Abstract
Objective
To systematically evaluate the literature addressing the role of MRI in the diagnosis and prognosis of early undifferentiated inflammatory arthritis and RA.
Methods
We performed a systematic literature review of the performance characteristics of MRI for diagnosing and prognosticating RA. We searched Ovid, supplementing this with manual searches of bibliographies, journals, meeting proceedings, and ClinicalTrials.gov website. To identify Diagnostic studies, we included studies of any duration that prospectively examined whether MRI findings predicted RA diagnosis and reported adequate information to calculate sensitivity and specificity. To identify Prognostic studies, we included prospective studies with at least 12 month follow-up that measured both baseline MRI findings and clinical and/or radiographic outcomes.
Results
In “Diagnostic Studies” (N=11), sensitivity and specificity of MRI findings for RA diagnosis ranged from 20–100% and 0–100%, respectively, depending upon criteria used. MRI diagnostic performance improved when lower quality or longer disease duration studies were excluded. In “Prognostic Studies” (N=17), MRI findings did not predict clinical remission and the ability to predict radiographic progression varied significantly (range 18–100% for sensitivity and 5.9–97% for specificity). Restricting the analysis to specific MRI findings or earlier disease improved MRI prognostic performance. The only prognostic study reporting 100% of a priori quality criteria found MRI bone edema the strongest predictor of radiographic progression.
Conclusion
Data evaluating MRI for the diagnosis and prognosis of early RA are currently inadequate to justify widespread use of this technology for these purposes, although MRI bone edema may be predictive of progression in certain RA populations.
Rheumatoid arthritis (RA) is a common debilitating disease (1). Treatment with disease modifying anti-rheumatic drugs (DMARDs) provides effective symptom control and decreased risk of disability. Evidence supports a ‘window of opportunity’, perhaps within three to six months of symptom onset, during which initiating treatment maximizes improvement in long-term outcomes (2, 3). Recent evidence suggests that early initiation of aggressive treatment might improve the chance of sustained remission (4). Some have even raised the possibility of cure (5). Therefore, methods to improve RA diagnosis and prognostication are of high priority because treating all patients would expose some individuals to unacceptable levels of risk from treatment. While DMARDs are effective in reducing inflammation and restoring function, they are not without cost, including infectious and other complications (6). MRI has been proposed as a means to improve rheumatologists’ ability to diagnose early RA and predict which patients will likely develop progressive disease and thus should receive more aggressive treatment. While utilization of MRI in RA is unknown, an unpublished national survey of rheumatologists found >30% had used MRI for management of RA patients within the last year (Marissa Blum, M.D., personal communication).
The ability of MRI to provide additional and more sensitive information than clinical examination or conventional radiography is well established (7, 8). MRI can identify bone erosions earlier than conventional radiography (7) and can detect bone marrow edema and synovitis, which may to be important precursors to erosive disease (9, 10). Given these properties, MRI has been proposed as a diagnostic tool among individuals with suspected inflammatory arthritis and as a prognostic tool among those with known RA. However, MRI performance characteristics in the diagnosis and prognostication of early RA are not well defined and false positive results may counteract the benefits of high MRI sensitivity. Given the importance of accurate early diagnosis and prognostication in early RA and the rising utilization of MRI in RA, our objective was to systematically evaluate published reports describing the diagnostic and prognostic capability of MRI findings in undifferentiated inflammatory arthritis and early RA, respectively.
Materials and Methods
The following describes the eligibility criteria, search strategies, a priori criteria for methodological quality, outcome measures, data extraction methods, and data analysis strategies for 1) diagnostic and 2) prognostic studies, respectively. We employed methods based upon Cochrane Collaboration guidelines, including systematic search strategies for all published literature to identify relevant articles, followed by comprehensive, standardized data extraction of relevant outcomes and study characteristics (11). We established a priori inclusion and exclusion criteria and employed both standard and topic-specific methodological quality assessments.
Diagnostic Studies: Eligibility Criteria
The eligibility criteria for included Diagnostic studies were the following:
Prospective English language studies of any duration that examined the ability of hand or wrist MRI findings to predict an RA diagnosis among adult patients with undifferentiated polyarthritis of the hand or wrist;
Used ACR 1987 revised criteria and/or clinical assessment by a rheumatologist as the diagnostic gold standard;
Reported adequate information to calculate sensitivity and specificity; and
Reported data for > 10 patients.
Undifferentiated polyarthritis was defined by published criteria (12, 13). These criteria included patients with characteristics, history, examination, or laboratory data suggesting an inflammatory arthritis, but without a specific diagnosis of rheumatic disorder. Presentations in this category include arthralgias in a distribution typical of RA, with or without abnormal inflammatory markers or positive rheumatoid factor, a dramatic response to corticosteroid medications, a convincing history of joint swelling, specific extraarticular features (e.g., nodules), or atypical joint swelling (e.g., asymmetric, oligoarticular or unusual joint patterns) (13–15). Where possible, data from mixed populations of undifferentiated polyarthritis, arthralgia, and early suspected RA, were examined separately.
Diagnostic Studies: Search Strategy & Study Selection
Using Ovid, one reviewer (LGS) searched the specialized Cochrane Central Register of Controlled Trials and Medline (through April week 1 2010). In order to capture all potential studies, we employed a broad search strategy using medical subject headings (MeSH) consisting of “exp Magnetic Resonance Imaging/” combined with “exp Arthritis/” or “exp Arthritis, Rheumatoid/”. Citation abstracts were searched by hand for studies meeting the above inclusion criteria. A secondary manual search included:
Bibliographies of all included studies and relevant review articles identified by the preceding search within three years;
Abstracts and meeting proceedings of journals and professional societies within three years (Table 1); and
ClinicalTrials.gov website.
Table 1.
