, as identified by circles in the transversal sections. (A H) Sections arranged in a rostral to caudal order. Abbreviations: Cb, cerebellum; CoA, anterior commissure; CP, posterior commissure; BST: bed nucleus of the stria terminalis, FA, fronto-arcopallial tract; FLM, fasciculus longitudinalis medialis (medial longitudinal bundle); GCt, mesencephalic central grey (periaqueductal grey); HA, accessory part of the hyperpallium; Hp, hippocampus; HVC, used as a proper name; ICo, intercollicular nucleus; IPc, parvocellular part of the isthmi nucleus; LaM, mesopallial lamina; LMAN, lateral magnocellular nucleus of the anterior nidopallium; LoC, locus ceruleus; M, mesopallium; MLd, lateral mesencephalic nucleus; MMAN, medial magnocellular nucleus of the anterior nidopallium; mPOA, median preoptic area; N, nidopallium; NC, caudal nidopallium; NCM, caudal medial nidopallium; NIf, nucleus interface of the nidopallium; NIII, oculomotor nerve; OL, optic lobe; OM, occipito-mesencephalic tract; PSL, pallial-subpallial lamina; RA, robust nucleus of arcopallium; Rt, nucleus rotundus; Ru, red nucleus; SCd, dorsal subceruleus nucleus; SFL, superior frontal lamina; SL, lateral septal nucleus; SNc, substantia nigra, pars compacta; StM, medial striatum; StL, lateral striatum; TnA, nucleus taeniae of the amygdala; UVA, nucleus uveaformis; V, ventricle; VMN, ventromedial nucleus of the hypothalamus; VTA, ventral tegmental area; X, Area X.
Table 1.
Number (mean ± SEM), estimated weight (mean ± SEM) and protein content (mean ± SEM) of punches from the 13 different brain regions.
| Number of punches | Estimated weight (mg) | Protein content (mg) | ||
|---|---|---|---|---|
| High aromatase | mPOA | 4.85 ± 0.17 | 0.93 ± 0.03 | 0.055 ± 0.003 |
| Hp | 3.30 ± 0.21 | 0.63 ± 0.04 | 0.035 ± 0.003 | |
| VMN | 1.50 ± 0.11 | 0.29 ± 0.02 | 0.019 ± 0.004 | |
| BST | 1.45 ± 0.11 | 0.28 ± 0.02 | 0.011 ± 0.001 | |
| NCM | 4.35 ± 0.23 | 0.83 ± 0.04 | 0.055 ± 0.004 | |
| TnA | 2.45 ± 0.17 | 0.47 ± 0.03 | 0.026 ± 0.003 | |
|
| ||||
| Low aromatase | Area X | 2.35 ± 0.19 | 0.45 ± 0.04 | 0.026 ± 0.003 |
| MMAN | 2.37 ± 0.16 | 0.45 ± 0.03 | 0.026 ± 0.003 | |
| HVC | 2.80 ± 0.17 | 0.54 ± 0.03 | 0.039 ± 0.003 | |
| RA | 1.94 ± 0.20 | 0.37 ± 0.04 | 0.027 ± 0.003 | |
| GCt | 1.80 ± 0.12 | 0.34 ± 0.02 | 0.020 ± 0.002 | |
| OL | 3.35 ± 0.18 | 0.64 ± 0.03 | 0.036 ± 0.003 | |
| Cb | 3.30 ± 0.24 | 0.63 ± 0.05 | 0.041 ± 0.003 | |
Note: These values correspond to punches obtained from one side of the brain only. The left side was used to determine aromatase activity and the right side for E2 concentration. For each region within a subject, the number of punches collected from the right and left sides of the brain was always identical.
Because the very small amount of tissue could introduce an error in the weight measurement, tests were performed to estimate the weight of a single punch. Briefly, 3 pools of punches (each containing 75 punches) were obtained from 3 sparrow brains. The weight of each pool was recorded and used to calculate the weight of one pun ch. The average weight of one punch was calculated to be 0.1919 mg, and this value was used to estimate the wet weight of punches from the right side of the brain (Table 1). Note that some of the punches were not complete due to the proximity of ventricles (e.g. Hp, HVC and TnA). However, for each region analyzed, no weight difference was observed between CON and STI groups (Student’s t-tests, p>0.1).
Brain punches from the left side were homogenized in the microcentrifuge tubes with a specific pestle (CTFE/stainless steel “Pellet Pestle,” #749516-0500, Kimble/Kontes) with 200 μl of TEK (10 mM Tris HCl pH 7.2, 1 mM Na-EDTA, 150 mM KCl). Protein concentration was measured using the Bradford method from Bio-Rad (microassay procedure following manufacturer’s instructions). The protein content was approximately 6% of the estimated wet weight, similar to studies with Japanese quail brain (39). Again, no difference in protein concentration was observed between CON and STI groups (Student’s t-tests, p>0.1). Moreover, the protein concentration (from the left side) was highly correlated with the estimated weight (from the right side) (r=0.96, p<0.0001), further supporting the validity of using estimated weight.
