Abstract
Congenital heart block (CHB) is a passively acquired autoimmune disease due to the transfer of maternal autoantibodies anti-SSA/Ro –SSB/La to the fetus resulting in atrioventricular (AV) block and sinus bradycardia. We previously established a murine model for CHB where pups born to immunized wild type (WT) mothers exhibited electrocardiographic abnormalities similar to those seen in CHB and demonstrated inhibition of L-type Ca channels (LTCC) by maternal antibodies. Here, we hypothesize that overexpression of LTCC should rescue, whereas knockout of LTCC should worsen the electrocardiographic abnormalities in mice.
Methods and Results
Transgenic (TG) mice were immunized with SSA/Ro and SSB/La antigens. Pups born to immunized WT mothers had significantly greater sinus bradycardia and AV block compared to pups from nonimmunized WT. TG pups overexpressing LTCC had significantly less sinus bradycardia and AV block compared to their non-TG littermates and to pups born to immunized WT mothers. All LTCC knockout pups born to immunized mothers had sinus bradycardia, advanced degree of AV block and decreased fetal parity. No sinus bradycardia or AV block were manifested in pups from control nonimmunized WT mothers. IgG from mothers with CHB children, but not normal IgG, completely inhibited intracellular Ca transient ([Ca]iT) amplitude.
Conclusions
Cardiac specific overexpression of LTCC significantly reduced the incidence of AV block and sinus bradycardia in pups exposed to anti-SSA/Ro -SSB/La autoantibodies, whereas exposure of LTCC knockout pups to these autoantibodies significantly worsened the electrocardiographic abnormalities. These findings support the hypothesis that maternal antibodies inhibit LTCC and [Ca]iT thus contributing to the development of CHB. Altogether, the results are relevant to the development of novel therapies for CHB.
Keywords: congenital heart block, calcium channels, systemic lupus erythematosis, autoantibodies, genetics
Introduction
Congenital heart block (CHB) detected at or before birth in a structurally normal heart is associated with autoantibodies against the intracellular ribonucleoproteins SSA/Ro 52 kDa, SSA/Ro 60 kDa, and SSB/La 48 kDa 1. The autoantibodies gain access to the fetal circulation and cause injury to different organs such as the heart, liver, and skin 2. CHB is usually detected between 16 to 24 weeks of gestation, with varying degrees of atrioventricular (AV) block 3, 4 and sinus bradycardia5, 6. Complete AV block is irreversible resulting in 30% mortality, with most of the infants requiring lifelong pacemakers 7. The autoantigens SSA/Ro and SSB/La are intracellular and are inaccessible to the circulating autoantibodies in a normal cardiomyocytes suggesting that cross reactivity with a protein (s) on the sarcolemma 8–11 such as the muscarinic receptor 12, laminin 8, the serotonin receptor, 5-HT4 9, and the L-type Ca channels (LTCC) 11, 13.
However, access by the circulating antibodies to the intracellular antigens requires a trigger to translocate the antigens to the cell surface. These include viral infection 14, ultraviolet light exposure 15 and apoptosis, 16.
Two subtypes of cardiac LTCC are encoded by Cav1.2 and Cav1.3 pore-forming subunits 17, 18. Cav1.2 is ubiquitously expressed in the heart and mediates excitation-contraction coupling. Cav1.3 is highly expressed in the sinoatrial (SAN) and AV node (AVN) 19, 20. Cav1.2 Ca current (ICa-L) activates at −40 to −30 mV and accounts for the electrogenesis of the action potential at the AVN, whereas Cav1.3 ICa-L activates between −60 to −40 mV, at a range corresponding to the diastolic depolarization in the SAN 21. Interestingly, the Cav1.3 knockout mice exhibit sinus bradycardia and AV block; a phenotype similar to that seen in CHB 19, 20, 22, 23. A pathological interaction between LTCC and anti-SSA/Ro and -SSB/La autoantibodies is well documented 10, 11, 24–27. IgG from mothers with CHB children inhibited ICa-L and this inhibition could account for the AV block and sinus bradycardia seen in CHB 11, 24–27. Impulse generation in the SAN and conduction through the AVN critically depends on ICa-L 10, 19, 20.
A murine model for CHB has been established by the immunization of WT female mice with recombinant SSA/Ro or SSB/La proteins 13, 28. Electrocardiographic abnormalities in the pups included sinus bradycardia and various degrees of AV block (I°, II°, and III°, with the latter being rare) 13, 28. In this study, two transgenic (TG) mouse models were utilized: Knockout of Cav1.3 LTCC 23 and cardiac specific overexpression of Cav1.2 LTCC 29. We hypothesized that the increase in the Cav1.2 ICa-L density should minimize the inhibition caused by the autoantibodies and subsequently reduce or prevent the manifestation of the electrocardiographic abnormalities seen in the pups of immunized mice. Although the knockout of Cav1.3 per se results in sinus bradycardia and AV block, not all pups are affected 19, 20, 23, 30. We therefore hypothesized that because Cav1.3 knockout mice already exhibit cardiac phenotypes of CHB, immunization should worsen the electrocardiographic abnormalities in the pups.
Methods
Ethical Approval
Animal Protocol: All experiments were performed in accordance with the IACUC at the VA New York Harbor Healthcare System and conform to the NIH guidelines (NIH Publication 86-23).
IgGs and Preparation of recombinant antigens
Recombinant full length human SSA/Ro52, SSA/Ro60, and SSB/La48 were prepared as previously described 28. IgGs were kindly provided by Dr. Jill Buyon (NYU School of Medicine, NY) from the U.S. Research Registry for Neonatal Lupus established by the National Institute for Arthritis, Musculoskeletal and Skin Diseases.
Immunization
FVB and C57BL/6 mice were selected because of the established FVB mice that overexpress Cav1.2 LTCC 29, and C57BL/6 mice because of the established C57BL/6 Cav1.3 LTCC knockout mice 23. Unfortunately, Cav1.2 knockout mice are embryonic lethal and Cav1.3 overexpression mice are not available; therefore, they were not used in this study. The mice overexpressing Cav1.2 LTCC were kindly provided by Dr. A. Schwartz (University of Cincinnati, Cincinnati, Ohio) and the Cav1.3 knockout mice were kindly provided by Dr. R. Flavell (Yale University, New Haven, CT) with the concurrence of Dr. J. Striessnig (Innsbruck, Austria). Mature 6–8 week old female mice were immunized with 50 µg of each of the recombinant proteins in Complete Freud’s Adjuvant (CFA) intraperitoneally, and 25 µg of the same preparation subcutaneously (s.c.) in the scapular region. Subsequent booster injections in Incomplete Freud’s Adjuvant (IFA) were continued every 3 weeks. The mice were then mated after an immune response was established.
Groups of mice (n=6) and their genotype
| GROUPS (Mothers) | GENOTYPE (Pups) |
|---|---|
| Control Wild Type Nonimmunized (FVB) | WT |
| Wild Type Immunized (FVB) | WT |
| Cav1.2 Overexpression Nonimmunized (FVB) | TG, NTG |
| Cav1.2 Overexpression Immunized (FVB) | TG, NTG |
| Cav1.3 KO Nonimmunized (C57BL/6) | KO, Heterozygous, NTG |
| Cav1.3 KO Immunized (C57BL/6) | KO, Heterozygous, NTG |
WT: Wild Type; TG: Transgenic; NTG: Non-Transgenic; KO: Knockout.