MRI diagnostic test performance in included studies*
| Reference | N | Mean Follow-up, months (range) |
RF positive (%) |
anti- CCP positive (%) |
Plain radiograph erosions (%) |
Quality Criteria Reported (%) |
MRI Definition of RA | Sensitivity | Specificity | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugimoto 1996 (29) | 27 | 2.7 (NR) | 37 | NR | 0 | 53 | presence of MRI synovitis† | 100% | 72.7% | 84.2% | 94.1% | 3.56 | 0.04 |
| Sugimoto 2000 (30)‡ | 48 | 25 (4–72) | 73 | NR | 0 | 69 | presence of bilateral MRI synovitis (periarticular enhancement) | 96.2% | 86.4% | 89.3% | 95.0% | 7.05 | 0.05 |
| Klarlund 2000 (20) | 13 | 12 | 49 | NR | 6 | 62.5 | presence of MRI tenosynovitis | 60.0% | 62.5% | 50.0% | 71.4% | 40.00 | 0.80 |
| presence of MRI bone erosions | 20.0% | 100.0% | 100.0% | 66.7% | 0.60 | 0.64 | |||||||
| Boutry 2005 (28) | 47 | 29 (4–73) | NR | NR | 0 | 88 | presence of OMERACT MRI synovitis | 100.0% | 0.0% | 59.6% | 50.0% | 1.01 | 0.68 |
| presence of OMERACT MRI bone erosions§ | 60.7–100.0% | 15.8–52.6% | 63.6–65.4% | 47.6–85.7% | 1.17–1.28 | 0.75–0.11 | |||||||
| presence of OMERACT MRI bone edema | 39.3–71.4% | 84.2–94.7% | 78.6–95.2% | 48.5–69.2% | 2.49–13.57 | 0.30–0.72 | |||||||
| Solau-Gervais (21) | 30 | 30.6 (12-NR) | 30 | NR | 0 | 88 | OMERACT RAMRIS erosion score > 15 | 70.0% | 64.0% | - | - | - | - |
| presence of MRI carpus or MCP erosions | 66.7–80.0% | 27.3–53.8% | 55.6–66.7% | 27.3–70.0% | 1.73 | 0.37 | |||||||
| Tamai 2006 (22) | 113 | 12 (NR) | 39 | 24 | NR | 63 | presence of ≥ 2 of anti-CCP or RF antibodies, MRI bone edema or MRI erosions, and symmetric MRI synovitis | 82.5% | 84.8% | 93.0% | 66.7% | 5.43 | 0.21 |
| Narvaez 2008 (23) | 40 | 20 (12–42) | 0 | 23 | 0 | 87 | presence of OMERACT MRI synovitis with MRI bone edema or MRI erosions | 100.0% | 77.8% | 93.9% | 93.3% | 4.43 | 0.02 |
| Duer 2008 (24) | 41 | 24 (NR) | 34 | NR | 0 | 73 | presence of MRI synovitis | 100.0% | 60.0% | 48.0% | 100.0% | 2.50 | 0.00 |
| presence of MRI erosions | 64.0% | 77.0% | 50.0% | 85.0% | 2.78 | 0.47 | |||||||
| presence of MRI synovitis or erosions | 100.0% | 50.0% | 42.0% | 100.0% | 2.00 | 0.00 | |||||||
| presence of MRI synovitis and erosions | 64.0% | 87.0% | 64.0% | 87.0% | 4.92 | 0.41 | |||||||
| Mori 2008 (26) | 21 | 27.4 (13–40) | 59 | 24 | NR | 71 | presence of MRI symmetrical synovitis** | 100.0% | 75.0% | 62.5% | 100.0% | 2.00 | 0.18 |
| Eshed 2009 (25) | 99 | 8†† (6–41) | 35 | 26 | 0 | 69 | presence of MRI flexor tenosynovitis | 60.3% | 73.2% | 76.1% | 56.6% | 2.25 | 0.54 |
| presence of MRI extensor tenosynovitis | 24.1% | 87.8% | 73.7% | 45.0% | 1.98 | 0.86 | |||||||
| presence of MRI MCP synovitis | 82.8% | 39.0% | 65.7% | 61.6% | 1.36 | 0.44 | |||||||
| presence of anti-CCP antibodies and MRI tenosynovitis | 78.9% | 73.0% | - | - | - | - | |||||||
| Tamai 2009 (27)‡‡ | 129 | > 12 (NR) | 43 | 36 | NR | 63 | presence of MRI symmetrical synovitis | 74.7% | 59.3% | 71.8% | 62.7% | 1.84 | 0.43 |
| presence of MRI bone edema | 41.3% | 90.7% | 86.1% | 52.7% | 4.44 | 0.65 | |||||||
| presence of MRI erosions | 29.3% | 90.7% | 81.5% | 48.0% | 3.15 | 0.78 | |||||||
| presence of anti-CCP antibodies and MRI bone edema | 50.7% | 100.0% | 100.0% | 59.3% | 31.97 | 0.71 |
All studies used 1987 ACR criteria as RA diagnostic gold standard; All characteristics represent baseline prevalence; anti-CCP positive = baseline prevalence of anti-cyclic citrullinated peptide antibodies; RF positive = baseline rheumatoid factor antibodies; PPV = Positive predictive value; NPV = Negative predictive value; LR+ = Likelihood Ratio Positive; LR− = Likelihood ratio Negative; NR = Not reported; MCP = metacarophalangeal;
Defined as periarticular enhancement
Patient population overlaps with Sugimoto et al, 1996
Represents range of values for considering wrist and MCP joints separately
Addition of the presence of RF and/or anti-CCP antibodies did not alter test performance
Standard deviation
Population overlaps with Tamai et al, 2006
Diagnostic Studies: Methodological Quality Assessment
To identify and account for potential sources of selection and measurement biases, we assessed methodological quality based upon recently updated recommendations for assessing the methodological quality of diagnostic studies (16, 17). We used the 14 items of the Quality Assessment of Diagnostic Accuracy Studies or QUADAS checklist (16) (Appendix) to assess study quality. The Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) group also published the RA MRI Scoring system (RAMRIS) that combines MRI evidence of erosions, edema and synovitis into a validated, reproducible scoring system (18) and recommended minimum core sequences to improve the quality of research in this field (19). Therefore, we also considered whether or not the study obtained minimum core MRI sequences recommended by the recent OMERACT working group and/or employed a validated MRI scoring method (i.e., OMERACT RAMRIS).
Appendix.
Criteria for Methodological Quality Assessment of Included Studies*
| Criteria for Diagnostic Studies |
| A. Was spectrum of patients representative of patients who will receive the test in practice? |
| B. Were selection criteria clearly described? |
| C. Is reference standard likely to correctly classify target condition? |
| D. Is time period between reference standard and index test short enough to be reasonably sure that target condition did not change between two tests? |
| E. Did whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? |
| F. Did patients receive the same reference standard regardless of index test result? |
| G. Was reference standard independent of index test (i.e. the index test did not form part of the reference standard)? |
| H. Was execution of index test described in sufficient detail to permit replication? |
| I. Was execution of reference standard described in sufficient detail to permit replication? |
| J. Were index test results interpreted without knowledge of reference standard results? |
| K. Were reference standard results interpreted without knowledge of index test results? |
| L. Were same clinical data available when test results were interpreted as would be available when test is used in practice? |
| M. Were missing/uninterpretable/intermediate test results reported? |
| N. Were withdrawals from study explained? |
| O. Were minimum core OMERACT MRI sequences used? |
| P. Was validated MRI scoring method (i.e., OMERACT RAMRIS) used? |
| Criteria for Prognostic Studies |
| A. Was there clear description of inclusion and exclusion criteria? |
| B. Were subject enrolled consecutively? |
| C. Was there random treatment allocation? |
| D. Was there uniform, standardized treatment? |
| E. Was treatment allocation blinded? |
| F. Was receipt of treatment blinded? |
| G. Was there clear description of variables & outcomes assessed? |
| H. Was outcome measurement blinded? |
| I. Were minimum core OMERACT MRI sequences used? |
| J. Was validated MRI scoring method (i.e., OMERACT RAMRIS) used? |
| K. Was MRI measurement blinded? |
| L. Were missing/uninterpretable/intermediate test results reported? |
| M. Were withdrawals from study explained? |
| N. Was intention to treat analysis used? |
| O. Were analyses adjusted for baseline disease severity? |
Each item scored as Yes/No/Unknown where Yes = Sufficient information and a positive assessment; No = Sufficient information and a negative assessment; and Unknown = Insufficient information.
Diagnostic Studies: Outcome Measure
For diagnosis studies, the primary outcome was ability to predict RA diagnosis, defined as fulfilling ACR 1987 revised criteria and/or clinical assessment by a rheumatologist, at follow-up, reported as sensitivity and specificity.
Diagnostic Studies: Data Extraction
All articles were abstracted in duplicate by two independent reviewers using standard abstraction forms. Data abstracted from diagnostic studies included:
Study features (design, sample size, handling of missing data);
Baseline patient demographics, clinical, plain radiographic and MRI data; and
Sensitivity and Specificity (as reported and/or calculated from information provided).
Discrepancies were resolved by consensus discussion between reviewers.
Diagnostic Studies: Statistical Analysis
The following measures of test accuracy were computed for each study: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratio positive (LR+), and likelihood ratio negative (LR−). Sensitivity and specificity for test thresholds identified in each study were used to plot a summary receiver operating characteristic(SROC) curve and calculate the area under the curve (AUC). If appropriate, Cochran’s Q statistic was used to determine homogeneity in measures of test accuracy across studies (p>0.1). Absent heterogeneity, hierarchical summary ROC model and bivariate random-effects model were used to calculate average sensitivity and specificity values. We also stratified the analysis by study and patient characteristics, including quality criteria, specific MRI parameters and disease duration.