Aromatase activity
Aromatase activity was quantified by measuring the production of tritiated water from [1β-3H]-androstenedione as described by (40, 41) and validated for songbird brains (42, 43). On an ice bath, 50 μl of TEK buffer, 50 μl of 100 nM [1β-3H]-androstenedione, and 50 μl of 4.8 mM NADPH were added to aliquots (50 μl) of homogenate. The final volume of the reaction was 200 μl, the final concentration of [3H]-AE was 25 nM, and the final concentration of NADPH was 1.2 mM. These concentrations were previously used for 1–8 mg of tissue per tube (42, 43) while our quantification was performed on 0.07 to 0.23 mg of tissue (table 1). Therefore, we are confident that substrate was not limiting in our assays and was present at saturating concentrations. Samples were quantified in duplicate. Background values were obtained for each sample by processing brain samples in the presence of an excess (final concentration, 40 μM) of the potent and specific aromatase inhibitor, fadrozole (gift from Novartis). All these steps were conducted at 4°C in 1.5-ml microcentrifuge tubes, which were then quickly capped and incubated for 20 min at 37°C. The reaction was stopped by cooling the samples in an ice/water bath and adding 0.4 ml ice-cold 10% trichloroacetic acid containing 2% activated charcoal. After centrifugation at 1200 g for 15 min, supernatants were applied to small columns made of Pasteur pipettes plugged with glass beads and filled (3 cm high) with a Dowex cation exchange resin (AG 50W-X4, 100–200 mesh, Biorad, Richmond, CA). Distilled water (3 × 0.6 ml) was then added to the columns. Effluents were collected in scintillation vials, and 10 ml of scintillation liquid were added. Vials were counted for 3 min on a liquid scintillation counter (Beckman Coulter LS6500). Enzyme activity was expressed as fmol/hr/mg of protein, after correction of the dpm for recovery (95%), background values, and percentage of tritium in β-position in the substrate (76.8%).
Solid phase extraction
To measure E2 in brain samples (Experiment 1), tissue was homogenized in the microcentrifuge tubes with the Pellet Pestle with 50μl ice-cold deionised water, and then 250 μl ice-cold HPLC-grade methanol was quickly added. Samples were left overnight at 4°C. Steroids were then extracted with solid phase extraction (31, 44). Briefly, C18 columns (500 mg sorbent, 6 ml column volume, United Chemical Technologies) were primed with HPLC-grade ethanol and equilibrated with deionised water. Then 10 ml of deionised water was added to the brain samples before loading onto the C18 columns. After sample loading, the C18 columns were washed with deionised water (10 ml), and the steroids were eluted with 90% HPLC-grade methanol (5 ml). The eluates were dried (Speedvac) prior to resuspension. We resuspended samples in 350 μl PBSG (phosphate buffered saline with 0.1% gelatin) containing 0.7% absolute ethanol.
To measure E2 in plasma samples (Experiment 2), we also used solid phase extraction with C18 columns (Sep-Pak Vac C18 cartridge, 500 mg sorbent, 3 ml column volume, Waters). For logistical reasons, the C18 columns used for plasma samples were from a different manufacturer but, importantly, contain the same type of sorbent (C18) and the same amount of sorbent (500 mg). Columns were primed and equilibrated as above. After sample loading (29.2 μl of plasma + 10 ml deionised water), the columns were washed with 40% HPLC-grade methanol (10 ml). Steroids were eluted with 90% HPLC-grade methanol (5 ml), and eluates were dried and resuspended as above. In preliminary studies with the Sep-Pak C18 columns, we measured E2 in zebra finch plasma, and the results matched our previous results with C18 columns from United Chemical Technologies (30 and unpub. results). Moreover, the C18 columns from both manufacturers gave similar results for standards containing known amounts of E2, water blanks, and recovery (see below).
Œstradiol radioimmunoassay
Resuspended samples were then assayed as singletons (to maximize the number of detectable samples) with a double-antibody 125I-E2 radioimmunoassay (DSL-4800, Beckman Coulter Canada, Inc.) that we modified and validated for songbird brain samples (31). Briefly, 100 μl of diluted anti-œstradiol antiserum (dilution: 1 ml stock antibody + 2.5 ml PBSG) was added to 300 μl of sample, the tubes were quickly vortexed and incubated at room temperature for 4 hr. Then 100 μl of diluted 125I-E2 (dilution: 1 ml stock tracer + 2 ml PBSG) was added, and the tubes were vortexed and incubated for 24 hr at 4°C. Then 500 μl of precipitating reagent was added, and tubes were vortexed and incubated for 20 min at room temperature. The tubes were centrifuged at 1500g for 15 min at 4°C, the supernatant was decanted, and tubes were counted.
The E2 antibody has a low cross-reactivity with strone (2.4%), striol (0.64%), 17α-œstradiol (0.21%), 17β-œstradiol-3-glucuronide (2.56%), 17β-œstradiol-17-glucuronide (<0.01%), œstradiol-3-sulfate (0.17%), testosterone (<0.01%) and DHEA (<0.01%), as per the manufacturer. The lowest point on the standard curve was 0.1875 pg per tube. E2 values below the lowest point on the standard curve were set to zero. In the assays with brain samples, water blank values were 0.138 ± 0.057 pg/tube (n=5), and in the assay with plasma samples, water blanks were 0.055 ± 0.036 pg/tube (n=2). For low (0.375 pg per tube) and high (1.125 pg per tube) standards, intra-assay variation was 0.92% and 4.37%, and inter-assay variation was 5.46% and 5.36%, respectively. The low intra-assay variation confirms the validity of using singletons to maximize the number of detectable samples. We also examined the recovery of a known quantity of radioinert E2 (0.375 pg per tube) added to brain tissue (dorso-medial telencephalon containing NCM) or plasma before solid phase extraction. We calculated recovery by comparing the quantity of E2 in spiked (n = 5) and unspiked samples (n = 5). Data were corrected for recovery (brain samples: 75.95%, plasma samples: 84.00%), similar to our previous results (31).
Nissl staining
After the punch collection, sections were mounted on glass slides, dried overnight and Nissl-stained to confirm the location of the punches. Briefly, sections were brought to room temperature and postfixed in 4% paraformaldehyde for 15 min. The sections were rinsed 3 times in PBS (0.1M) and stained in 0.2% toluidine blue in Walpole solution (0.3M sodium acetate, 0.12% acetic acid) for 2 min. The sections were rinsed in deionised water, destained in Walpole solution, and the stain fixed in 0.04M ammonium molybdate. The sections were dehydrated with increasing concentrations of ethanol, and incubated in acetone and then xylene before coverslipping (Permount ®, Fisher).