Blood Collection and Genotyping
Blood was obtained by orbital bleeding in adult mice and by cardiocentesis in pups (1–3 days old). The serum was isolated by centrifugation for 10 minutes at 8000 rpm and ELISA was used to detect antibody levels. Genotyping of TG mice was performed after an electrocardiogram (ECG) was recorded. For the Cav1.2 genotyping, the primers used were:
sense 5’- GACTGTGGAGATGGTCGCATTG-3’;
antisense 5’- CACTCTTAGCAAACCTCAGGC-3’.
For the Cav1.3 genotyping, primers to detect WT, heterozygous:
Sense 5’- GCAAACTATGCAAGAGGCACC-3’;
antisense 5’- GGGAGAGAGAGATCCTACAGGTG -3’.
For the heterozygous and knockout, the primers were:
Sense 5’- CATTCCACTATACTAATGCAGG-3’;
antisense 5’- TTCCATTTGTCACGTCCTGCACCA-3’
Enzyme-Linked Immunosorbent Assay (ELISA) for the detection of antibody levels in adults and pups
The recombinant proteins were coated overnight, washed in PBST, blocked with BSA/PBST and probed in diluted serum followed by anti-mouse IgG alkaline phosphatase. The plates were developed with disodium p-nitrophenyl phosphate. All samples were run in triplicates. Results were expressed as the optical density (O.D.) at 405 nm minus that of the reagent blank. An O.D. value was considered positive if it was >2SD above the value obtained for controls.
Electrocardiographic recordings
After delivery, ECGs were recorded 28, 31. Pups were placed on a heated pad to maintain body temperature (36° – 37°C). Lead I was obtained using Acqkowledge software (Biopac MP100 system, CA). Recordings were analyzed for heart rate, PR interval, and conduction abnormalities (including I°, II°, and III° degree AV block). The AV block percentage was counted as 100% only when all pups had at least I°, II°, or III° degree AV block. Bradycardia was defined as 40% slower heart rate than control.
Intracellular Ca measurements
Adult ventricular myocytes were obtained from age- and sex-matched mice (C57/B6). Hearts were enzymatically dissociated and myocytes isolated as previously described 30. Extracellular Ca was sequentially increased, and the final solution consisted of (in mM): 137 NaCl, 5.4 KCl, 1.0 CaCl2, 1 MgCl, 0.33 NaH2PO4, 10 HEPES and 10 glucose (pH 7.4). Myocytes were incubated in a 5 µM solution of fura-2 acetoxymethylester ester (Molecular Probes) for 30 minutes at room temperature, followed by resuspension in fura-2-free solution for additional 30 minutes. Cells were subsequently plated in a perfusion chamber placed on the stage of an inverted microscope continually superfused with Tyrode solution and field-stimulated (0.5 Hz, 3-ms duration at 37°C) through two electrodes immersed in the bath of the superfusion chamber. Cells were then illuminated at 340 and 380nm. Fluorescence emission was monitored at 510 nm, was captured with a photomultiplier (DeltaRam; PTI, South Brunswick, NJ) and converted to intracellular Ca transient ([Ca]iT). Analysis of [Ca]iT was performed offline using Felix3.4 and Origin7.0 software.
Data Analysis
Statistical comparisons were evaluated using either unpaired Student's t-test or ANOVA. Data are presented as means ± SE. A P < 0.05 value is considered significant.
Results
Establishment of autoantibody responses
The active model of CHB was established by immunizing female WT FVB, C57BL/6, and the two TG lines (Cav1.2 and Cav1.3) with SSA/Ro52, SSA/Ro60, and SSB/La48 antigens. To ensure that the antibodies were generated in the mothers and crossed the placenta to the pups, mothers were bled and the sera obtained were tested separately for reactivity to each of the recombinant antigens by ELISA.
Both the immunized mothers and the pups had high levels of antibodies reactive with SSA/Ro52, SSA/Ro60, and SSB/La48 confirming the generation and transfer of the antibodies from the mother to the pups. All immunized groups (mothers and pups) had high titers of antibodies (Supplementary Table 1). The antibody titer within 10 days following the primary injection was high (0.85–0.98) and remained high (1.15–1.175) with continuous booster throughout the experimental period.
Electrocardiographic parameters of pups from the immunized WT mice
In this study, we immunized WT mice to serve as a control for the two TG mice to detect improvement or worsening of conduction abnormalities. Table 1 and supplementary table 2 summarize the ECG parameters in the WT groups. Pups from the WT nonimmunized FVB (n=48), had no electrocardiographic abnormalities (neither sinus bradycardia nor AV block). Pups from the WT immunized mothers, had 19.7% sinus bradycardia and 25 % AV block (10 out of 76 had I° AV block, 6 had II° AV block, and 3 had III° AV block).
Table 1.
Electrocardiographic parameters of pups from the nonimmunized and immunized Cav1.2 L-type Ca channel overexpression mice
| Immunized Mothers (n) |
Pregnancy (n) |
Deliveries (n) |
Total Pups (n) |
Genotype (n) |
Bradycardia n(%) |
Abnormality | AVB (%) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| I°AVB (n) |
2°AVB (n) |
3°AVB (n) |
||||||||
| WT Nonimmunized FVB | 5 | 8 | 8 | 48 | WT (n=48) | 0(0%) | 0 | 0 | 0 | 0 |
| WT Immunized FVB | 8 | 12 | 12 | 76 | 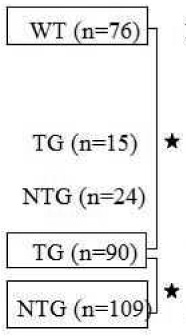 |
15(19.7%) | 10 | 6 | 3 | 25 |
| Cav1.2 Overexpression | 5 | 7 | 7 | 39 | 0(0%) | 0 | 0 | 0 | 0 | |
| Nonimmunized | 0(0%) | 0 | 0 | 0 | 0 | |||||
| Cav1.2 Overexpression | 30 | 36 | 36 | 199 | 9(10%) | 7 | 1 | 0 | 8.8 | |
| Immunized | 24(22%) | 24 | 4 | 2 | 27.5 | |||||
n, number of animals; WT, wild type; TG, transgenic; NTG, nontransgenic. The AV block percentage was counted as 100% only when all pups had at least I°, II°, III° degree AV block. Connection bar compares sinus bradycardia and AV block between TG pups from the immunized Cav1.2 overexpression mothers with the NTG littermates and WT immunized.
Denotes statistical significance p<0.05.
Electrocardiographic parameters of pups from the immunized mice overexpressing Cav1.2 L-type Ca channel
The Cav1.2 overexpression TG pups had significantly fewer abnormalities [10% (p<0.05) sinus bradycardia and 8.8% (p<0.05) AV block] compared to the NTG littermates and pups from immunized WT mothers (Table 1). Of these, 7 had I° AV block, 1 had II° AV block, and 0 had III° AV block. Of the 109 NTG pups, 22% had sinus bradycardia and 27.5% had AV block with 24 pups exhibiting I° AV block, 4 pups II° AV block, and 2 pups III° AV block. Figure 1 (and supplementary table 2) summarizes the heart rates and PR intervals. TG pups from the immunized Cav1.2 overexpression mothers showed significantly fewer abnormalities than pups from the immunized WT mothers. The average heart rate and PR interval of TG pups from the immunized Cav1.2 overexpression was 315±36 bpm (n=90) and 48±10 ms (n=90), respectively. The average heart rate and PR interval of WT pups from the immunized WT mothers was 282±32 bpm (n=76) and 64±14 ms (n=76), respectively. Interestingly, NTG littermate pups (n=109) born to the same immunized Cav1.2 overexpression mother were not protected and showed sinus bradycardia (268±21 bpm) and prolonged PR interval (71±12 ms).
Figure 1. Heart rate and PR interval in WT FVB and Cav1.2 overexpression groups.
