Abstract
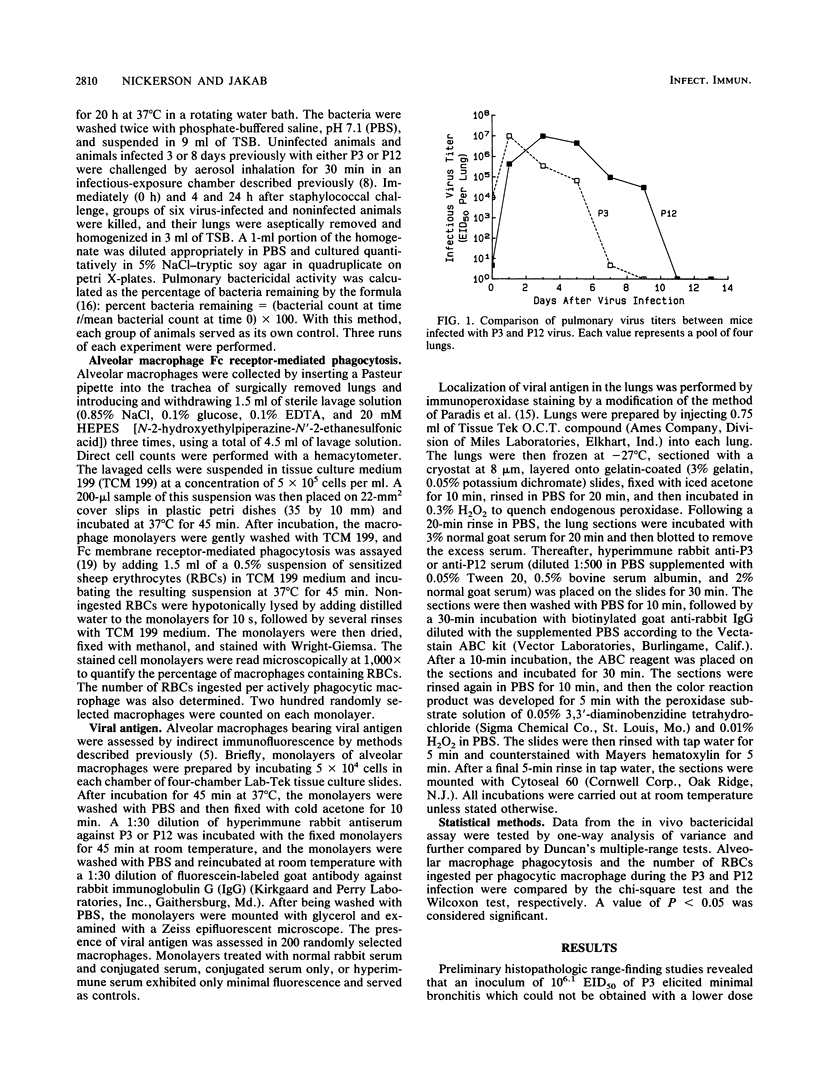
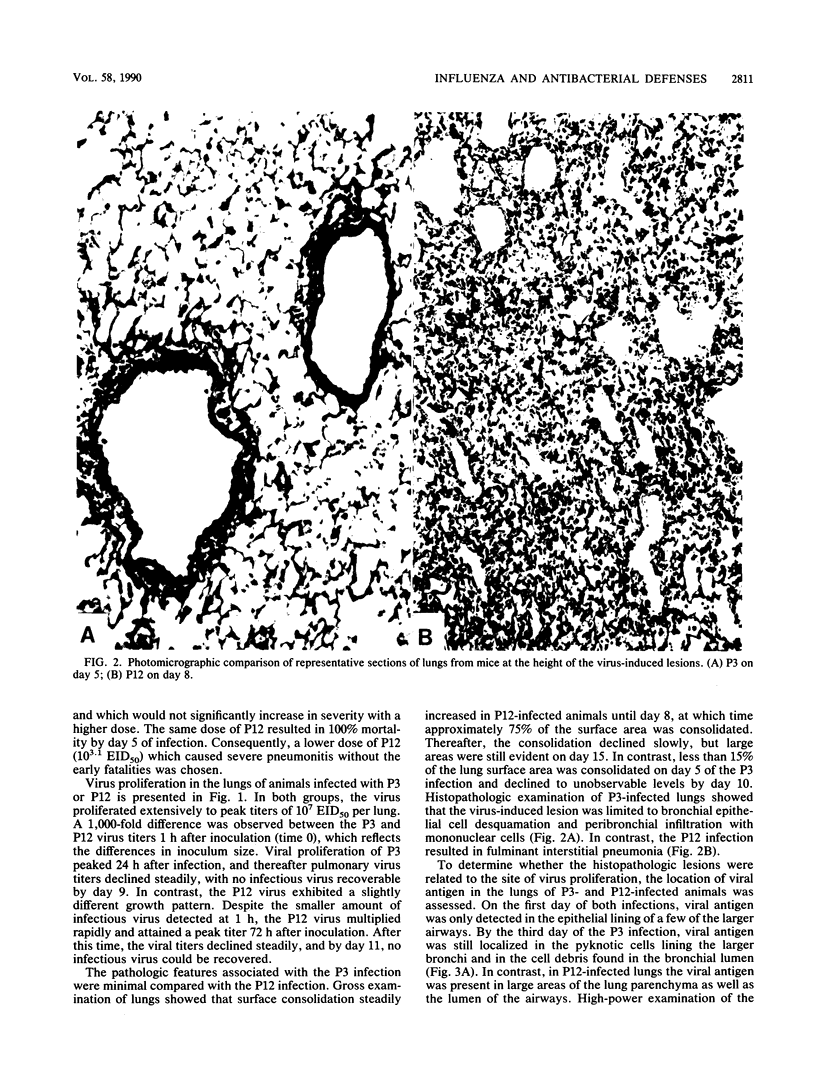
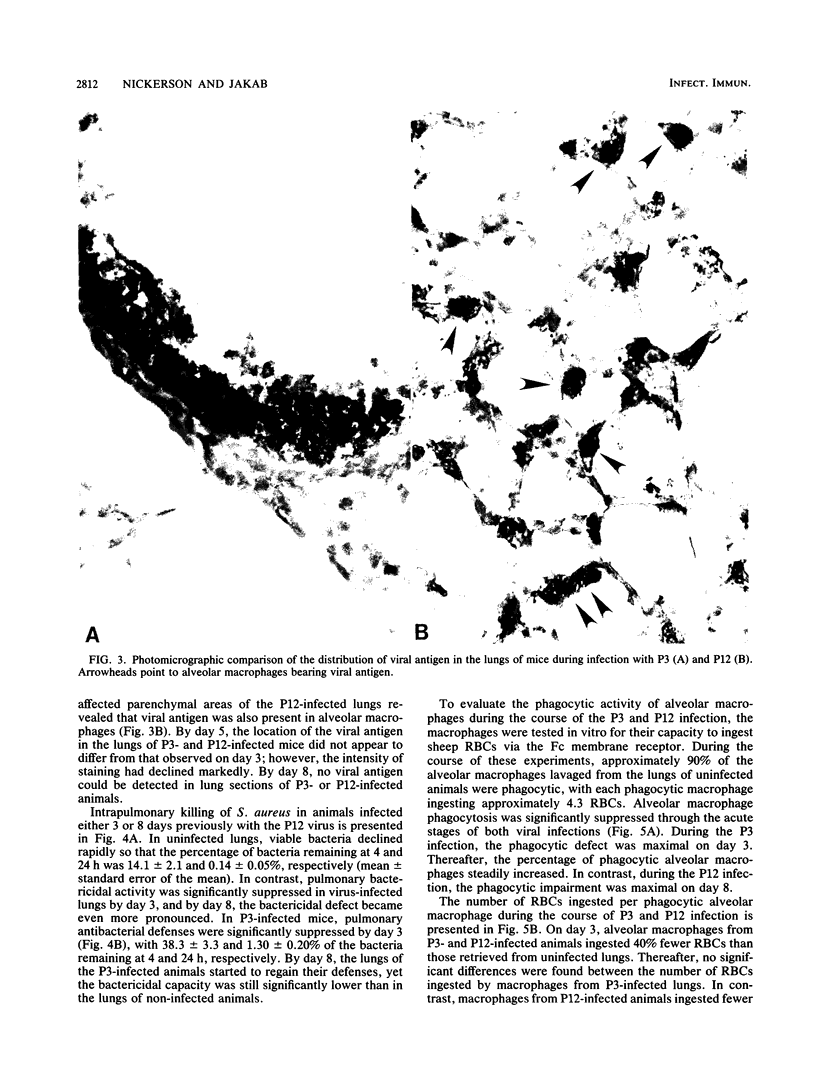
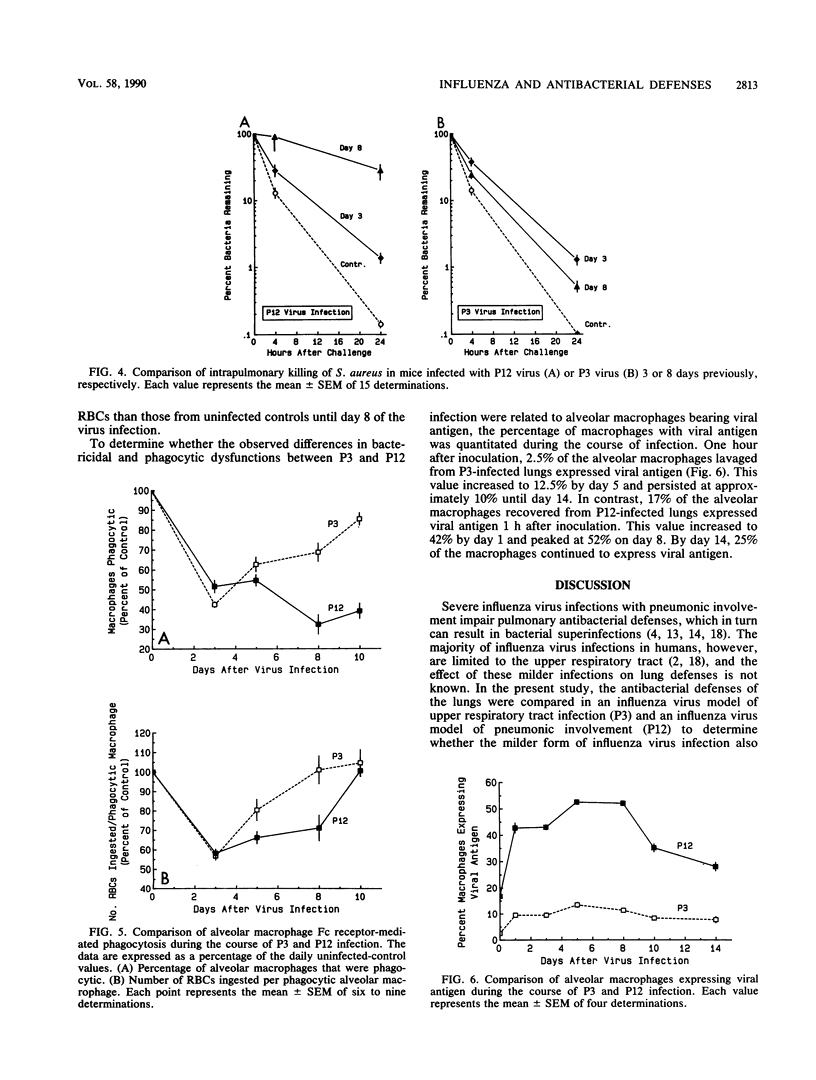
Severe influenza virus infections with pneumonic involvement are known to predispose the lungs to bacterial superinfections due to dysfunctions in the alveolar macrophage (AM) phagocytic system. To determine whether milder forms of influenza without pneumonic involvement have a similar outcome, pulmonary antibacterial defenses and AM phagocytosis were compared in murine models of mild and severe influenza virus A/HK/68 infections. Bactericidal activity was quantitated by the intrapulmonary killing of Staphylococcus aureus following aerosol challenge, whereas the functional capacity of the AMs was determined by Fc-receptor-mediated phagocytosis. With the severe virus infection, maximal suppression of bactericidal activity occurred on day 8 of infection and correlated with impairment of AM phagocytosis. A lesser but significant degree of suppression of pulmonary antibacterial defenses and AM phagocytosis was observed on the third day of the mild virus infection. The data demonstrate that mild influenza virus infections that are limited to the upper respiratory tract also impair pulmonary antibacterial defenses and may predispose the lungs to bacterial superinfections.