Statistical analysis
All data are presented as their mean ± SEM. Data analysis included Student’s t-tests, Mann-Whitney tests, one-way ANOVAs, and mixed-design two-way ANOVAs, and these were performed using Statview (MacIn version 5.0.1, Abacus Concept Inc., Berkeley, CA, USA). When appropriate, ANOVA tests were followed by post hoc Tukey’s HSD tests. Differences were considered significant for p ≤ 0.05.
RESULTS
Morphology
CON and STI groups did not differ with respect to body mass (CON: 24.00 ± 0.85 g, STI: 24.18 ± 0.78 g, df=18, t=0.151, p=0.88) and left tarsus length (CON: 20.36 ± 0.29 mm, STI: 20.65 ± 0.28 mm, df=18, t=0.703, p=0.49). The testes were starting to regress, and there was no difference between groups in total testes mass (CON: 361 ± 53 mg, STI: 379 ± 30 mg, df=18, t=0.309, p=0.76) or the length of the left testis (CON: 8.81 ± 0.61 mm, STI: 8.77 ± 0.31 mm, df=18, t=0.059, p=0.95).
Behaviour
The STI elicited a robust aggressive response (Table 2). STI significantly decreased the latency to approach the cage, increased the number of songs, increased the number of flights, and increased the time spent within 1 meter and 5 meters of the decoy (Table 2). Trills and wing waves were rarely observed and not significantly different between the groups (number of trills: CON: 0.00 ± 0.00, STI: 1.54±1.36, Z=0.684, p=0.49; number of wing waves: CON: 0.00±0.00, STI: 0.91±0.73, Z=0.684, p=0.49). The aggression score (PC1) was significantly different between groups (CON: -2.15±0.19, STI: 1.25±0.21, df=18, t=11.875, p<0.0001).
Table 2.
Aggressive responses and endocrine measures (mean±SEM) in CON (n=9) and STI (n=11) subjects.
| CON | STI | t or Z | p | |
|---|---|---|---|---|
| Behaviour | ||||
| Latency (sec) | 1010.44 ± 292.69 | 55.70 ± 25.12 | Z=2.531 | *p=0.01 |
| Number of songs | 13.89 ± 7.14 | 110.82 ± 21.85 | Z=2.773 | *p=0.006 |
| Number of flights | 0.78 ± 0.57 | 49.27 ± 7.02 | Z=3.761 | *p=0.0002 |
| Time in 1m (sec) | 0.00 ± 0.00 | 862.73 ± 159.00 | Z= 3.419 | *p=0.0006 |
| Time in 5m (sec) | 3.33 ± 3.33 | 1647.64 ± 44.54 | Z=3.761 | *p=0.0002 |
|
| ||||
| Endocrine measures | ||||
| Plasma CBG (nM) | 141.30 ± 17.67 | 214.03 ± 30.94 | Z=1.937 | (*)p=0.053 |
|
| ||||
| Plasma corticosterone (nM) | 27.14 ± 8.72 | 87.50 ± 18.16 | Z=2.849 | *p=0.004 |
| (ng/ml) | 9.39 ± 3.02 | 40.25 ± 6.28 | ||
|
| ||||
| Plasma progesterone (nM) | 2.17 ± 0.55 | 2.18 ± 0.40 | t=0.003 | p=0.998 |
| (ng/ml) | 0.68 ± 0.17 | 0.69 ± 0.12 | ||
|
| ||||
| Plasma testosterone (nM) | 4.94 ± 3.17 | 2.92 ± 1.74 | t=0.584 | p=0.564 |
| (ng/ml) | 1.42 ± 0.87 | 0.84 ± 0.24 | ||
Note: Plasma CBG is expressed in nM, and steroid concentrations in both nM and ng/ml.
Endocrine measurements
In Experiment 1, we examined endocrine measurements obtained from the brachial plasma (Table 2). Total plasma corticosterone levels were significantly elevated in the STI group, and there was a strong trend for plasma CBG levels to increase in the STI group. No changes in plasma progesterone or testosterone levels were observed in this subset of birds.
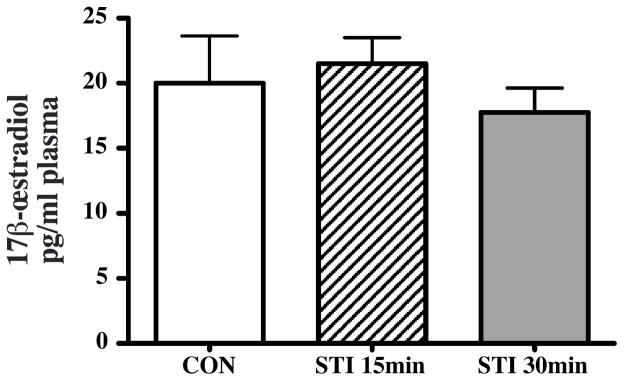
In a separate group of subjects (Experiment 2), exposure to STI for 15 or 30 min did not affect systemic E2 levels in brachial plasma compared to the CON group (F2,18=0.522, p=0.602, Figure 2). In general, plasma E2 levels were low but above the detection limit in all samples.
Figure 2.
Bar graphs representing plasma 17β-œstradiol concentrations in controls (CON, white) and subjects exposed to a simulated territorial intrusion (STI) for 15 min (dashed) or 30 min (grey). Subjects were wild adult male white-crowned sparrows, in the late breeding season.