Prognostic Studies: Eligibility Criteria
The eligibility criteria for including studies on prognosis were:
Prospective English language study of at least twelve months’ duration that collected and reported hand, wrist and/or foot MRI and plain radiographic, and any clinical data on early RA patients;
RA was defined by 1987 ACR or equivalent classification criteria;
“Early” RA was broadly defined as <60 months disease duration in order to capture all relevant studies;
Reported data for > 10 patients.
Prognostic Studies: Search Strategy & Study Selection
To identify prognostic studies, we used the identical search strategy described above for diagnostic studies. Citation abstracts were searched by hand for studies meeting the above prognostic study inclusion criteria. We performed a secondary manual search of bibliographies, meeting proceedings, journals, and the ClinicalTrials.gov website to further identify prognostic studies.
Prognostic Studies: Methodological Quality Assessment
Although recommendations for the assessment of methodological quality for prognostic studies do not exist, we sought to identify and account for potential sources of selection, measurement and, where relevant, intervention biases in the included studies. We combined recommendations for the assessment of diagnostic study methodological quality listed above with relevant criteria for assessment of clinical trials and observational studies (11), including clear descriptions of methods, inclusion and exclusion criteria, blinding, handling of missing data and losses to follow-up, and use of OMERACT core sequences and/or validated MRI scoring (Appendix). Intervention biases are of particular concern in prognostic studies as administration of treatment may alter the disease course. Therefore, we also assessed whether uniform, standardized treatment protocols were employed to all or a subset of the study population and/or whether analyses adjusted for baseline disease severity.
Prognostic Studies: Outcome Measures
For the prognosis studies, the primary outcome of interest was ability to predict radiographic outcomes (Sharp or Larsen scores) at 12 months. Secondary outcome measures included the same radiographic outcomes at ≥ 12 months as well as clinical status (measured by ACR20 and/or its components) and functional ability and/or quality of life (measured by HAQ score, SF-36 or other validated measure) at ≥ 12 months.
Prognostic Studies: Data Extraction
All articles were abstracted in duplicate by two independent reviewers using a standard abstraction form. Data abstracted from prognosis studies included:
Study features (controls, randomization, sample size, therapeutic intervention, handling of missing data);
Baseline patient demographics, clinical and MRI data; and
Plain radiographic and MRI outcomes data as well as clinical and functional outcomes (including ACR20 response, Disease Activity Score or DAS, HAQ or SF-36 scores).
Discrepancies were resolved by consensus discussion between reviewers.
Prognostic Studies: Statistical Analysis
The primary comparison across studies was the effect size of the correlation between baseline MRI findings and 12 month radiographic progression as measured by Sharp, modified Sharp or Larsen score. In addition, we examined the sensitivity, specificity, PPV, NPV, LR+, and LR− of MRI findings to predict radiographic and clinical outcomes at ≥ 12 months. This information was plotted as an SROC curve and the AUC was calculated. If appropriate, Cochran’s Q statistic was used to determine homogeneity (p>0.1). Absent heterogeneity, we planned to pool effect sizes. Stratified analyses, according to the study and patient characteristics, were also performed. Correlations for secondary outcomes were similarly compared.
Results
Search results for all included Diagnostic and Prognostic studies are presented in Figure 1 and described below. The most common reasons for exclusion were a non-RA population, no hand, wrist or foot imaging, <10 patients, and < 12 months of follow-up.
Figure 1.
Flowchart of Literature Search for Diagnostic and Prognostic Studies
Diagnostic Studies
Our search for Diagnostic studies yielded 11 studies comprising 606 individual patients. The mean/median disease duration was ≤ 18 months for the eight studies (20–27) that reported this information (range 0.5–180 months) and the mean follow-up was < 20 months (range 4–73 months). The study populations ranged from 67–100% female participants and the mean/median ages ranged from 40 to 57.7 years (range 13–80 years). Four studies (20, 22, 26, 27) did not report the prevalence of baseline radiographic erosions in their cohorts and the remainder excluded individuals with plain radiographic erosions at baseline. Two studies (24, 25) used low-field MRIs (0.2 Tesla, versus 1.0–1.5 Tesla for the rest of the studies).
There was marked variation among studies regarding the MRI classification criteria used to diagnose RA (Table 1). Three studies (21, 23, 28) used the OMERACT RAMRIS scoring system, but only one (21) reported a specific cut-off for positivity and the remaining studies used the OMERACT definitions for synovitis, erosions and bone edema without reporting score cutoffs. Eight studies (21–23, 25–27, 29, 30) considered the presence of anti-cyclic citrullinated peptide or rheumatoid factor antibodies in their analysis; two (29, 30) studies examined only synovitis or contrast enhancement and one (21) examined only erosion scores or the presence of erosions.
Overall, sensitivity and specificity of MRI findings varied broadly (range 20–100% for sensitivity and 0–100% for specificity), even for comparable MRI definitions of RA (i.e., symmetrical synovitis). Increasingly restrictive diagnostic criteria (i.e., requiring the combined presence of multiple MRI and/or other clinical or laboratory findings) improved specificity at the expense of sensitivity. However, Sugimoto, et. al. (29) found decreased specificity with a diagnostic algorithm where rheumatoid factor and joint count assessment preceded MRI. MRI was less informative in differentiating between inflammatory conditions. Boutry, et. al. (28) compared findings among individuals eventually found to have RA, systemic lupus erythematosis and Sjogrens and found no significant differences in MRI findings, including OMERACT erosion scores. Although other data (21) found this score differed significantly between individuals eventually diagnosed with RA versus those with all other diseases pooled.