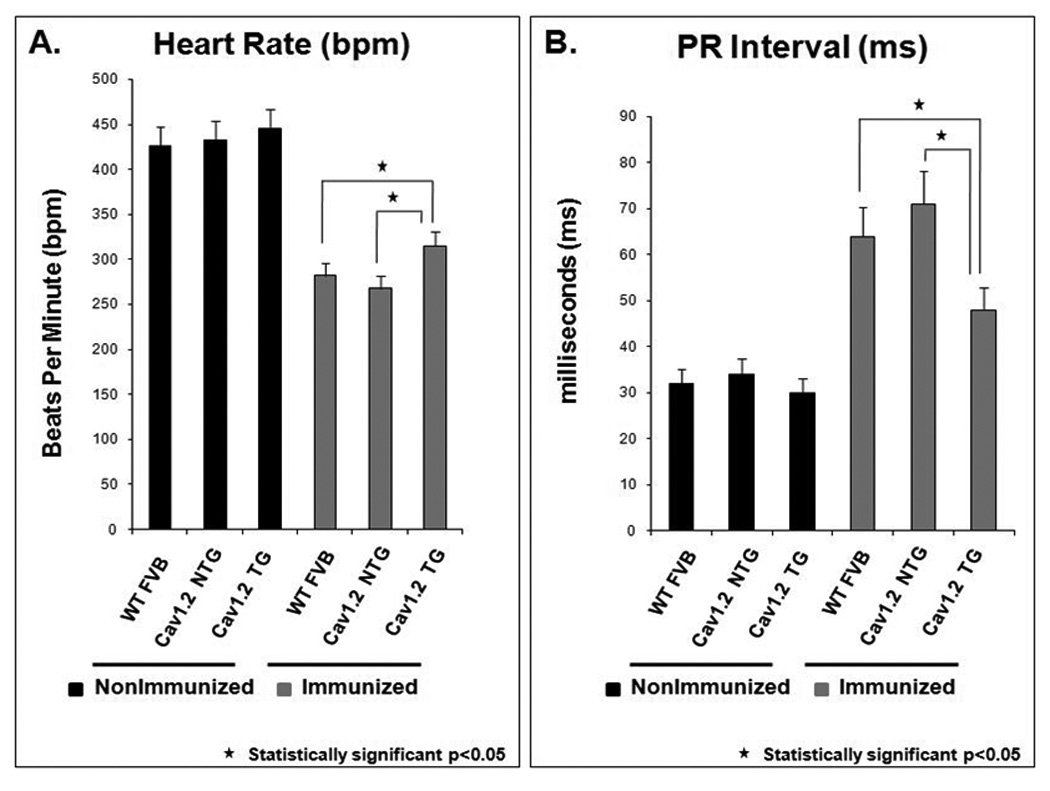
Panel A shows the heart rate, beats per minute (bpm) in the wild type (WT) FVB and Cav1.2 transgenic mice (TG). The average heart rate of pups from the immunized transgenic (TG) Cav1.2 overexpression was 315±36 bpm (n=90) compared to immunized WT FVB with an average heart rate of 282±32 bpm (n=76). Non-Transgenic (NTG) littermate pups born to the same immunized Cav1.2 overexpression showed sinus bradycardia (268±21 bpm, n=109). Pups from the nonimmunized WT FVB and the nonimmunized Cav1.2 overexpression had similar heart rates (426±41 bpm, n=48 and 445±21 bpm, n=15 respectively). Panel B represents the PR interval in the WT FVB and Cav1.2 TG. The average PR interval in the pups from the nonimmunized WT FVB and the nonimmunized Cav1.2 overexpression was similar 32±8 ms (n=48) and 30±7 ms (n=15), respectively. The pups from the immunized WT FVB had a prolonged mean PR interval of 64±14 ms (n=76). The NTG immunized littermates in the Cav1.2 overexpression were similarly not protected and showed an increase in PR interval to 71±12 ms (n=109). The average PR interval of pups from the immunized TG Cav1.2 overexpression improved to 48±10 ms (n=90, p<0.05 compared to the immunized WT FVB and immunized NTG littermates).
Representative control ECG from a pup born to a nonimmunized WT mother with a normal sinus rate of 454 bpm and a PR interval of 46 ms is shown in Figure 2, panel A. Upon immunization of WT mothers, pups developed II° AV block (upper trace of panel B) and III° AV block with complete AV dissociation (lower trace of panel B). TG pups that had an overexpression of the Cav1.2 Ca channel showed fewer electrocardiographic abnormalities: normal ECG (upper trace of panel C) and sinus bradycardia (296 bpm) with I° AV block (PR interval of 70 ms, lower trace of panel C).
Figure 2. ECG traces from the FVB immunized and nonimmunized groups within 1 to 3 days of birth.
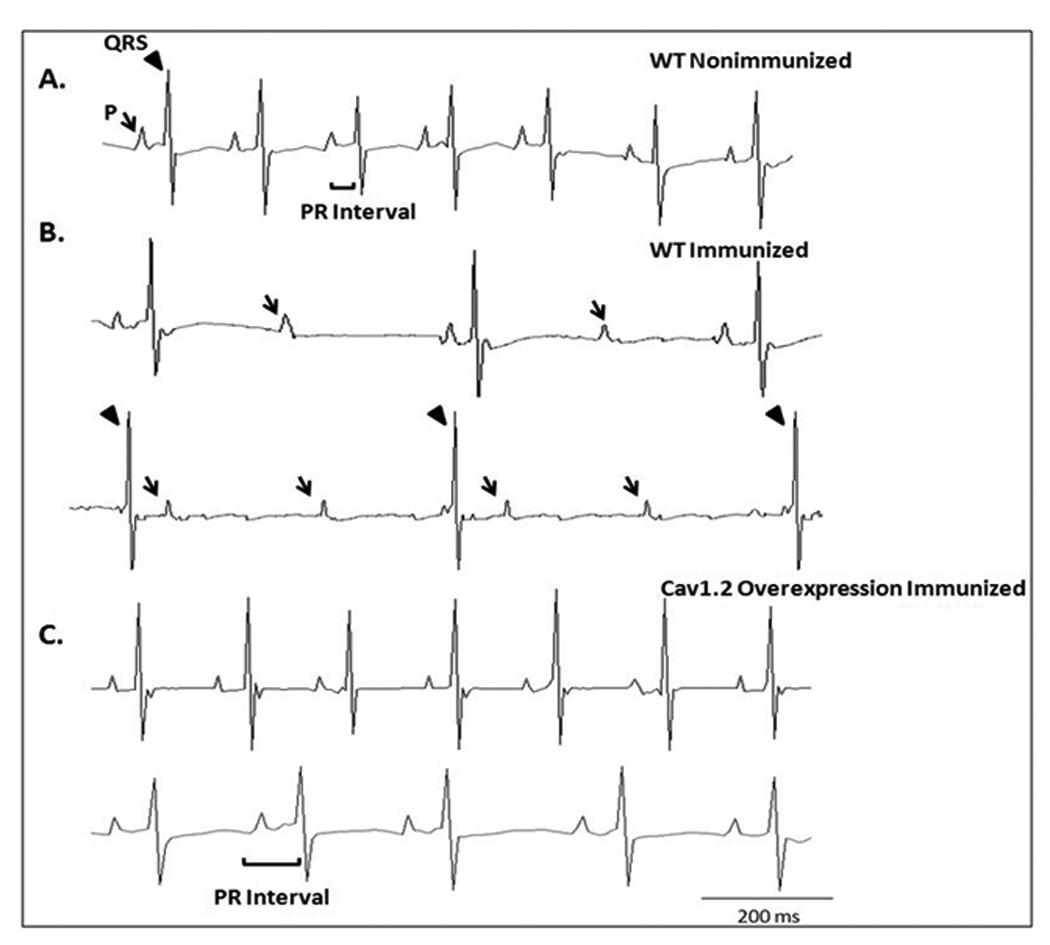
Panel A represents a control ECG from a pup born to a nonimmunized WT mother with a normal sinus rhythm of 454 and a PR interval of 42 ms. The ECG showed a normal PR interval followed by a QRS complex. Panel B shows increased electrocardiographic abnormalities in the pups as the result of immunization of WT mothers [II° AV block (upper trace of panel B) and III° AV block with complete AV dissociation (lower trace of panel B)]. Panel C shows the fewer electrocardiographic abnormalities in the TG pups from the immunized mice overexpressing Cav1.2 L-type Ca channel as illustrated by a normal sinus rhythm, 438 bpm and normal PR interval, 44 ms (upper trace of panel C), and sinus bradycardia (296 bpm) with I° AV block (PR interval 70 ms). The arrow indicates the P wave and the arrowhead indicates the QRS complex.