Brain aromatase activity
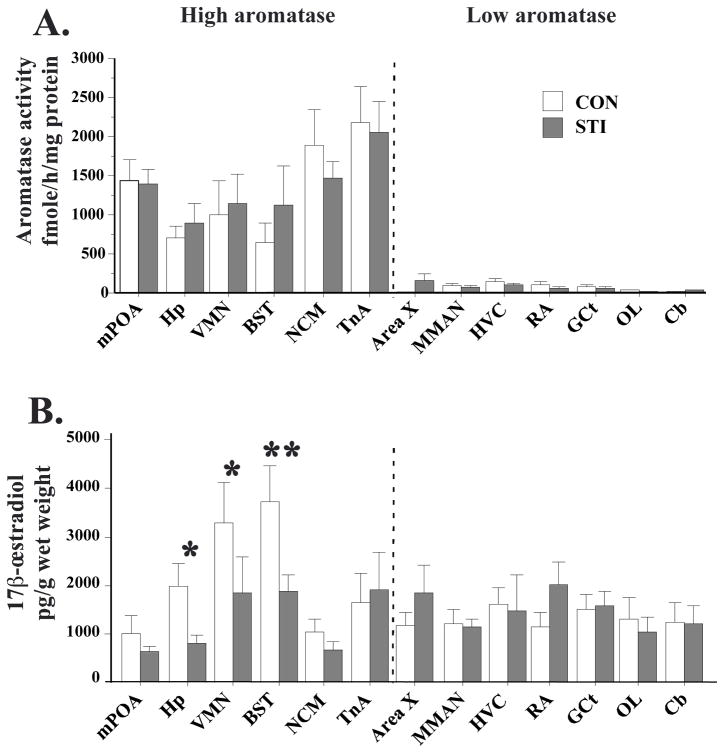
We analyzed the effect of STI on aromatase activity by a mixed-design two-way ANOVA with a between-subjects factor (Treatment) and a within-subjects factor (Region). This analysis revealed no main effect of Treatment (F(1,13)=2.510, p=0.137), an effect of Region (F(1,12)=13.924, p<0.0001) and no interaction between the two factors (F(12,156)=0.450, p=0.940). As expected from previous reports, high aromatase activity was detected in the mPOA, Hp, VMN, BST, NCM and TnA (Figure 3A). Low or non-detectable aromatase activity was detected in Area X, MMAN, HVC, RA, GCt, OL and Cb. It should be noted that separate t-tests were also performed for each brain region, and here again, no significant differences were found between CON and STI groups (p>0.15 in all cases).
Figure 3.
Bar graphs representing aromatase activity (A) and 17β-œstradiol concentration (B) in 13 brain regions in the controls (CON, white) and subjects exposed to a simulated territorial intrusion (STI, grey) for 30 min. Subjects were wild adult male white-crowned sparrows, in the late breeding season. * p<0.05; **p<0.01.
Brain 17β-œstradiol concentrations
The use of singletons for E2 quantification increased the number of detectable samples compared to what was previously obtained in zebra finch [compare Table 3 and (31)]. A mixed-design two-way ANOVA of E2 concentrations revealed no main effect of Treatment (F(1,12)=0.056, p=0.817; Figure 3B), an effect of Region (F(1,12)=3.818, p<0.0001) and a strong trend for an interaction between the two factors (F(12,144)=1.816, p=0.0506). E2 concentrations varied across brain regions, albeit less than aromatase activity did. Among the regions with high aromatase activity, higher levels of E2 were found in the VMN and BST, while lower E2 concentrations were observed in the mPOA and NCM.
Table 3.
Percentage of detectable samples for aromatase activity and œstradiol in the 13 different brain regions for CON and STI groups.
| Aromatase activity | Œstradiol | ||||
|---|---|---|---|---|---|
| CON | STI | CON | STI | ||
| High aromatase | mPOA | 100 | 100 | 78 | 91 |
| Hp | 100 | 100 | 100 | 73 | |
| VMN | 100 | 100 | 89 | 64 | |
| BST | 67 | 100 | 100 | 91 | |
| NCM | 100 | 100 | 78 | 91 | |
| TnA | 100 | 100 | 78 | 82 | |
|
| |||||
| Low aromatase | Area X | 57 | 80 | 71 | 80 |
| MMAN | 87 | 90 | 75 | 100 | |
| HVC | 100 | 91 | 100 | 73 | |
| RA | 100 | 90 | 71 | 80 | |
| GCt | 75 | 60 | 87 | 80 | |
| OL | 87 | 36 | 78 | 80 | |
| Cb | 78 | 64 | 78 | 82 | |
Because we expected a priori stronger modulation by STI of E2 concentrations in aromatase-rich regions, a mixed-design two-way ANOVA was also used to assess the effects of STI specifically in the 6 brain regions with high aromatase activity (mPOA, Hp, VMN, BST, NCM and TnA). This ANOVA revealed a trend for an effect of Treatment (F(1,16)=3.054, p=0.099), a strong effect of Region (F(1,5)=9.758, p<0.0001) and a significant interaction (F(5,80)=2.465, p=0.0396). Tukey’s post hoc tests revealed statistically significant reductions of E2 in Hp (~60%; p<0.05), VMN (~50%; p<0.05) and BST (~50%; p<0.01) in the STI group (Figure 3B). No significant change was observed in mPOA, NCM or TnA.
Importantly, E2 concentrations in the brain punches (Figure 3B) were much higher than those in the plasma (~40× higher in mPOA and NCM, and up to ~200× higher in BST; compare the axes of Figures 2 and 3B). When comparing brain and plasma concentrations, note that 1 ml of plasma weighs very close to 1 g (45, 46).
