Gerstmann-Sträussler-Scheinker (GSS) disease is a rare inherited neurodegenerative disease due to a mutation in the prion protein (PRNP) gene, resulting in dementia and/or ataxia associated with mandatory amyloid plaques visible in the cerebral and/or cerebellar cortices on specimens stained with haematoxylin-eosin. Such amyloid plaques are dichroic in polarized light after Congo red staining and well marked by immunohistochemistry to prion protein (PrP). Almost every GSS family carries a PRNP missense mutation and almost half of them are due to a P102L mutation present in the original Austrian family [1], and also in a large family from central England [2–4]. GSS cases were also reported with a P105L mutation in Japan [5], with an A117V mutation in French-Alsacian [6], American [7] and English [8] families, and with a F198S mutation in the Indiana Kindred [9]. Other rare mutations have briefly been reported [3]. Three GSS cases due to an octapeptide repeat insertion (OPRI) in their PRNP between codons 51 and 91 [3] have also been investigated, eight in two unrelated French families [10– 13] and seven in a Dutch family [14]. We report here a French case with a novel six OPRI.
The patient was a 65-year-old French woman, with no familial history of neurological disorder. She was admitted to the neurology department because of rapidly progressive cognitive deterioration, with almost complete mutism. Her family reported moderate memory problems that had started two months earlier, but the patient had not been investigated at that time. At admission, clinical examination showed cerebellar ataxia, extra-pyramidal rigidity and myoclonus in upper limbs. Cranial CT found cortico-subcortical atrophy predominating in the posterior cranial fossa and EEG showed diffuse slow waves with periodic paroxystic activity. A search for 14.3.3 protein in the cerebrospinal fluid remained negative. The neurological state worsened progressively with dementia, and the patient died seven months after presentation.
An autopsy restricted to the brain was performed 10 hours after death. The brain weighed 1050 g. Specimens from the frontal, temporal, occipital and cerebellar cortices were frozen, and the whole brain was fixed with 10% buffered formalin. Preliminary examination was carried out on haematoxylin-eosin stained paraffin sections, completed by thioflavine S and Congo red stainings. PrP-immunostaining was performed with 12F10 antibody (Spibio, Montreal, Canada) after proteinase K (PK) digestion. Immunohistochemistry was also performed with anti-glial fibrillary acidic protein (GFAP), anti-beta A4 amyloid and anti-tau antibodies (Dako, Trappes, France).
The coding sequence of the PRNP gene was PCR-amplified and the two fragments obtained were eluted from a polyacrylamide gel and directly sequenced on both strands as described previously [13]. The family originated from Gascony in the South-West of France and declined any further study of the PRNP gene.
Frozen tissues from frontal cortex, temporal cortex, occipital cortex and cerebellum were homogenized in lysis buffer to prepare 10% (w/v) brain homogenates as described previously [15]. Samples were resolved on 15% Tris-HCl Criterion pre-cast gels (Bio-Rad, Richmond, CA, USA) for SDS (sodium dodecyl sulfate) polyacrylamide gel electrophoresis at 150 V for ~80 min. The proteins on the gels were transferred to PVDF (polyvinylidene fluoride, Millipore, Billerica, MA, USA) for 2 h at 70V. The membranes were incubated for 2 hours at room temperature with 3F4 (1:40,000) as the primary antibody for probing the PrP molecule [16]. Following incubation with HRP (horseradish peroxidase) -conjugated sheep anti-mouse IgG at 1:3000, the PrP bands were visualized on Kodak film by the ECL Plus (Enhanced Chemiluminescence) in accordance with the manufacturer's protocol (GE Healthcare, Piscataway, NJ, USA).