Electrocardiographic parameters of pups from the immunized Cav1.3 L-type Ca channel knockout mice
Table 2 summarizes the data obtained from the Cav1.3 knockout mice (C57BL/6). No electrocardiographic abnormalities were found in NTG pups (n=20) from the nonimmunized Cav1.3 knockout mothers. Heterozygous pups (n=28) from the nonimmunized Cav1.3 knockout mothers had 3.6% bradycardia and 3.6% AV block (one I° AV block). However, the knockout pups (n=9) from the nonimmunized Cav1.3 knockout mothers had 100% sinus bradycardia and 100% AV block (8 I°AV block, 1 II° AV block, but 0 III° AV block).
Table 2.
Electrocardiographic parameters of the pups from nonimmunized and immunized Cav1.3 L-type Ca channel knockout mice
| Immunized Mothers (n) |
Pregnancy (n) |
Deliveries (n) |
Total Pups(n) |
Genotype (n) |
Bradycardia n (%) |
Abnormality | AVB (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| I°AVB(n) | 2°AVB(n) | 3°AVB(n) | ||||||||
| Cav1.3 KO c57BL/6 | 8 | 13 | 12 | 57 | 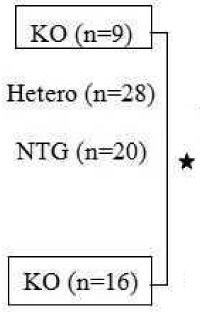 |
9(100) | 8 | 1 | 0 | 100 |
| Nonimmunized | 1(3.6) | 1 | 0 | 0 | 3.6 | |||||
| 0(0) | 0 | 0 | 0 | 0 | ||||||
| Cav1.3 KO c57BL/6 | 29 | 39 | 31 | 101 | 16(100) | 9 |  |
100 | ||
| Immunized | Hetero(n=52) | 18(34.6) | 16 | 5 | 1 | 42.3 | ||||
| NTG(n=33) | 6(18) | 5 | 1 | 0 | 18 | |||||
n, number of animals; WT, wild type; KO, knockout. The AV block percentage was counted as 100% only when all pups had at least I°, II°, III° degree AV block. Highlighted Rectangle denotes advanced degrees of AV block Connection bar compares sinus bradycardia and AV block between KO pups from the nonimmunized and immunized Cav1.3 mothers.
Denotes statistical significance p<0.05.
Sinus bradycardia occurred at a rate of 18.2% in NTG pups, 34.6% in heterozygous pups, and 100% in knockout pups. Eighteen percent of NTG pups had AV block that included 5 I° AV block, 1 II° AV block, and 0 III° AV block. The heterozygous pups had 42.3% AV block, which included 16 I° AV block, 5 II° AV block, and 1 III° AV block. The knockout pups on the other hand had 100% AV block with 9 I° AV block, 4 II° AV block, and 3 III° AV block. Overall, pups from the immunized Cav1.3 knockout mothers had the most severe degrees of AV block, the highest of all groups with 3 out of 16 pups (19%) presenting III° AV block.
The Cav1.3 knockout pups from the immunized Cav1.3 knockout mothers had severe sinus bradycardia (232±30 bpm; n=16), and prolonged PR intervals (112±21 ms, n=16) compared to the Cav1.3 knockout pups from the nonimmunized Cav1.3 knockout mothers (sinus bradycardia was 265±33 bpm and PR-intervals were 93±15 ms, n=9) (Figure 3 and supplementary table 2).
Figure 3. Heart rate and PR interval in Cav1.3 knockout groups.
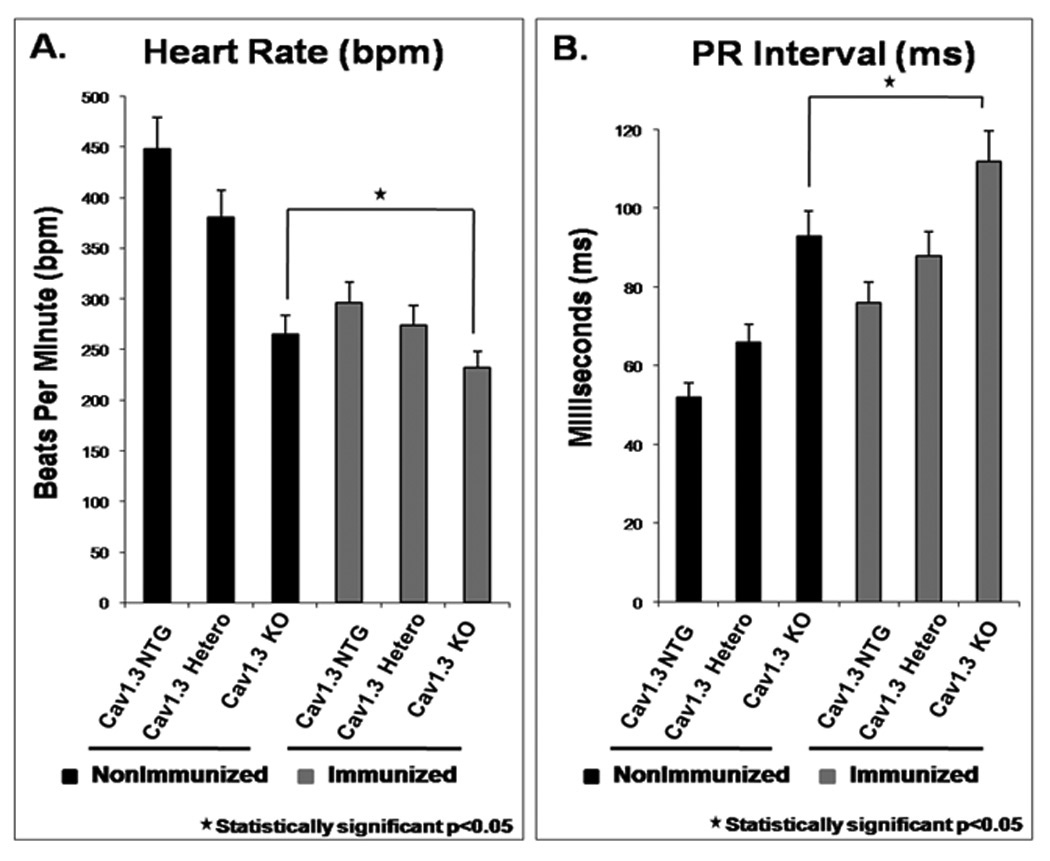
Panel A: The Cav1.3 knockout pups had significantly slower average heart rates 265±33 bpm (n=9) vs. the non-transgenic (NTG) littermates 448±56 bpm (n=20). The KO pups from the immunized Cav1.3 knockout mothers had severe sinus bradycardia with an average heart rate of 232±30 bpm (n=16). Panel B: PR interval (ms) in the Cav1.3 knockout pups. The NTG pups had a PR interval of 52±6 ms and the Cav1.3 knockout pups from nonimmunized mothers had a PR interval of 93±15 (n=9). The pups from the heterozygous immunized mothers had a mean PR interval of 88±16 ms. The KO pups from the Cav1.3 knockout immunized mothers had a very prolonged average PR interval of 112±21 ms (n=16).