Correlations between brain aromatase activity and brain E2
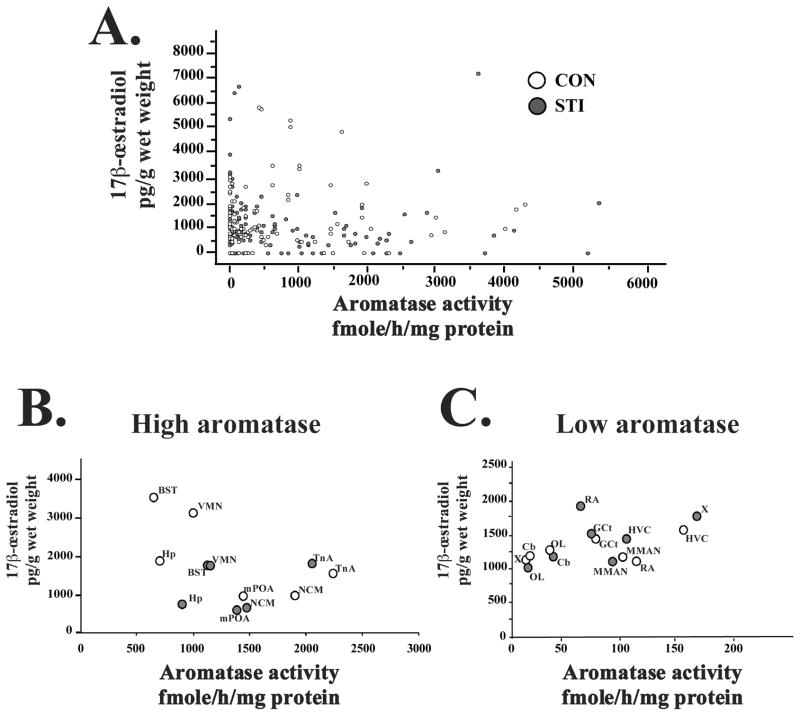
We found no correlations between aromatase activity and brain E2 concentration (all data points: n=245, r=0.029, p=0.647, Figure 4A). The correlational analyses were also performed separately for the CON and STI groups, as well as for individual brain regions. Here again, no significant correlations were found (p>0.1 in all cases). The analysis of the average aromatase activity and E2 concentration from individual nuclei, in CON and STI groups, and separately in high aromatase regions (Figure 4B) and low aromatase regions (Figure 4C) did not reveal any correlations (p>0.2).
Figure 4.
Graphs showing the absence of correlations between brain aromatase activity (fmoles/h/mg of protein) and brain 17β-œstradiol concentration (pg/g of wet weight) in CON (empty circles) and STI (grey circles) animals. (A) Graph that includes all data from all subjects. (B and C) Aromatase activity (group average) as a function of œstradiol concentration (group average) for high aromatase and low aromatase regions in CON (empty circles) and STI (grey circles) subjects.
Unexpectedly, our results suggest that brain E2 concentration is not always directly linked to aromatase activity. Indeed, relatively low E2 levels are observed in the aromatase-rich NCM, and E2 concentrations are similar between aromatase-rich TnA and low aromatase regions such as HVC, OL and Cb.
We also analyzed the association of brain E2 or aromatase activity from each individual brain regions with the endocrine (corticosterone, progesterone, testosterone and CBG) and behavioural (latency, flight, song, PCI) measures. Here again, no significant correlations were found (p>0.1 in all cases).
DISCUSSION
This is one of the first studies to measure E2 in microdissected brain regions [see also (31)], and the first study to do so in free-ranging animals in their natural habitat. To our knowledge, this is also the first study to examine the effects of aggressive interactions on E2 levels in the brain. Moreover, we measured both brain aromatase activity and brain E2 concentrations in the same subjects, which has not been done before. Brain aromatase activity levels were similar in the control and STI groups, while brain E2 concentrations were significantly reduced in Hp, BST and VMN in the STI group. Surprisingly, brain E2 concentrations were not correlated with aromatase activity. These data demonstrate that social interactions have rapid effects on local E2 concentrations in specific brain regions.
Brain aromatase activity
Regional differences in aromatase activity
Very little information exists on regional differences in aromatase activity in the brain of wild songbirds. Aromatase activity has been studied using relatively large brain regions, such as the telencephalon or diencephalon (43, 47). In the closely-related Gambel’s white-crowned sparrow caught in the non-breeding season, aromatase activity was high in the telencephalon and relatively low in the diencephalon (48). However, aromatase-expressing cells are present in very discrete locations in the diencephalon, and thus the use of whole diencephalon dilutes aromatase-expressing cells with the surrounding tissue.
The Palkovits punch technique allowed us to study aromatase activity in specific brain nuclei. While this technique was previously used in 2 domesticated bird species, the Japanese quail and the zebra finch (37, 49), the present study is the first to investigate aromatase activity in microdissected brain regions from a wild bird species. We showed here that two hypothalamic nuclei (mPOA and VMN) as well as several telencephalic regions (BST, Hp, NCM and TnA) have high levels of aromatase activity. Note that subjects were caught late in the breeding season, and aromatase activity in some brain regions (e.g., mPOA) might be higher earlier in the breeding season (37, 48, 50).
Relationship between aromatase activity and aggressive behaviour
Aromatization of testosterone is critical for the regulation of male aggression in birds and mammals (51, 52). In birds, seasonal changes in territorial aggression are associated with seasonal changes in aromatase in certain brain regions, such as the diencephalon and TnA (42, 47, 50, 53, 54). Further, aromatase inhibitor treatments reduce aggression, and this effect can be rescued by E2 replacement (3, 55, 56). In rodents, aromatase is also important in the control of aggressive behaviour. For instance, aggressive behaviour is reduced in aromatase-deficient mice (57, 58) and in mice treated with an aromatase inhibitor (59). E2 treatment increases aggressive behaviour in house mice (58, 60) and California mice housed under short photoperiods (26). Most of these studies have focused on the effects of long-term changes in aromatase activity, and little is known about rapid changes in aromatase activity associated with aggression [see however (26, 27)].