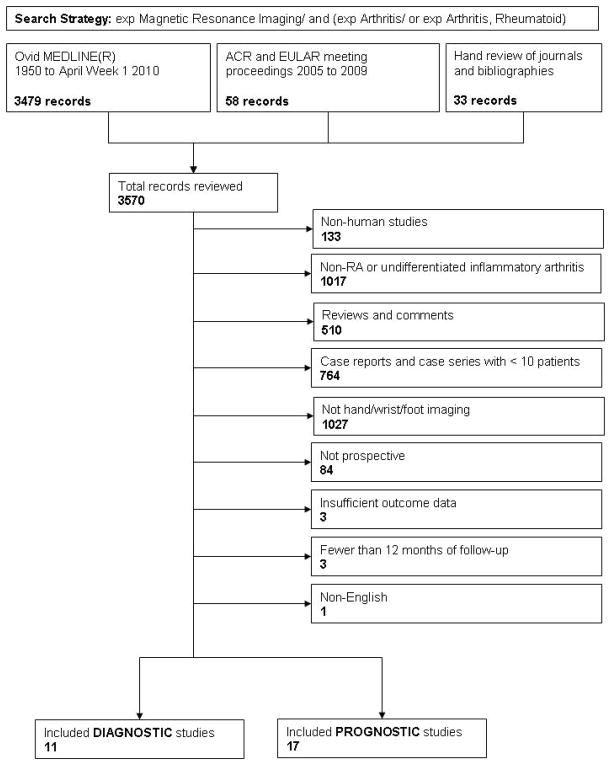
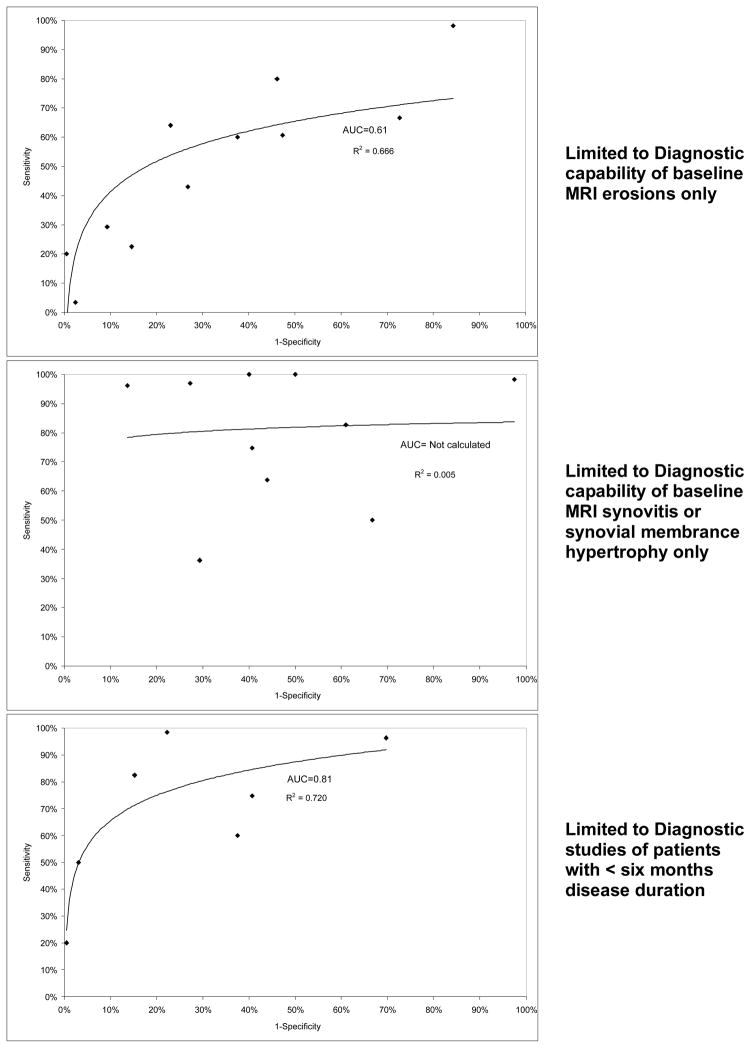
There was considerable variability in methodological quality. Four studies (21, 23, 24, 28) met criteria for the minimum recommended MRI sequences, and blinding, handling of missing data and losses to follow up were adequately reported by four or fewer studies. Where reported, missing data was handled by exclusion. Given apparent heterogeneity of MRI diagnostic criteria and study designs, we chose to provide stratified data, rather than pool diverse studies. Each graph in Figure 2 shows the sensitivity and specificity of included studies plotted in ROC space with a regression line and R2 value provided to demonstrate fit. The 1st graph shows the results for all 11 included studies, some of which provided data for multiple MRI RA definitions (21, 25–28). The 2nd graph shows data from studies receiving the highest quartile of quality assessment (i.e., those studies with 80% or greater scores out of a possible 100% for quality) (21, 23, 28). The 3rd provides data from studies (22, 25, 27) in the highest size quartile (> 86 participants), none of which were in the highest quality quartile. The 4th graph shows data for MRI erosions, while the 5th, data for measures of MRI synovitis. There were an insufficient number of studies to allow subgroup analysis of those using OMERACT RAMRIS scoring or examining MRI bone edema or mixed arthritis populations. The 6th graph shows data from studies (20, 22, 23, 27) examining patients with < six months of disease.
Figure 2.
Sensitivity and Specificity of included Diagnostic studies in ROC space
While limiting the analysis to only those studies in the highest quality quartile or earliest disease improved MRI performance (AUCs for all, highest quality quartile, and < six months disease duration studies were 0.77, 0.80, and 0.82, respectively), limiting analysis to studies in the highest size quartile or to specific MRI parameters appeared to decrease MRI performance (AUC for highest size quartile and MRI erosion studies 0.70 and 0.61, respectively; AUC not calculated for MRI synovitis studies due to extreme heterogeneity of results). Only one study (28) examined the diagnostic capability of MRI bone marrow edema independent of other parameters.
Prognostic Studies
Seventeen Prognostic studies, comprising 710 individual patients, seven randomized clinical trials (31–37) and ten observational studies (9, 10, 38–45), met our inclusion criteria. An additional study (20) examined the prognostic capability of MRI in both early RA and undifferentiated arthritis patients, but did not provide sufficient data for the RA cohort to allow inclusion. The mean follow-up was 24.4 months and the mean/median disease duration was < 12 months (range 0.4–20.6 months) for all but three studies (31, 38, 44) who reported mean/median disease durations ≤ 25 months (range 3–264 months). Women comprised 56–80% of study participants with mean/median age 38–60 years (range 20–83). Six studies (9, 31, 32, 35, 36, 41) reported the prevalence of baseline plain radiographic erosions, which ranged from 24–62% of patients and Cohen, et. al. (37) used the presence of baseline radiographic erosions as an inclusion criteria in order to study a high risk population. One study (41) used low-field (0.2 Tesla) MRI machines for all examinations and Hetland, et. al. (36) used machines with a range of Tesla (0.2–1.5) for their study; the remainder of studies used 1.5 Tesla MRIs.
One study (38) reported the prognostic capability of MRI to predict clinical outcomes (remission as defined by ACR criteria), but found no significant association. Sensitivity and specificity of MRI findings to predict radiographic progression, defined as either new erosions or increased Sharp score, varied broadly (range 18–100% for sensitivity and 5.9–97% for specificity), even for comparable MRI findings, such as baseline MRI erosions (range 60–88.9% for sensitivity and 5.9–94% for specificity) (Table 2).
Table 2.