A representative control ECG with a heart rate of 442 bpm and a PR interval of 48 ms from a NTG pup born to nonimmunized Cav1.3 knockout mother is shown in Figure 4 panel A. The Cav1.3 Ca channel knockout pup born to a nonimmunized mother exhibited sinus bradycardia (266 bpm) with I° AVB (PR interval 96 ms) (Panel B). Upon immunization of the Cav1.3 knockout mother, the pup showed advanced degree of AV block (III°, panel C).
Figure 4. ECG traces from the C57BL/6 Cav1.3 immunized and nonimmunized groups within 1 to 3 days of birth.
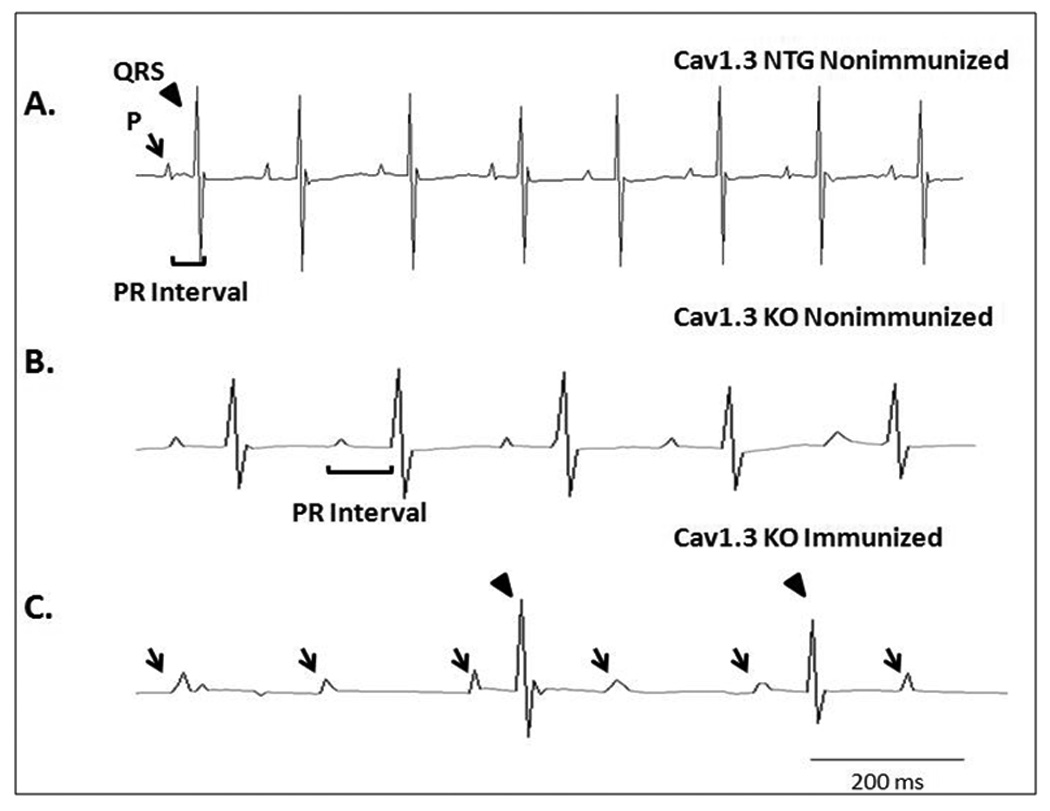
Panel A shows a control ECG with sinus rhythm (442 bpm) from a pup born to nonimmunized Cav1.3 NTG. Panel B shows sinus bradycardia (266 bpm) with I° AV block (PR interval of 96 ms) from a KO pup born to nonimmunized Cav1.3 knockout mother.
Panel C shows worsening of AV conduction abnormalities and development of III° AV block in a KO pup following immunization of Cav1.3 knockout mothers. The arrow indicates the P wave and the arrowhead indicates the QRS complex.
Fetal parity
The fetal parity was significantly lower (p<0.05 using ANOVA) in the immunized Cav1.3 knockout mothers (Figure 5). Immunized Cav1.3 knockout mothers had the lowest fetal parity (3.2±0.8 litters per pregnancy) likely due to in utero death related to bradycardia and AV block. Furthermore, in 18 out of 31 births, dead pups were delivered by the immunized Cav1.3 knockout mothers. However, the fetal parity in the nonimmunized and immunized WT and/or Cav1.2 overexpression mothers ranged between 5.83±1.2 and 6.3±1.6 litters per pregnancy (p=NS).
Figure 5. Fetal parity/average number of litters per pregnancy in the Cav1.2 and Cav1.3 immunized and nonimmunized groups.
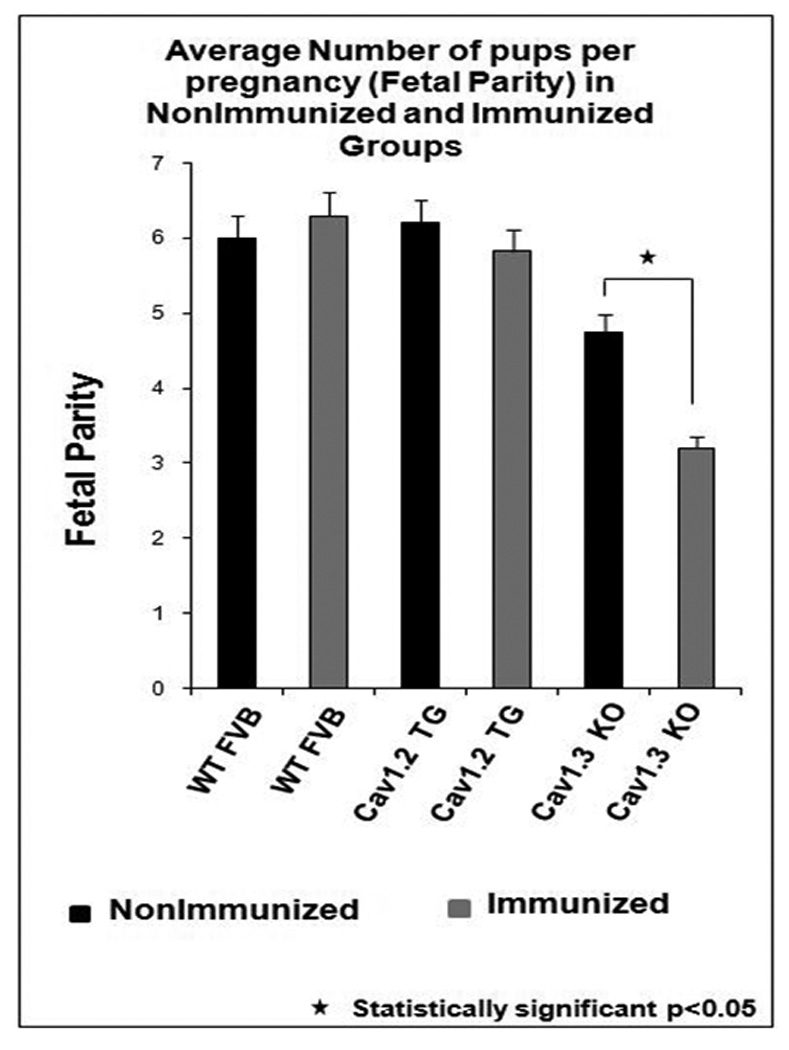
The fetal parity in the nonimmunized and immunized WT and/or Cav1.2 mothers ranged between 5.83±1.2 and 6.3±1.6 pups per pregnancy (p = not significant). However, immunized Cav1.3 knockout mothers had the lowest fetal parity (3.2±0.8 pups per pregnancy) and was much lower compared to the nonimmunized Cav1.3 knockout mothers (p<0.05).