Effects of aggressive behaviour on aromatase activity
Here, aromatase activity was not different between the CON and STI groups in any region. Several reasons could explain the lack of a group difference. First, we might have missed a transient change in aromatase activity by analyzing aromatase activity after 30 min of STI. It is possible that aromatase activity is modulated, for example, within 10 min and returns to baseline by 30 min. Indeed, aromatase activity in male quail hypothalamus is significantly reduced after 2 to 15 min of exposure to a female, with a recovery to baseline levels after 30 min (17). Second, the aromatase protein itself might not be rapidly modulated by aggressive encounters, but rather its cofactor (NADPH) might be modulated. Our assay included a saturating level of cofactor and would have missed this difference. A similar scenario has been suggested for 3β–HSD and its cofactor (61). It should be emphasized that the endogenous substrate, testosterone (total from the plasma or free extrapolated from changes in CBG, progesterone and corticosterone), did not vary after STI (see also 32). Third, perhaps the rapid initiation of territorial behaviour is independent of rapid synthesis of E2. In this case, aromatase activity simply might not be rapidly modulated by aggressive interactions.
Plasma and brain œstradiol levels
E2 has numerous effects on behaviours associated with reproduction and aggression. In songbirds specifically, song production, song perception, and the song control system are sensitive to sex steroids, such as E2 (28, 62–65). Our results show that E2 is present at high concentrations within the male songbird brain, relative to plasma. Low plasma levels of E2 were also detected in other adult male songbirds (66, 67). In wintering male Gambel’s white-crowned sparrows, levels of plasma estrogens (E2 and estrone combined) are ~250 pg/ml, also lower than the brain E2 levels seen here (68). The origin of plasma E2 in male white-crowned sparrows is unclear. In male zebra finches, E2 in the general circulation originates mainly from the brain, not from the gonads or other organs (68). However, in other avian species, low aromatase activity was detected in the liver (43). In the brain, E2 in brain regions with low to non-detectable aromatase activity might be the result of passive diffusion away from high aromatase regions or of sequestration (specific or non-specific).
Region-specific changes in E2
Our data demonstrate that E2 concentrations are reduced following STI in three brain nuclei: VMN, BST, and Hp. Besides its well-known role in the regulation of female sexual behaviour, the ventromedial nucleus of the hypothalamus (VMN) also regulates other social behaviours (69). Immediate early gene induction is observed in VMN after agonistic encounters (69, 70). Further, lesions, electrical stimulation or neuropeptide injection within VMN modulate aggression (71–73). The bed nucleus of the stria terminalis (BST) is involved in the control of social behaviours, including inter-male aggression, sexual behaviour, pair-bonding and parental behaviour (74, 75). The hippocampus (Hp) plays a major role in spatial orientation (76–78), and a change in E2 in the Hp might relate to remembering the location of the intruder within the territory. The Hp shows major structural and activity changes following social interaction and social stress (79) and is important for social recognition in mammals (80). Both strogen receptor alpha and beta are present in these 3 regions (81–83).
It is surprising that no group difference was observed in NCM E2 levels. Using microdialysis, Remage-Healey and colleagues showed an increase in E2 content in the zebra finch NCM following playback exposure (30). The microdialysis samples were collected over 30 min, rendering direct comparison with our data difficult. In addition, in the control subjects, E2 levels are low in the aromatase-rich NCM, which was also unexpected. Our previous data from zebra finches suggest that E2 concentration was highest in the NCM (31). Note, however, that aromatase activity is similar in the POA and NCM of white-crowned sparrows (present study), whereas aromatase activity in the telencephalon far exceeds the activity in the preoptic area of zebra finches (43).
Decreases in brain E2 concentrations: possible mechanisms
The decreases in E2 levels in VMN, BST and Hp were unexpected, given that strogens typically stimulate aggressive behaviour [but see (26)]. Also surprising is the absence of correlations between brain aromatase activity and brain E2 concentrations. There are several possible explanations. First, aromatase activity might have been transiently reduced during the start of the STI, leading to a subsequent decrease in E2 content in specific brain regions. Second, decreases in local E2 levels might be the result of decreases in other steroidogenic enzymes in the brain [e.g. 3β-HSD or CYP17; (61)]. Third, decreases in local E2 concentrations could be due to mechanisms designed to increase local androgen levels. In mice, androgens act via neural androgen receptors to mediate the “winner effect,” whereby winners of aggressive encounters are more likely to win future aggressive encounters (84, 85). Here, in the late breeding season, no increase in circulating T levels, either total or free, was detected in the STI group [present data and (32)]. Fourth, the reductions in E2 concentrations could result from increased catabolism of E2, subsequent to estrogen signaling. Similar mechanisms have been shown for dopamine signaling. A reduction in extracellular dopamine, along with an increase in the dopamine metabolites HVA or DOPAC, results in an increased HVA/dopamine or DOPAC/dopamine ratio, indicating an increase in dopaminergic signaling (86–88). The catabolism of strogens occurs in the brain, as well as the liver, and is a key mechanism to control local strogen levels (19, 89).
Conclusions
There is considerable evidence in a wide variety of species that strogens play an important role in the regulation of social and aggressive behaviours. The causes and consequences of rapid variations in local steroid levels, however, remain largely unknown, and studies of wild animals in their natural environments should help elucidate these important issues. The lack of a simple correlation between brain E2 concentrations and brain aromatase activity suggests complex mechanisms for the production and metabolism of this brain steroid. The present data clearly demonstrate that social interactions have rapid effects on local E2 concentrations in specific brain regions, independent of systemic E2 concentrations.