MRI prognostic test performance in included studies*
| Reference | Study Design |
N | Mean Follow-up, months |
RF positive (%) |
Anti- CCP positive (%) |
Plain radiograph erosions (%) |
Quality Criteria Reported (%) |
MRI Prognosis Findings | Sensitivity | Specificity | PPV | NPV | Odds Ratio (95% CI) |
Correlation (p value) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee 1997 (38) | open label trial† | 10 | 14 | 90 | NR | NR | 38 | No significant association between MRI synovial proliferation, bone edema or erosions and ACR-defined remission | - | - | - | - | - | - |
| Ostergaard 1999 (31) | Open RCT‡ | 26 | 12 | 58 | NR | 46 | 50.0 | MRI erosions at baseline predicted radiographic erosions at follow-up | 88.9% | 5.9% | 33.3% | 50.0% | - | - |
| MRI synovial membrane hypertrophy score significantly correlated with progression of radiographic erosions | - | - | - | - | - | 0.42 (<0.05) | ||||||||
| MRI synovial membrane hypertrophy score ≥ 5 cm3 predicted progression of radiographic erosions | 100.0% | 29.4% | 42.9% | 100.0% | - | - | ||||||||
| MRI synovial membrane hypertrophy score ≥ 10 cm3 predicted progression of radiographic erosions | 44.4% | 64.7% | 40.0% | 68.8% | - | - | ||||||||
| MRI AUC synovial membrane hypertrophy ≥ 4 predicted progression of radiographic erosions | 100.0% | 41.2% | 52.6% | 100.0% | - | - | ||||||||
| MRI AUC synovial membrane hypertrophy ≥ 8 predicted progression of radiographic erosions | 80.0% | 93.8% | 88.9% | 88.2% | - | - | ||||||||
| McQueen 1999 (39) | observational | 42 | 12 | 90 | NR | 36 | 50 | MRI erosions predicted radiographic erosions | 83.3% | 70.0% | 52.6% | 91.3% | 11.6 (NR)§ | - |
| MRI erosion score ≥ 6 predicted radiographic erosions | 93.3% | 81.8% | 93.0% | - | 63 (7.7–513) | - | ||||||||
| MRI erosion score ≥ 13 predicted radiographic erosions | 82% | 73% | 53% | 92% | - | - | ||||||||
| MRI bone edema predicted radiographic erosions | - | - | - | - | 6.47 (3.2–13.1) | - | ||||||||
| MRI synovitis predicted radiographic erosions | - | - | - | - | 2.14 (1.3–3.7) | - | ||||||||
| McQueen 2001 (40) ** | observational | 42 | 24 | 90 | NR | 36 | 50 | MRI erosion score ≥ 13 predicted radiographic erosions at follow-up | 80% | 76% | 67% | 86% | 13.4 (2.65–60.5) | - |
| Conaghan 2003 (32) | RCT†† | 42 | 12 | 60 | NR | 45 | 75 | No significant prognostic findings reported‡‡ | - | - | - | - | - | - |
| McQueen 2003 (9) §§ | observational | 42 | 72 | 90 | NR | 36 | 50 | MRI bone edema significantly associated with 6 year radiographic progression | 18.0% | 96.8% | - | - | - | - |
| Quinn 2005 (33) | RCT*** | 20 | 24 | 65 | NR | NR | 81 | No significant prognostic findings reported††† | - | - | - | - | - | - |
| Lindegaard 2006 (41) | observational ‡‡‡ | 24 | 12 | 95 | NR | 24 | 56 | OMERACT MRI baseline erosions or bone edema predicted radiographic progression at follow-up | 80.0% | 57.9% | 33.3% | 79.2% | - | - |
| OMERACT MRI baseline erosion score significantly correlated with radiographic progression at follow-up | - | - | - | - | - | 0.69 (<0.001) | ||||||||
| Jarrett 2006 (34) | RCT§§§ | 39 | 6 | 77 | NR | NR | 75 | No significant prognostic findings reported | - | - | - | - | - | - |
| Durez 2007 (35) | RCT**** | 44 | 12 | 77 | 70 | 27 | 75 | No significant prognostic findings reported | - | - | - | - | - | - |
| Hetland 2009 (36) | RCT†††† | 130 | 24 | 67 | 61 | 62 | 100 | OMERACT baseline MRI bone edema only significant predictor of radiographic progression at follow-up‡‡‡‡ | - | - | - | - | - | 0.50–0.64 (<0.001) |
| Cohen 2008 (37) §§§§ | RCT | 218 | 12 | 78 | NR | NR | 81 | No significant prognostic findings reported | - | - | - | - | - | - |
| Haavardsholm 2008 (42) | observational | 84 | 12 | 44 | 55 | NR | 69 | OMERACT baseline MRI bone edema score > 2 predicted radiographic progression at follow- up***** | - | - | - | - | 2.77 (1.06–7.21) | - |
| Boyesen 2008 (10) ††††† | observational | 89 | 36 | 42 | 63 | NR | 69 | AUC of OMERACT baseline MRI synovitis score predicted radiographic progression at follow-up‡‡‡‡‡ | - | - | - | - | - | 0.53 (0.004) |
| Syversen 2008 (43) | observational | 82 | 12 | 44 | 55 | NR | 71 | MRI bone edema significantly associated with 1 year radiographic progression§§§§§ | - | - | - | - | - | - |
| Mundwiler 2009 (44) | observational | 46 | 24 | 90 | 76 | NR | 50 | OMERACT baseline MRI erosions predicted radiographic progression at 12 month follow-up | 60% | 94% | 10% | 99.5% | 32.2 (3.7–144.6) | - |
| OMERACT baseline MRI erosions predicted radiographic progression at 24 month follow-up | 75% | 94% | 17% | 99.5% | 43.6 (4.27–445) | - | ||||||||
| OMERACT baseline MRI bone edema predicted radiographic progression at 12 month follow-up | 67% | 97% | 50% | 99% | 68.0 (13.6–338.9) | - | ||||||||
| Hammer 2009 (45) ****** | observational | 58 | 12 | >25% | >50% | NR | 69 | No significant prognostic findings reported | - | - | - | - | - | - |
All characteristics represent baseline prevalence; anti-CCP positive = baseline prevalence of anti-cyclic citrullinated peptide antibodies; RF positive = baseline rheumatoid factor antibodies; PPV = Positive predictive value; NPV = Negative predictive value; LR+ = Likelihood Ratio Positive; LR− = Likelihood ratio Negative; NR = Not reported; DMARD = disease modifying anti-rheumatic drug; AUC = area under the curve; RCT = randomized controlled trial; ESR = erythrocyte sedimentation rate; 95% CI = 95% confidence interval
Standardized treatment protocol consisted of methotrexate 10 mg orally each week, 7.5 mg prednisone daily and hydroxycholoroquine 300–400 mg daily)
Treatment consisted of DMARD monotherapy versus DMARD combined with 30 mg daily prednisolone tapered to lowest dose tolerated
P value = 0.005
Population overlaps with McQueen et al, 1999
Treatment consisted of escalated methotrexate monotherapy with versus without intraarticular corticosteroids
No prognostic associations noted as all patients demonstrated both significant clinical improvement and the absence of radiographic progression over the study period
Population overlaps with McQueen et al, 1999 and McQueen et al, 2001
Treatment consisted of escalated methotrexate monotherapy with versus without infliximab infusion (3 mg/kg)
No prognostic associations noted as all patients demonstrated significant clinical improvement and the relationship of baseline MRI findings to clinical or radiographic progression over the study period was not reported
All patients received escalated methotrexate monotherapy with maximum oral prednisolone 7.5 mg daily; additional DMARDs were not permitted
Treatment consisted of escalated methotrexate monotherapy with versus without zoledronic acid infusion (5 mg)
Treatment consisted of escalated methotrexate monotherapy alone versus with infliximab infusion (3 mg/kg) versus with intravenous methylprednisolone
Treatment consisted of escalated methotrexate monotherapy with versus without cyclosporine (2.5–4.0 mg/kg)
Multivariable analysis adjusted for age, gender, smoking status, HLA status, baseline disease activity, presence of anti-CCP antibodies, and baseline MRI erosion and synovitis scores; model predicted 25% and 41% of variance among all 130 subjects with wrist MRI and 84 subjects with both wrist and MCP MRI, respectively
Treatment consisted of escalated methotrexate monotherapy, with or without other DMARDs, versus denosumab 60 mg or 180 mg at 0, 6 months
Multivariable analysis adjusted for age, gender, baseline disease activity, presence of anti-CCP antibodies, baseline Sharp score, and baseline MRI bone edema, erosion, synovitis and tensosynovitis scores; model correctly classified 76% of radiographic progressors (OR 10.8)
Abstract only; population overlaps with Haavardsholm et al, 2008
Multivariable analysis adjusted for age, gender, baseline ESR and presence of anti-CCP antibodies (R2 = 0.35)
In regression model including baseline MRI bone edema, change in serum eotaxin level, gender, age and baseline disease activity, MRI bone edema remained significantly associated with 1 year radiographic progression (model prediction accuracy 71%)
Population overlaps with Haavardsholm et al, 2008 and Boyesen et al, 2008
There was marked variation in methodological quality among studies, with the percentage of adequately addressed quality criteria ranging from 38% to 100% (Table 2). Nine studies used uniform treatment administration over the course of the study, either as randomized clinical trials (seven) (31–37) or with standardized treatment protocols (two) (38, 41), however, only two (31, 36) found MRI findings (synovitis and bone edema) were significantly associated with subsequent radiographic progression and only one reported sufficient information to calculate sensitivity and specificity of MRI to predict radiographic erosions at one year (31). Hetland, et. al. (36) found MRI bone edema was the only statistically significant predictor of radiographic progression by Sharp score at two years in a multivariable model adjusting for age, gender, smoking status, HLA status, baseline disease activity, presence of anti-CCP antibodies, and baseline MRI erosion and synovitis scores. This model predicted 25% and 41% of variance among 130 subjects with wrist MRIs and 84 subjects with both wrist and MCP MRIs, respectively.