Effect of antibodies on intracellular calcium transient, [Ca]iT
To test whether maternal IgG containing SSA/Ro and SSB/La antibodies (positive IgG) leads to intracellular Ca dysregulation, cardiomyocytes were subjected to positive IgG from a mother whose children have CHB. Figure 6A shows [Ca]iT recordings from myocytes stimulated at 0.5Hz during basal conditions and after the addition of control (normal) IgG (150 µ/ml) from a healthy mother with no SSA/Ro and SSB/La antibodies and a healthy child. No significant changes of [Ca]iT amplitude were observed (Figure 6A). However, in Figure 6B, the application of positive IgG at much lower concentration (26 µg/ml) than control IgG (150 µ/ml) resulted in a progressive decrease in [Ca]iT amplitude followed by complete inhibition (p<0.04, n=15, Figure 6C). These effects were only partially reversible upon positive IgG washout. Figure 6C illustrates averaged data from 15 myocytes each for control IgG and positive IgG and their effect on [Ca]iT amplitude.
Figure 6. Effect of IgGs on Fura-2 loaded mouse ventricular myocytes.
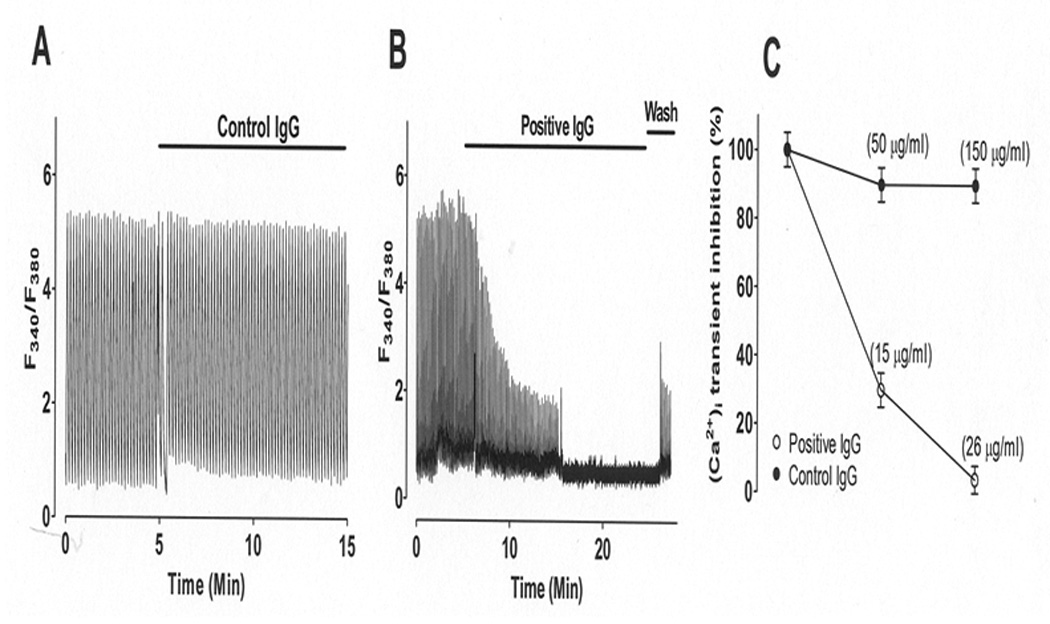
Intracellular Ca transient ([Ca]iT) was recorded during baseline and IgG application from field-stimulated single mouse ventricular myocytes electrically stimulated at 0.5 Hz. Panel A: Control IgG (normal IgG, 150 µg/ml) from a healthy mother with no SSA/Ro or SSB/La antibodies and with healthy children had no effect on [Ca]iT. Panel B: Addition of positive IgG (26 µg/ml) from a mother with SSA/Ro and SSB/La antibodies and children with CHB caused a progressive decrease of peak [Ca]iT until complete inhibition. Panel C: Dose-response summary from panel A and B. Data is shown as mean ± SE (n=15 each).
Discussion
In this study, overexpression of Cav1.2 L-type Ca channel in mice significantly reduced electrocardiographic abnormalities and the knockout of Cav1.3 L-type Ca channel significantly worsened the electrocardiographic abnormalities in pups born to immunized mothers. Together, these findings support the hypothesis that L-type Ca channels play an important role in the pathogenesis of CHB.
Immunization
A main requirement in establishing an immune response is the demonstration of high antibody response in the mothers and the placental transfer of the antibody to the pups. All immunized mothers developed a high titer of antibodies for each of the recombinant antigens SSA/Ro52, SSA/Ro60, and SSB/La48 (supplementary table 1). The placental transfer of the antibodies from the mothers to the pups was also demonstrated (supplementary table 1).
Immunization and overexpression of Cav1.2 L-type Ca channel
Ca channels are functionally inhibited by maternal autoantibodies11, 13 and these antibodies interact directly with the Cav1.2 Ca channel protein31. We hypothesized that if inhibition of the Ca current is critical in cardiac conduction disorders found in CHB patients, then upregulation of the Ca current should rescue or reverse the electrocardiographic abnormalities seen in CHB. The cardiac specific overexpression of the Cav1.2 α1C subunit of the L-type Ca channel was used. Detailed molecular, hemodynamic and electrophysiological characteristics of these TG mice are reported elsewhere 29. Briefly, these TG mice had no overt phenotype by observation, and litter sizes and pup survivals were similar to non-TG littermates. Cav1.2 α1C transcript was increased by 2.8-fold without any changes in the accessory subunits, α2/δ and β subunits of L-type Ca channel. Similarly, the density of ICa-L increased by 44% to 52% in cardiac myocytes from the TG mice. This percentage increase is ideal since maternal autoantibodies inhibit ICa-L within the same range, 40–60% 10, 11. Therefore, we postulated that immunization of TG mice overexpressing Cav1.2 should give birth to pups with no or fewer electrocardiographic abnormalities. Indeed, a lesser degree of sinus bradycardia (10% in TG pups from immunized mothers overexpressing Cav1.2 vs. 22% in NTG littermates vs. 19.7% in pups from WT immunized mothers), and fewer AV conduction abnormalities (8.8% in TG pups from mothers overexpressing Cav1.2 vs. 25% in pups from WT immunized mothers vs. 27.5% in NTG littermates) were observed in TG pups from immunized mothers overexpressing Cav1.2. These data support the concept that maternal antibodies inhibit the Cav1.2 Ca channel, and consequently conduction abnormalities occur, as expressed in the ECG. The Cav1.2 knockout mice are embryonic lethal at E14.5 and, therefore, could not be utilized in this study. The finding that TG pups from Cav1.2 overexpression immunized mothers had reduced conduction abnormalities points to the importance of finding suitable Ca channel agonists that could increase Ca channel density and thus ameliorate or reverse the severity of conduction abnormalities seen in CHB infants. In this regard, we have recently shown that Bay K8644, an L-type Ca channel agonist, was not only able to reverse the inhibitory effect of maternal antibodies of Ca current but restored it above the basal level 10.