Acknowledgments
We would like to thank Dr. Joanne Weinberg for sharing equipment and Dr. Chunqi Ma, Nora Prior, Annika Sun, and Ilan Ruhr for technical help. We also would like to thank Drs. Jacques Balthazart, Charlotte Cornil and Brian Trainor, as well as 2 anonymous reviewers, for helpful comments on the manuscript. This research was supported by grants from the Canadian Institutes of Health Research (CIHR), Canada Foundation for Innovation, and the Michael Smith Foundation for Health Research (MSFHR) to KKS, and from NIH (R01NS042767) to CJS. TDC was a FNRS postdoctoral researcher and is currently Research Associate at the University of Liège. AEMN was a NSERC and MSFHR fellow, SAH is a CIHR and MSFHR postdoctoral fellow and KWLP was a NSERC USRA fellow.
References
- 1.Naftolin F, Ryan KJ, Davies IJ, Petro Z, Kuhn M. The formation and metabolism of estrogens in brain tissue. Adv Biosci. 1975;15:105–121. [PubMed] [Google Scholar]
- 2.Roselli CE, Liu M, Hurn PD. Brain Aromatization: classic roles and new perspectives. Sem Reprod Med. 2009;27:207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlinger BA, Callard GV. Aromatization mediates aggressive behavior in quail. Gen Comp Endocrinol. 1990;79:39–53. doi: 10.1016/0016-6480(90)90086-2. [DOI] [PubMed] [Google Scholar]
- 4.Soma KK, Scotti MA, Newman AE, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol. 1997;61:365–374. [PubMed] [Google Scholar]
- 6.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical reexamination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 7.Wagner CK, Morrell JI. Neuroanatomical distribution of aromatase mRNA in the rat brain: indications of regional regulation. J Steroid Biochem Mol Biol. 1997;61:307–314. [PubMed] [Google Scholar]
- 8.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- 10.Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 12.Balthazart J. Steroid control and sexual differentiation of brain aromatase. J Steroid Biochem Mol Biol. 1997;61:323–339. [PubMed] [Google Scholar]
- 13.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endoc rRev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 14.Balthazart J, Baillien M, Ball G. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Bioch Molec Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 15.Balthazart J, Baillien M, Charlier TD, Ball G. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 16.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 17.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter. TINS. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly MJ, Moss RL, Dudley CA. The effects of microelectrophoretically applied estrogen, cortisol and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of female rat. Exp Brain Res. 1977;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- 22.Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord LD, Bond J, Thompson RR. Rapid steroid influences on visually guided sexual behavior in male goldfish. Horm Behav. 2009;56:519–526. doi: 10.1016/j.yhbeh.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;66:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. Photoperiod reverses the effects of estrogens on male aggression via genomic and nongenomic pathways. Proc Natl Acad Sci U S A. 2007;104:9840–9845. doi: 10.1073/pnas.0701819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53:192–199. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlier TD, Po KW, Newman AE, Shah AH, Saldanha CJ, Soma KK. 17beta-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2010;167:18–26. doi: 10.1016/j.ygcen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlier TD, Underhill C, Hammond GL, Soma KK. Effects of aggressive encounters on plasma corticosteroid-binding globulin and its ligands in white-crowned sparrows. Horm Behav. 2009;56:339–347. doi: 10.1016/j.yhbeh.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Lynn SE, Hahn TP, Breuner CW. Free-living male mountain White-crowned Sparrows exhibit territorial aggression without modulating total or free plasma testosterone. Condor. 2007;109:173–180. [Google Scholar]
- 34.Wingfield JC, Hahn TP. Testosterone and territorial behavior in sedentary and migratory sparrrows. Anim Behav. 1994;47:77–89. [Google Scholar]
- 35.Petrinovitch L, Patterson TL. The response of white-crowned sparrows to songs of different dialects and subspecies. Z Tierpsychol. 1981;57:1–14. [Google Scholar]
- 36.Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- 37.Vockel A, Prove E, Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990;511:291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- 38.Nixdorf-bergweiler BE, Bischof H-J. Stereotaxic atlas of the brain of the zebra finch, Taeniopygia guttata with special emphais on telencephalic visual and song system nuclei in transverse and sagital sections. 2007 [Google Scholar]
- 39.Schumacher M, Contenti E, Balthazart J. Testosterone metabolism in discrete areas of the hypothalamus and adjacent brain regions of male and female Japanese quail. Brain Res. 1983;278:337–340. doi: 10.1016/0006-8993(83)90267-6. [DOI] [PubMed] [Google Scholar]
- 40.Thompson EA, Jr, Siiteri PK. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J Biol Chem. 1974;249:5364–5372. [PubMed] [Google Scholar]
- 41.Roselli CE, Resko JA. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B, editor. Neuroendocrine research methods. Chur, Switzerland: Harwood Academic Publisher; 1991. pp. 937–951. [Google Scholar]
- 42.Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm Behav. 2004;45:225–234. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen Comp Endocrinol. 2000;117:34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- 44.Newman AE, Chin EH, Schmidt KL, Bond L, Wynne-Edwards KE, Soma KK. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen Comp Endocrinol. 2008;155:503–510. doi: 10.1016/j.ygcen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt KL, Soma KK. Cortisol and corticosterone in the songbird immune and nervous systems: local vs. systemic levels during development. Am J Physiol-Regy Integ Comp Physiol. 2008;295:R103–R110. doi: 10.1152/ajpregu.00002.2008. [DOI] [PubMed] [Google Scholar]