Other methodological quality criteria varied across the included studies. While the included randomized clinical trials provided the highest quality assessments, they rarely provided adequate data regarding the prognostic capability of MRI findings within uniform treatment groups to predict radiographic or clinical outcomes. One randomized clinical trial (32) that attempted to report data on the ability of MRI to predict radiographic outcomes was limited by the absence of plain radiographic progression in their cohort. In addition, most clinical trials studies found either clinical stability or uniform improvement over time and thus were not able to assess the prognostic capability of MRI to predict clinical progression.
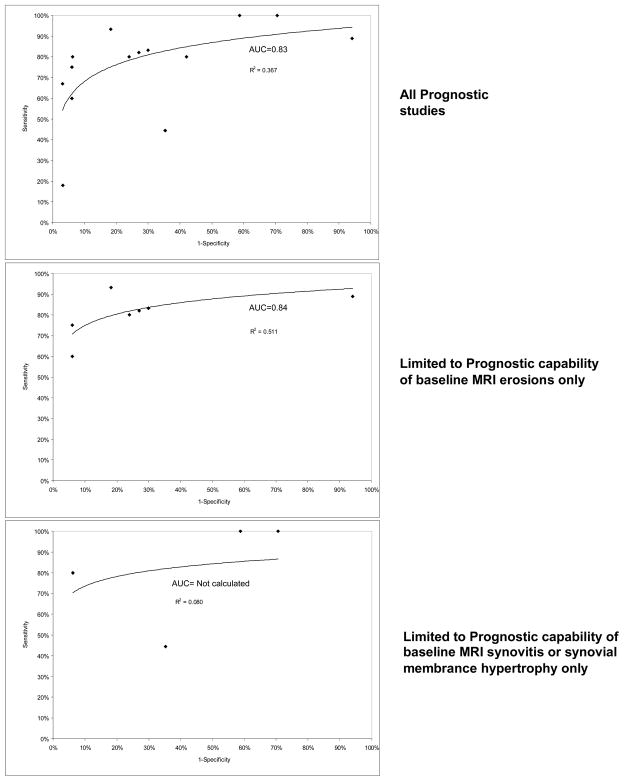
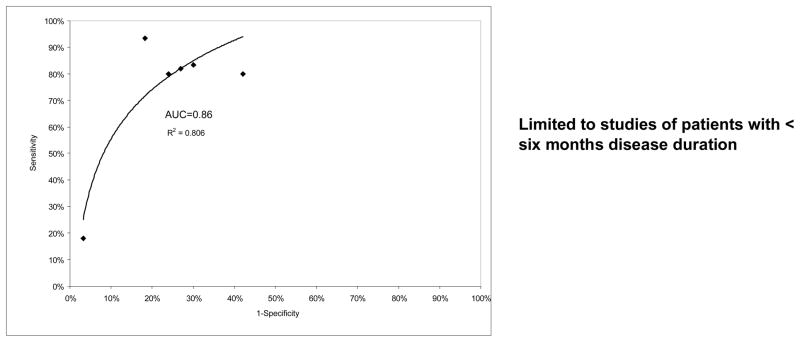
Similar to Diagnostic studies, given apparent heterogeneity in MRI prognostic criteria, we chose to stratify analyses, rather than pool diverse studies. The 1st first graph in Figure 3 shows the sensitivity and specificity from all applicable studies (9, 31, 39–41, 44) plotted in ROC space with a regression line and R2 value provided to demonstrate fit (AUC 0.83). No study in the highest size or quality (i.e., 75% or greater quality scores) quartile reported sensitivity and specificity data. The 2nd and 3rd graphs show data examining MRI erosions and measures of synovitis, respectively. The 4th graph shows data (9, 10, 36, 39–43, 46) on patients with disease duration < six months. There were insufficient data provided to pool odds ratios for studies that did not provide sensitivity and specificity. Limiting the analysis to studies examining only the presence of baseline MRI erosions or patients with disease duration < six months slightly improved overall MRI performance (AUCs for MRI erosions and < six months disease duration 0.84 and 0.86, respectively).
Figure 3.
Sensitivity and Specificity (to predict radiographic outcomes at 1 or more years) of included Prognostic studies in ROC space
Discussion
We performed a systematic review of published studies assessing the diagnostic and prognostic capability of MRI findings in undifferentiated inflammatory arthritis and early RA, respectively. To our knowledge, this is the first such systematic review. An exhaustive literature search found few published studies supporting the use of MRI for either of these roles. We found 11 studies addressing RA diagnosis and 17 evaluating prognosis, however small study size, variability in methodological quality and lack of uniform treatment limited our ability to make robust statements about the utility of MRI in clinical practice.
The sensitivity and specificity of early MRI findings for RA diagnosis ranged from 20–100% and 0–100%, respectively, depending upon MRI criteria used. Among diagnostic studies, excluding lower quality studies or studies of patients with longer symptom duration improved performance, while excluding small studies or examining individual MRI parameters, such as MRI erosions or synovitis, decreased MRI performance. No diagnostic study met 100% of our a priori methodological quality criteria. Among prognostic studies, the ability of MRI to predict progressive radiographic damage varied widely (range 18–100% for sensitivity and 5.9–97% for specificity). Only one high quality study examined the prognostic capability of MRI.
While data examining the utility of MRI in RA diagnosis and prognostication exist, there is no consensus on definitive MRI criteria for RA diagnosis. In addition, among prognostic studies, the only study to achieve a perfect rating for methodological quality (36) found MRI bone edema was the only significant predictor of radiographic progression. This study examined 130 early RA patients with disease duration under six months receiving standardized treatment, making it a compelling statement in favor of the capability of MRI bone edema to predict radiographic erosions. However, despite the short disease duration of the study population, 62% of participants had baseline plain radiographic erosions. As data suggest the prevalence of erosions in early RA ranges from 1% to 34% (32, 36, 39, 41, 45–47), this study may not be broadly generalizable. The utility of MRI to predict radiographic progression among individuals with no baseline radiographic erosions or to predict clinical outcomes such as remission remains undefined.
There are limitations to this analysis. We did not include unpublished data in this review. Patient-level meta-analysis of randomized clinical trial results where baseline MRI data were collected might improve our understanding of the role of MRI in predicting both response to therapy and likelihood of clinical as well as radiographic progression. In addition, due to the rapidly expanding nature of this field, this analysis provides a temporary assessment of currently available data and will hopefully soon be superseded by definitive studies of the optimal role for MRI in early RA management. However, due to the uptake of MRI into clinical practice without adequate evidence to guide clinical decision making, reviews such as this are important additions to the literature.
The use of MRI in musculoskeletal diseases is expanding rapidly. According to the Medicare Payment Advisory Commission (MedPAC), a nearly 50% increase in spending on imaging occurred between 2000 and 2003, most of which was due to increases in CT and MRI spending (48). Although national estimates of the utilization of MRI in rheumatoid arthritis are not available, office-based extremity MRI units are being directly marketed to rheumatologists for use in the management of early RA (49). Our findings suggest that, while data support the use of MRI for both the diagnosis and risk stratification of early RA, there are discordant results of which MRI findings are most accurate at diagnosing RA and/or predictive of subsequent joint damage. Available data are limited by inconsistent MRI scoring systems, small sample and effect sizes, short follow-up and lack of adjustment for disease severity and treatment.