Immunization and knockout of Cav1.3 L-type Ca channel
Biochemical and functional evidences show that the Cav1.3 Ca channel is expressed in the human fetal heart and that anti-SSA/Ro – SSB/La autoantibodies from mothers of children with CHB inhibit Cav1.3 ICa-L 10. The autoantibodies also recognize the Cav1.3 Ca channel protein by Western blot 10 and specifically bind to the extracellular loop between transmembrane region between S5–S6 in domain I 24. These data along with the unique localization of the Cav1.3 in the conductive tissue established the Cav1.3 Ca channel as a novel target in the pathogenesis of CHB. We propose that together with the knockout of Cav1.3, the inhibition of the Cav1.2 ICa-L by anti-SSA/Ro –SSB/La autoantibodies could account for the worsening of the electrocardiographic abnormalities seen in the Cav1.3 knockout pups.
Further evidence comes from the fact that pups from the Cav1.3 knockout mothers have similar electrocardiographic abnormalities even in the absence of immunization 20, 23, 30. Pups from nonimmunized Cav1.3 knockout mothers showed sinus bradycardia and I°/II° AV block, while the heterozygous had minimal abnormalities (3.6% sinus bradycardia and only I° AV block); no electrocardiographic abnormalities were seen in the NTG.
Immunized Cav1.3 knockout mothers had decreased parity with 3.2 litters per pregnancy and only 31 deliveries in 39 pregnancies. This low parity could be due to in-utero death because of the complete AV block. Of the pups that survived, 100% had sinus bradycardia and I°/II°/III° AV block. It is important to mention the high incidence of III° AV block seen in the pups from immunized Cav1.3 knockout mothers. The unique localization and the higher expression of Cav1.3 Ca channel in the supraventricular conductive tissue compared to Cav1.2 Ca channel may explain why complete AV block occurred in 19% of pups (3 out of 16 pups) in the Cav1.3 knockout model. In addition, Cav1.3 knockout mice are more responsive to the anti-SSA/Ro –SSB/La antibodies likely because of the genetic deletion of the Cav1.3 together with the inhibition of the remaining Cav1.2 Ca channel.
These data support the hypothesis that further inhibition of L-type Ca channels by maternal antibodies generated by the immunization worsens the electrocardiographic abnormalities already seen in the pups from the Cav1.3 knockout mothers. Furthermore, the immunized Cav1.3 knockout mice provide a novel animal model of CHB where therapeutic drugs could be tested. Previous models 28, 32 showed the incidence of III° AV block to be very low (0% III° AV block, and 5.8% III° AV block from the group immunized with 52 Ro β and 1.8% III° AV block in the 52 Ro α immunized group).
Maternal antibodies and intracellular Ca transient
Maternal antibodies containing SSA/Ro and SSB/La (positive IgG) inhibited CaiT in a time and dose-dependent manner resulting in a complete inhibition at 26 µg/ml. These findings are consistent with the recent report demonstrating that positive IgG also significantly inhibited [Ca]iT in cardiomyocytes derived from human embryonic stem cell 33. The inhibition of [Ca]iT by positive IgG can be attributed to the inhibition of L-type Ca current by positive IgG that is expected to reduce the amount of Ca entry to the cell and subsequently the amount of Ca release by the sarcoplasmic reticulum resulting in reduced [Ca]iT. Salomonsson et al.34 elegantly showed that using spontaneously beating rat cardiac myocytes in culture exposed to a monoclonal antibody (S3A8) with binding specificity for SSA/Ro52 and p200 peptide, resulted in an initial phase of increased frequency of [Ca]iT, followed by a progressive decrease in the rate of [Ca]iT oscillations, resulting in the accumulation of intracellular Ca and loss of Ca oscillations and contraction. The exact mechanism of the observed differences in the intracellular Ca is unclear but could be, in part, attributed to the fact that the experimental conditions are not identical. We used paced freshly isolated mouse myocytes and they used spontaneously beating neonatal rat moycytes in culture. In addition, we used IgG from mothers whose children have CHB and they used monoclonal antibodies generated by screening phage display libraries derived from peripheral B cells of Ro52 antibody-positive patients. Nevertheless, our data and the data from Salomonsson et al.34 both lead to loss in [Ca]iT.
The role of L-type Ca channels in the pathophysiology of CHB is supported by several studies 10, 11, 13, 25, 26. Maternal antibodies from mothers with CHB children induced: 1) complete AV block in the Langendorff perfused human fetal heart 13 and rat heart 11; 2) suppressed AV node action potentials and inhibited ICa-L 11. Since conduction in the AV node is dependent on ICa–L, AV block should be expected from interventions that reduce ICa-L. Data imply an interaction between autoantibodies and Ca channel proteins that results in perturbation of sarcolemmal Ca fluxes. Maternal antibodies from mothers with CHB children, but not control antibodies from healthy mothers with healthy children, cross-reacted with the native 26 and the expressed 10, 24, 26 Cav1.2 and Cav1.3 L-type Ca channels by immunoprecipitation and Western blot. However, maternal antibodies failed to affect the fast cardiac Na11 and K11 channels indicating specificity to Ca channels.
In summary, the animal models used in this study provide evidence for a pathogenic interaction of anti-SSA/Ro and/or anti-SSB/La antibodies with the L-type Ca channel in the development of CHB. We propose that the maternal autoantibodies of children with CHB are causally related to the abnormalities in the developing conduction system and that chronic exposure of Ca channels to these maternal autoantibodies during pregnancy leads to Ca channels inhibition11, 13 , Ca channels downregulation26, 31, intracellular Ca inhibition and dysregulation 34, and cell death 2, 4, 35. An overexpression of the Cav1.2 rescued the electrocardiographic abnormalities, whereas a deletion of the Cav1.3 exacerbated the abnormalities. These data suggest that the development of pharmacotherapy targeted at agents that can act as fetal Ca channel agonist may prove to be beneficial in the reduction or reversal of conduction abnormalities seen in CHB. The future clinical use of Ca channel agonists in CHB will require the development of agonist that will have cardiac selectivity over the vascular tissue, thus eliminating the vasoconstrictor effect from the cardiostimulant properties. In this regard, a group of hybrid Ca channel modulators were developed 36 where the isopropyl ester moiety of isopropyl 1,4-dihydro-2,6-dimethyl-3-nitro-4-(2,1,3-benzoxadiazol-4-yl)pyridine-5-carboxylate (PN 202–791) was replaced by a variety of nitric oxide (*NO) donor nitrooxyalkyl ester substituents. These compounds exhibited more potent and selective in vitro Ca channel antagonist activity on guinea pig ileum longitudinal smooth muscle and most potent cardiac Ca channel agonist (positive inotropic) activity on guinea pig left atrium. Such compounds may prove to be useful clinically if there safety and efficacy in pregnant women is established.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01-HL-077494) and the Veterans Affairs MERIT grants to Dr. Boutjdir and the VA-MREP grant to Dr. Qu.
Footnotes
No disclosures.