- 46.Taves MD, Schmidt KL, Ruhr IM, Kapusta K, Prior NH, Soma KK. Steroid concentrations in plasma, whole blood and brain: effects of saline perfusion to remove blood contamination from brain. PLoS One. 2011;5:e15727. doi: 10.1371/journal.pone.0015727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soma KK, Schlinger BA, Wingfiel JC, Saldanha CJ. Brain aromatase, 5α-reductase and 5β-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- 48.Schlinger BA, Slotow RH, Arnold AP. Plasma Estrogens and Brain Aromatase in Winter White-Crowned Sparrows. Ornis Scandinavica. 1992;23:292–297. [Google Scholar]
- 49.Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990;2:675–683. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 50.Wacker DW, Wingfield JC, Davis JE, Meddle SL. Seasonal Changes in Aromatase and Androgen Receptor, but not Estrogen Receptor mRNA Expression in the Brain of the Free-Living Male Song Sparrow, Melospiza melodia morphna. J Comp Neurol. 2010;518:3819–3835. doi: 10.1002/cne.22426. [DOI] [PubMed] [Google Scholar]
- 51.Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: how interaction between aromatase and the environment modulate agression. Front Neuroendocrinol. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soma KK. Testosterone and aggression: Berthold, birds and beyond. J Neuroendocrinol. 2006;18:543–551. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. Proc Biol Sci. 2000;267:1089–1096. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soma KK, Bindra RK, Gee J, Wingfield JC, Schlinger BA. Androgen-metabolizing enzymes show region-specific changes across the breeding season in the brain of a wild song-bird. J Neurobiol. 1999;41:176–188. [PubMed] [Google Scholar]
- 55.Harding CF, Walters MJ, Collado D, Sheridan K. Hormonal specificity and activation of social behavior in male red-winged blackbirds. Horm Behav. 1988;22:402–418. doi: 10.1016/0018-506x(88)90011-6. [DOI] [PubMed] [Google Scholar]
- 56.Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J Comp Physiol A. 2000;186:759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77:416–424. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- 58.Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001;168:217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- 59.Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor alpha in select brain nuclei are associated with individual differences in aggression. Horm Behav. 2006;50:338–345. doi: 10.1016/j.yhbeh.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon NG. Hormonal processes in the development and expression of aggressive behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain, and behavior. New York: Academic Press; 2002. pp. 339–392. [Google Scholar]
- 61.Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 2010;57:381–389. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol. 2004;58:413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- 63.Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003;57:130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- 64.Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marler P, Peters S, Ball GF, Dufty AM, Wingfield JC. The role of sex steroids in the acquisition and production of birdsong. Nature. 1988;336:770–772. doi: 10.1038/336770a0. [DOI] [PubMed] [Google Scholar]
- 67.Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 68.Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci U S A. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc Biol Sci. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- 71.Kruk MR, Van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AH. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- 72.Kruk MR. Ethology and Pharmacology of Hypothalamic Aggression in the Rat. Neurosci Biobehav Rev. 1991;15:527–538. doi: 10.1016/s0149-7634(05)80144-7. [DOI] [PubMed] [Google Scholar]
- 73.Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 74.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 75.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 76.Jeffery KJ. Self-localization and the entorhinal-hippocampal system. Curr Opin Neurobiol. 2007;17:684–691. doi: 10.1016/j.conb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Bingman VP, Able KP. Maps in birds: representational mechanisms and neural bases. Curr Opin Neurobiol. 2002;12:745–750. doi: 10.1016/s0959-4388(02)00375-6. [DOI] [PubMed] [Google Scholar]
- 78.Moser EI, Moser MB. A metric for space. Hippocampus. 2008;18:1142–1156. doi: 10.1002/hipo.20483. [DOI] [PubMed] [Google Scholar]
- 79.Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 81.Shugrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor alpha and beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 82.Ball GF, Bernard DJ, Foidart A, Lakaye B, Balthazart J. Steroid sensitive sites in the avian brain: Does the distribution of the estrogen receptor alpha and beta types provide insight into their function? Brain Behav Evol. 1999;54:28–40. doi: 10.1159/000006609. [DOI] [PubMed] [Google Scholar]
- 83.Hodgson ZG, Meddle SL, Christians JK, Sperry TS, Healy SD. Influence of sex steroid hormones on spatial memory in a songbird. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:963–969. doi: 10.1007/s00359-008-0369-4. [DOI] [PubMed] [Google Scholar]
- 84.Fuxjager MJ, Mast G, Becker EA, Marler CA. The 'home advantage' is necessary for a full winner effect and changes in post-encounter testosterone. Horm Behav. 2009;56:214–219. doi: 10.1016/j.yhbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Trainor BC, Bird IM, Marler CA. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm Behav. 2004;45:115–121. doi: 10.1016/j.yhbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 86.Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 87.Karstaedt PJ, Kerasidis H, Pincus JH, Meloni R, Graham J, Gale K. Unilateral destruction of dopamine pathways increases ipsilateral striatal serotonin turnover in rats. Exp Neurol. 1994;126:25–30. doi: 10.1006/exnr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 88.Cransac H, Cottet-Emard JM, Pequignot JM, Peyrin L. Monoamines (norepinephrine, dopamine, serotonin) in the rat medial vestibular nucleus: endogenous levels and turnover. J Neural Transm. 1996;103:391–401. doi: 10.1007/BF01276416. [DOI] [PubMed] [Google Scholar]
- 89.Song WC, Melner MH. Steroid transformation enzymes as critical regulators of steroid action in vivo. Endocrinology. 2000;141:1587–1589. doi: 10.1210/endo.141.5.7526. [DOI] [PubMed] [Google Scholar]