Our findings suggest several approaches to improving the quality of literature in this field. Use of validated scoring systems, such as the OMERACT RAMRIS, and a uniform approach to combining radiographic and clinical information will significantly improve our understanding of the diagnostic role of MRI in undifferentiated inflammatory arthritis. In addition, future studies examining the utility of MRI in RA diagnosis should be concerned with study power. Larger studies with multi-year follow-up and adjustment for disease severity and treatment, as are now underway in the form of early RA randomized clinical trials, may provide valuable insights into the incremental prognostic capability of MRI over currently available prognostic markers. Data evaluating MRI for the diagnosis and prognosis of early RA are currently inadequate to justify widespread use of this technology for these purposes, although MRI bone edema may be predictive among patients with early, severe RA.
Footnotes
Disclosures: Dr. Suter is funded NIH K23 AR054095-01 Mentored Career Development and Arthritis Foundation Arthritis Investigator Awards. Dr. Fraenkel receives support from the NIH, Arthritis Foundation, American College of Rheumatology Research and Education Foundation, Donaghue Foundation, and the Department of Veterans Affairs. Dr. Braithwaite receives support from NIH, Robert Wood Johnson Foundation and Agency for Healthcare Research and Quality. There are no conflicts with this work and the funding sources had no role in study design or data interpretation. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Lisa G. Suter, Yale School of Medicine, New Haven, CT & VA Connecticut Healthcare System, West Haven, CT
Liana Fraenkel, Yale School of Medicine, New Haven, CT & VA Connecticut Healthcare System, West Haven, CT.
R. Scott Braithwaite, New York University School of Medicine, New York, NY.
References
- 1.Lawrence R, Helmick C, Arnett F, Deyo R, Felson D, Giannini E, Heyse S, Hirsch R, Hochberg M, Hunder G, Liang M, Pillemer S, Steen V, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis & Rheumatism. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Mottonen T, Hannonen P, Korpela M, Nissila M, Kautiainen H, Ilonen J, Laasonen L, Kaipiainen-Seppanen O, Franzen P, Helve T, Koski J, Gripenberg-Gahmberg M, Myllykangas-Luosujarvi R, Leirisalo-Repo M FIN-RACo Trial Group. FINnish Rheumatoid Arthritis Combination therapy. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis & Rheumatism. 2002;46(4):894–898. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 3.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. [comment] Arthritis & Rheumatism. 2003;48(7):1771–4. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- 4.Makinen H, Kautiainen H, Hannonen P, Mottonen T, Leirisalo-Repo M, Laasonen L, Korpela M, Blafield H, Hakola M, Sokka T. Sustained remission and reduced radiographic progression with combination disease modifying antirheumatic drugs in early rheumatoid arthritis. Journal of Rheumatology. 2007;34(2):316–21. [PubMed] [Google Scholar]
- 5.Sharp JT. Speculations on why early treatment of rheumatoid arthritis is uniquely effective. Journal of Rheumatology. 2008;35(11):2090–3. doi: 10.3899/jrheum.080671. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui MA. The efficacy and tolerability of newer biologics in rheumatoid arthritis: best current evidence. Current Opinion in Rheumatology. 2007;19(3):308–13. doi: 10.1097/01.bor.0000265447.48722.04. [DOI] [PubMed] [Google Scholar]
- 7.Ostendorf B, Scherer A, Modder U, Schneider M. Diagnostic value of magnetic resonance imaging of the forefeet in early rheumatoid arthritis when findings on imaging of the metacarpophalangeal joints of the hands remain normal. Arthritis & Rheumatism. 2004;50(7):2094–102. doi: 10.1002/art.20314. [DOI] [PubMed] [Google Scholar]
- 8.Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, Wakefield RJ, O’Connor PJ, Emery P. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis & Rheumatism. 2006;54(12):3761–73. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 9.McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, McLean L, Stewart N. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis & Rheumatism. 2003;48(7):1814–1827. doi: 10.1002/art.11162. [DOI] [PubMed] [Google Scholar]
- 10.Boyesen P, Haavardsholm EA, Ostergaard M, Kvien TK. MRI Synovitis Is Associated With Subsequent Joint Damage In Early Rheumatoid Arthritis Patients. American College of Rheumatology 2008 Annual Scientific Meeting, Abstract #1420; Boston, MA: American College of Rheumatology; [Google Scholar]
- 11.Higgins JPT, Green S, editors. Chichester, UK: John Wiley & Sons, Ltd; 2009. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- 12.Harrison BJ, Symmons DP, Barrett EM, Silman AJ. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis. American Rheumatism Association Journal of Rheumatology. 1998;25(12):2324–30. [PubMed] [Google Scholar]
- 13.Wolfe F, Ross K, Hawley DJ, Roberts FK, Cathey MA. The prognosis of rheumatoid arthritis and undifferentiated polyarthritis syndrome in the clinic: a study of 1141 patients. [see comment] Journal of Rheumatology. 1993;20(12):2005–9. [PubMed] [Google Scholar]
- 14.Zeidler H, Hulsemann JL. Benign polyarthritis and undifferentiated arthritis. An epidemiological terra incognita. Scandinavian Journal of Rheumatology. 1989;79(suppl 18):13–20. doi: 10.3109/03009748909092607. [DOI] [PubMed] [Google Scholar]
- 15.Mau W, Raspe HH, Mersjann H. Early arthritides: nosography, nosology, and diagnostic criteria. Scandinavian Journal of Rheumatology. 1989;79(suppl 18):3–12. doi: 10.3109/03009748909092606. [DOI] [PubMed] [Google Scholar]
- 16.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM Cochrane Diagnostic Test Accuracy Working G. Systematic reviews of diagnostic test accuracy. [see comment] Annals of Internal Medicine. 2008;149(12):889–97. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B, Bird P, Emery P, Genant H, Conaghan P. An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Annals of the Rheumatic Diseases. 2005;64(Supplement 1) doi: 10.1136/ard.2004.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, Shnier R, O’Connor P, Klarlund M, Emery P, Genant H, Lassere M, Edmonds J. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system.[erratum appears in J Rheumatol. 2004 Jan;31(1):198] Journal of Rheumatology. 2003;30(6):1385–6. [PubMed] [Google Scholar]
- 20.Klarlund M, Ostergaard M, Jensen KE, Madsen JL, Skjodt H, Lorenzen I. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. The TIRA Group. Annals of the Rheumatic Diseases. 2000;59(7):521–8. doi: 10.1136/ard.59.7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solau-Gervais E, Legrand JL, Cortet B, Duquesnoy B, Flipo RM. Magnetic resonance imaging of the hand for the diagnosis of rheumatoid arthritis in the absence of anti-cyclic citrullinated peptide antibodies: a prospective study. Journal of Rheumatology. 2006;33(9):1760–5. [PubMed] [Google Scholar]