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couch R. B. The effects of influenza on host defenses. J Infect Dis. 1981 Sep;144(3):284–291. doi: 10.1093/infdis/144.3.284. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Astry C. L., Warr G. A. Alveolitis induced by influenza virus. Am Rev Respir Dis. 1983 Oct;128(4):730–739. doi: 10.1164/arrd.1983.128.4.730. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. The effect of Sendai virus infection on bactericidal and transport mechanisms of the murine lung. J Clin Invest. 1972 Aug;51(8):1989–1998. doi: 10.1172/JCI107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J. Immune impairment of alveolar macrophage phagocytosis during influenza virus pneumonia. Am Rev Respir Dis. 1982 Nov;126(5):778–782. doi: 10.1164/arrd.1982.126.5.778. [DOI] [PubMed] [Google Scholar]
- Jakab G. J. Mechanisms of bacterial superinfections in viral pneumonias. Schweiz Med Wochenschr. 1985 Jan 19;115(3):75–86. [PubMed] [Google Scholar]
- Jakab G. J. Mechanisms of virus-induced bacterial superinfections of the lung. Clin Chest Med. 1981 Jan;2(1):59–66. [PubMed] [Google Scholar]
- Jakab G. J. Suppression of pulmonary antibacterial activity following Sendai virus infection in mice: dependence on virus dose. Arch Virol. 1975;48(4):385–390. doi: 10.1007/BF01317437. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A. Immune-enhanced phagocytic dysfunction in pulmonary macrophages infected with parainfluenza 1 (Sendai) virus. Am Rev Respir Dis. 1981 Nov;124(5):575–581. doi: 10.1164/arrd.1981.124.5.575. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A. The participation of antiviral immune mechanisms in alveolar macrophage dysfunction during viral pneumonia. Bull Eur Physiopathol Respir. 1983 Mar-Apr;19(2):173–178. [PubMed] [Google Scholar]
- Kavet R. I., Brain J. D. Methods to quantify endocytosis: a review. J Reticuloendothel Soc. 1980 Feb;27(2):201–221. [PubMed] [Google Scholar]
- LOURIA D. B., BLUMENFELD H. L., ELLIS J. T., KILBOURNE E. D., ROGERS D. E. Studies on influenza in the pandemic of 1957-1958. II. Pulmonary complications of influenza. J Clin Invest. 1959 Jan;38(1 Pt 2):213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli C. G. Influenza and the interaction of viruses and bacteria in respiratory infections. Medicine (Baltimore) 1973 Sep;52(5):369–384. doi: 10.1097/00005792-197309000-00001. [DOI] [PubMed] [Google Scholar]
- Mackowiak P. A. Microbial synergism in human infections (second of two parts). N Engl J Med. 1978 Jan 12;298(2):83–87. doi: 10.1056/NEJM197801122980206. [DOI] [PubMed] [Google Scholar]
- Paradis I. L., Merrall E. J., Krell J. M., Dauber J. H., Rogers R. M., Rabin B. S. Lymphocyte enumeration: a comparison between a modified avidin-biotin-immunoperoxidase system and flow cytometry. J Histochem Cytochem. 1984 Apr;32(4):358–362. doi: 10.1177/32.4.6368677. [DOI] [PubMed] [Google Scholar]
- Ruppert D., Jakab G. J., Sylwester D. L., Green G. M. Sources of variance in the measurement of intrapulmonary killing of bacteria. J Lab Clin Med. 1976 Mar;87(3):544–558. [PubMed] [Google Scholar]
- Sawyer W. D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- Warr G. A., Jakab G. J., Hearst J. E. Alterations in lung macrophage immune receptor(s) activity associated with viral pneumonia. J Reticuloendothel Soc. 1979 Oct;26(4):357–366. [PubMed] [Google Scholar]
- Warshauer D., Goldstein E., Akers T., Lippert W., Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):269–277. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- Wyde P. R., Couch R. B., Mackler B. F., Cate T. R., Levy B. M. Effects of low- and high-passage influenza virus infection in normal and nude mice. Infect Immun. 1977 Jan;15(1):221–229. doi: 10.1128/iai.15.1.221-229.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]