References
- 1.Buyon J. Neonatal Lupus Syndrome. In: Lahita R, editor. Systemic Lupus Erythromatosus. San Diego, CA: Elsevier Academic Press; 2004. pp. 449–484. [Google Scholar]
- 2.Buyon JP, CR . Neonatal Lupus. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 3.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, Lee LA, Provost TT, Reichlin M, Rider L, Rupel A, Saleeb S, Weston WL, Skovron ML. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DM, Rupel A, Buyon JP. Epidemiology, etiology, detection, and treatment of autoantibody-associated congenital heart block in neonatal lupus. Curr Rheumatol Rep. 2007;9:101–108. doi: 10.1007/s11926-007-0003-4. [DOI] [PubMed] [Google Scholar]
- 5.Menon A, Silverman ED, Gow RM, Hamilton RM. Chronotropic competence of the sinus node in congenital complete heart block. Am J Cardiol. 1998;82:1119–1121. doi: 10.1016/s0002-9149(98)00569-4. A1119. [DOI] [PubMed] [Google Scholar]
- 6.Brucato A, Cimaz R, Catelli L, Meroni P. Anti-Ro-associated sinus bradycardia in newborns. Circulation. 2000;102:E88–E89. doi: 10.1161/01.cir.102.11.e88. [DOI] [PubMed] [Google Scholar]
- 7.Michaelsson M, Riesenfeld T, Jonzon A. Natural history of congenital complete atrioventricular block. Pacing Clin Electrophysiol. 1997;20:2098–2101. doi: 10.1111/j.1540-8159.1997.tb03636.x. [DOI] [PubMed] [Google Scholar]
- 8.Horsfall AC, Li JM, Maini RN. Placental and fetal cardiac laminin are targets for cross-reacting autoantibodies from mothers of children with congenital heart block. J Autoimmun. 1996;9:561–568. doi: 10.1006/jaut.1996.0075. [DOI] [PubMed] [Google Scholar]
- 9.Eftekhari P, Salle L, Lezoualc'h F, Mialet J, Gastineau M, Briand JP, Isenberg DA, Fournie GJ, Argibay J, Fischmeister R, Muller S, Hoebeke J. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur J Immunol. 2000;30:2782–2790. doi: 10.1002/1521-4141(200010)30:10<2782::AID-IMMU2782>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Qu Y, Baroudi G, Yue Y, Boutjdir M. Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation. 2005;111:3034–3041. doi: 10.1161/CIRCULATIONAHA.104.517326. [DOI] [PubMed] [Google Scholar]
- 11.Boutjdir M, Chen L, Zhang ZH, Tseng CE, El-Sherif N, Buyon JP. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr Res. 1998;44:11–19. doi: 10.1203/00006450-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bacman S, Sterin-Borda L, Camusso JJ, Hubscher O, Arana R, Borda ES. Circulating antibodies against neurotransmitter receptor activities in children with congenital heart block and their mothers. FASEB J. 1994;8:1170–1176. doi: 10.1096/fasebj.8.14.7958624. [DOI] [PubMed] [Google Scholar]
- 13.Boutjdir M, Chen L, Zhang ZH, Tseng CE, DiDonato F, Rashbaum W, Morris A, el-Sherif N, Buyon JP. Arrhythmogenicity of IgG and anti-52-kD SSA/Ro affinity-purified antibodies from mothers of children with congenital heart block. Circ Res. 1997;80:354–362. doi: 10.1161/01.res.80.3.354. [DOI] [PubMed] [Google Scholar]
- 14.Baboonian C, Venables PJ, Booth J, Williams DG, Roffe LM, Maini RN. Virus infection induces redistribution and membrane localization of the nuclear antigen La (SS-B): a possible mechanism for autoimmunity. Clin Exp Immunol. 1989;78:454–459. [PMC free article] [PubMed] [Google Scholar]
- 15.LeFeber WP, Norris DA, Ryan SR, Huff JC, Lee LA, Kubo M, Boyce ST, Kotzin BL, Weston WL. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984;74:1545–1551. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clancy RM, Neufing PJ, Zheng P, O'Mahony M, Nimmerjahn F, Gordon TP, Buyon JP. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangoni ME, Couette B, Marger L, Bourinet E, Striessnig J, Nargeot J. Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog Biophys Mol Biol. 2006;90:38–63. doi: 10.1016/j.pbiomolbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 19.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- 21.Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation. 2005;112:1936–1944. doi: 10.1161/CIRCULATIONAHA.105.540070. [DOI] [PubMed] [Google Scholar]
- 23.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 24.Karnabi E, Qu Y, Wadgaonkar R, Mancarella S, Yue Y, Chahine M, R C, JP B, Boutjdir M. Congenital Heart Block: Identification of Autoantibody Binding Site on the Extracellular Loop (Domain I, S5–S6) of α1D L-type Ca Channel. Journal of Autoimmunity. 2009 doi: 10.1016/j.jaut.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu K, Qu Y, Yue Y, Boutjdir M. Functional basis of sinus bradycardia in congenital heart block. Circ Res. 2004;94:e32–e38. doi: 10.1161/01.RES.0000121566.01778.06. [DOI] [PubMed] [Google Scholar]
- 26.Qu Y, Xiao GQ, Chen L, Boutjdir M. Autoantibodies from mothers of children with congenital heart block downregulate cardiac L-type Ca channels. J Mol Cell Cardiol. 2001;33:1153–1163. doi: 10.1006/jmcc.2001.1379. [DOI] [PubMed] [Google Scholar]
- 27.Mazel JA, El-Sherif N, Buyon J, Boutjdir M. Electrocardiographic abnormalities in a murine model injected with IgG from mothers of children with congenital heart block. Circulation. 1999;99:1914–1918. doi: 10.1161/01.cir.99.14.1914. [DOI] [PubMed] [Google Scholar]
- 28.Miranda-Carus ME, Boutjdir M, Tseng CE, DiDonato F, Chan EK, Buyon JP. Induction of antibodies reactive with SSA/Ro-SSB/La and development of congenital heart block in a murine model. J Immunol. 1998;161:5886–5892. [PubMed] [Google Scholar]
- 29.Muth JN, Yamaguchi H, Mikala G, Grupp IL, Lewis W, Cheng H, Song LS, Lakatta EG, Varadi G, Schwartz A. Cardiac-specific overexpression of the alpha(1) subunit of the L-type voltage-dependent Ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem. 1999;274:21503–21506. doi: 10.1074/jbc.274.31.21503. [DOI] [PubMed] [Google Scholar]
- 30.Mancarella S, Yue Y, Karnabi E, Qu Y, El Sherif N, Boutjdir M. Impaired Calcium Homeostasis is Associated with Atrial Fibrillation in the {alpha} 1D L-Type Ca2+ Channel KO Mouse. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.00537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao GQ, Qu Y, Hu K, Boutjdir M. Down-regulation of L-type calcium channel in pups born to 52 kDa SSA/Ro immunized rabbits. FASEB J. 2001;15:1539–1545. doi: 10.1096/fj.01-0052com. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, Silverman ED, Wu X, Borges C, Zhao S, Isacovics B, Hamilton RM. Effect of maternal autoantibodies on fetal cardiac conduction: an experimental murine model. Pediatr Res. 2005;57:557–562. doi: 10.1203/01.PDR.0000155947.82365.E4. [DOI] [PubMed] [Google Scholar]
- 33.Gelernter-Yaniv L, Arbel G, Gepstein A, Itzhaki I, Toby ES, Irit H, Anat H, Clancy R, Buyon JP, Gepstein L. Abstract 21157: Establishing a Model for Congenital Atrioventricular Block using Human Embryonic Stem Cells. Circulation. 2010;122 A21157- [Google Scholar]
- 34.Salomonsson S, Sonesson SE, Ottosson L, Muhallab S, Olsson T, Sunnerhagen M, Kuchroo VK, Thoren P, Herlenius E, Wahren-Herlenius M. Ro/SSA autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med. 2005;201:11–17. doi: 10.1084/jem.20041859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–182. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 36.Shan R, Velazquez C, Knaus EE. Syntheses, calcium channel agonist-antagonist modulation activities, and nitric oxide release studies of nitrooxyalkyl 1,4-dihydro-2,6-dimethyl-3-nitro-4-(2,1,3-benzoxadiazol-4-yl)pyridine-5-ca rboxylate racemates, enantiomers, and diastereomers. J Med Chem. 2004;47:254–261. doi: 10.1021/jm030333h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