- 22.Tamai M, Kawakami A, Uetani M, Takao S, Rashid H, Tanaka F, Fujikawa K, Aramaki T, Nakamura H, Iwanaga N, Izumi Y, Arima K, Aratake K, Kamachi M, Huang M, Origuchi T, Ida H, Aoyagi K, Eguchi K. Early prediction of rheumatoid arthritis by serological variables and magnetic resonance imaging of the wrists and finger joints: results from prospective clinical examination. Annals of the Rheumatic Diseases. 2006;65(1):134–5. doi: 10.1136/ard.2005.043075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narvaez J, Sirvent E, Narvaez JA, Bas J, Gomez-Vaquero C, Reina D, Nolla JM, Valverde J. Usefulness of magnetic resonance imaging of the hand versus anticyclic citrullinated peptide antibody testing to confirm the diagnosis of clinically suspected early rheumatoid arthritis in the absence of rheumatoid factor and radiographic erosions. Seminars in Arthritis & Rheumatism. 2008;38(2):101–9. doi: 10.1016/j.semarthrit.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Duer A, Ostergaard M, Horslev-Petersen K, Vallo J. Magnetic resonance imaging and bone scintigraphy in the differential diagnosis of unclassified arthritis. Annals of the Rheumatic Diseases. 2008;67(1):48–51. doi: 10.1136/ard.2006.063792. [DOI] [PubMed] [Google Scholar]
- 25.Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, Backhaus M, Hermann KG. Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatology. 2009;48(8):887–91. doi: 10.1093/rheumatology/kep136. [DOI] [PubMed] [Google Scholar]
- 26.Mori G, Tokunaga D, Takahashi KA, Hojo T, Fujiwara H, Arai Y, Taniguchi D, Takatori R, Imai K, Otakara E, Ito H, Nishimura T, Kubo T. Maximum intensity projection as a tool to diagnose early rheumatoid arthritis. Modern Rheumatology. 2008;18(3):247–51. doi: 10.1007/s10165-008-0043-2. [DOI] [PubMed] [Google Scholar]
- 27.Tamai M, Kawakami A, Uetani M, Takao S, Arima K, Iwamoto N, Fujikawa K, Aramaki T, Kawashiri SY, Ichinose K, Kamachi M, Nakamura H, Origuchi T, Ida H, Aoyagi K, Eguchi K. A prediction rule for disease outcome in patients with undifferentiated arthritis using magnetic resonance imaging of the wrists and finger joints and serologic autoantibodies. Arthritis & Rheumatism. 2009;61(6):772–8. doi: 10.1002/art.24711. [DOI] [PubMed] [Google Scholar]
- 28.Boutry N, Hachulla E, Flipo RM, Cortet B, Cotten A. MR imaging findings in hands in early rheumatoid arthritis: comparison with those in systemic lupus erythematosus and primary Sjogren syndrome. [see comment] Radiology. 2005;236(2):593–600. doi: 10.1148/radiol.2361040844. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto H, Takeda A, Masuyama J, Furuse M. Early-stage rheumatoid arthritis: diagnostic accuracy of MR imaging. Radiology. 1996;198(1):185–92. doi: 10.1148/radiology.198.1.8539375. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto H, Takeda A, Hyodoh K. Early-stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology. 2000;216(2):569–75. doi: 10.1148/radiology.216.2.r00au20569. [DOI] [PubMed] [Google Scholar]
- 31.Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, Lorenzen I. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis.[see comment] Arthritis & Rheumatism. 1999;42(5):918–929. doi: 10.1002/1529-0131(199905)42:5<918::AID-ANR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Conaghan PG, O’Connor P, McGonagle D, Astin P, Wakefield RJ, Gibbon WW, Quinn M, Karim Z, Green MJ, Proudman S, Isaacs J, Emery P. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis & Rheumatism. 2003;48(1):64–71. doi: 10.1002/art.10747. [DOI] [PubMed] [Google Scholar]
- 33.Quinn MA, Conaghan PG, O’Connor PJ, Karim Z, Greenstein A, Brown A, Brown C, Fraser A, Jarret S, Emery P. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis & Rheumatism. 2005;52(1):27–35. doi: 10.1002/art.20712. [DOI] [PubMed] [Google Scholar]
- 34.Jarrett SJ, Conaghan PG, Sloan VS, Papanastasiou P, Ortmann CE, O’Connor PJ, Grainger AJ, Emery P. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis & Rheumatism. 2006;54(5):1410–4. doi: 10.1002/art.21824. [DOI] [PubMed] [Google Scholar]
- 35.Durez P, Malghem J, Nzeusseu Toukap A, Depresseux G, Lauwerys BR, Westhovens R, Luyten FP, Corluy L, Houssiau FA, Verschueren P. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis & Rheumatism. 2007;56(12):3919–27. doi: 10.1002/art.23055. [DOI] [PubMed] [Google Scholar]
- 36.Hetland ML, Ejbjerg B, Horslev-Petersen K, Jacobsen S, Vestergaard A, Jurik AG, Stengaard-Pedersen K, Junker P, Lottenburger T, Hansen I, Andersen LS, Tarp U, Skjodt H, Pedersen JK, Majgaard O, Svendsen AJ, Ellingsen T, Lindegaard H, Christensen AF, Vallo J, Torfing T, Narvestad E, Thomsen HS, Ostergaard M group Cs. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA) Annals of the Rheumatic Diseases. 2009;68(3):384–90. doi: 10.1136/ard.2008.088245. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W, Newmark R Denosumab Rheumatoid Arthritis Study G. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis & Rheumatism. 2008;58(5):1299–309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Lee SK, Suh JS, Yoon M, Song JH, Lee CH. Magnetic resonance imaging of the wrist in defining remission of rheumatoid arthritis. Journal of Rheumatology. 1997;24(7):1303–8. [PubMed] [Google Scholar]
- 39.McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, McLean L. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Annals of the Rheumatic Diseases. 1999;58(3):156–163. doi: 10.1136/ard.58.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McQueen FM, Benton N, Crabbe J, Robinson E, Yeoman S, McLean L, Stewart N. What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using x rays and magnetic resonance imaging over the first two years of disease. Annals of the Rheumatic Diseases. 2001;60(9):859–868. [PMC free article] [PubMed] [Google Scholar]
- 41.Lindegaard HM, Vallo J, Horslev-Petersen K, Junker P, Ostergaard M. Low-cost, low-field dedicated extremity magnetic resonance imaging in early rheumatoid arthritis: a 1-year follow-up study. Annals of the Rheumatic Diseases. 2006;65(9):1208–12. doi: 10.1136/ard.2005.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haavardsholm EA, Boyesen P, Ostergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Annals of the Rheumatic Diseases. 2008;67(6):794–800. doi: 10.1136/ard.2007.071977. [DOI] [PubMed] [Google Scholar]
- 43.Syversen SW, Goll GL, Haavardsholm EA, Boyesen P, Lea T, Kvien TK. A high serum level of eotaxin (CCL 11) is associated with less radiographic progression in early rheumatoid arthritis patients. Arthritis Research & Therapy. 2008;10(2):R28. doi: 10.1186/ar2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mundwiler ML, Maranian P, Brown DH, Silverman JM, Wallace D, Khanna D, Louie J, Furst DE, Weisman MH. The utility of MRI in predicting radiographic erosions in the metatarsophalangeal joints of the rheumatoid foot: a prospective longitudinal cohort study. Arthritis Research & Therapy. 2009;11(3):R94. doi: 10.1186/ar2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammer HB, Haavardsholm EA, Boyesen P, Kvien TK. Bone erosions at the distal ulna detected by ultrasonography are associated with structural damage assessed by conventional radiography and MRI: a study of patients with recent onset rheumatoid arthritis. Rheumatology. 2009;48(12):1530–2. doi: 10.1093/rheumatology/kep283. [DOI] [PubMed] [Google Scholar]
- 46.Hoving JL, Buchbinder R, Hall S, Lawler G, Coombs P, McNealy S, Bird P, Connell D. A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis.[see comment] Journal of Rheumatology. 2004;31(4):663–75. [PubMed] [Google Scholar]
- 47.de Vries-Bouwstra J, Le Cessie S, Allaart C, Breedveld F, Huizinga T. Using predicted disease outcome to provide differentiated treatment of early rheumatoid arthritis. Journal of Rheumatology. 2006;33(9):1747–53. [PubMed] [Google Scholar]
- 48.Medicare Payment Advisory Commission. Private insurers’ strategies for purchasing imaging services. Public Meeting; 2004 March 18, 10:00 AM – 11:30 AM; Ronald Reagan Building: The International Trade Center; Mar 18, 2004. [Google Scholar]
- 49.Applause™: Affordable, Portable In-Office MRI. Madison, WI: GE Medical Systems; 2003. [Google Scholar]