Microscopic examination of the frontal, temporal and occipital areas revealed marked spongiosis, but moderate neuronal loss in the neocortex and hippocampus. Spongiosis was also marked in the caudate and putamen, where a few amyloid deposits were identified. These amyloid deposits were kuru-plaque-like with radially arranged bundles. The thalamus appeared less damaged. Midbrain, pons and medulla were unremarkable on routine haematoxylin-eosin stained sections. Numerous and multicentric amyloid deposits, each surrounded by radially arranged bundles, were localized mainly in the molecular layer (Fig.1a) and less frequently the granular layer of the cerebellum. Fluorescence under UV light after thioflavine S staining (Fig.1b) and yellow-green birefringence after Congo red staining (Fig.1c and d) attested to the β-sheet conformation of amyloid fibrils. PrP-immunostaining was strongly positive on kuru-plaque-like in the striatum and on multicentric plaques in the cerebellum. The core of most of these amyloid plaques remained negative with the 12F10 antibody (Fig.1e). There were also numerous and scattered non-amyloid PrP deposits in the neo-cortex, hippocampus and thalamus. PrP deposits were mild in midbrain and were absent in the pons and medulla. Astrocytic gliosis, immunostained by anti-GFAP, was marked in the molecular layer of the cerebellum (Fig.1f). No significant patterns were revealed by anti-beta A4 and anti-tau immunohistochemistry. Only scarce beta A4-positive senile plaques were observed in the neo-cortex.
Fig. 1.
a Haematoxylin-eosin stained section of the cerebellum showing multicentric amyloid plaques in the molecular layer, each surrounded by radially arranged bundles (insert); b these deposits are fluorescent under UV light after thioflavine S staining; c they are stained by Congo red, d with a yellow-green birefringence; e PrP-immunostaining is strongly positive on the periphery of amyloid plaques; f GFAP-positive astrocytic gliosis is marked in the molecular layer of the cerebellum (bar a, e, f 80 μm; b 65 μm; a insert, c, d 40μm)
Molecular investigation of the PRNP gene revealed a new 144-bp insertional mutation into the N terminal repeat region. The mutation having the standardized nomenclature NM_000311.3c.226_227ins144; p.Pro76_His77ins48 consisted in the addition of six octarepeat motifs to the five normally present in the protein. Indeed, the repeat stretch had the following unrecorded sequence: R1-R2-R2-[R3-R2-R3g-R2-R2-R2]-R3-R4, in the nomenclature according to Goldfarb et al [10]. The patient was methionine/valine heterozygous at codon 129 (SNP rs1799990) and codon 129 encoded valine on the mutant allele.
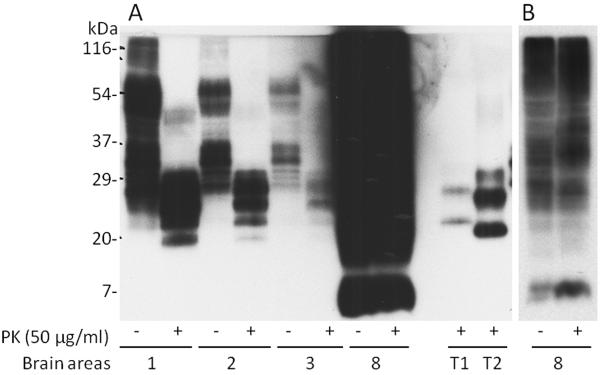
Western-blot analysis detected PK-resistant prion protein (PrPSc) in the four available samples (Fig. 2). A large number of poorly resolved bands spanning approximately 116 kDa and 25 kDa were observed in the PK-untreated preparations from tissue samples 1 (frontal cortex), 2 (temporal cortex) and 3 (occipital cortex). In contrast, PK-untreated preparations from sample 8 (cerebellum) essentially generated a smear migrating slightly faster than the corresponding kDa preparations from samples 1, 2 and 3 but also containing a distinct band of 7 kDa. After PK treatment, samples 1, 2 and 3 revealed 4–5 bands with a mobility approximately comprised between 29 kDa and 20 kDa. This profile was quite different from that of sample 8 that after PK-treatment revealed a profile essentially similar to that of the untreated preparation. Furthermore, all the electrophoretic profiles of the PK-treated preparations from the propositus were different from those of PrPSc type 1 or type 2 from sporadic Creutzfeldt-Jakob disease (CJD).
Fig. 2.
A: Western blot analysis of insoluble PrP on brain homogenates labeled as 1 (frontal cortex), 2 (temporal cortex), 3 (occipital cortex), and 8 (cerebellum). A 5 μl brain homogenate each was detected by Western blotting with 3F4 after treated with or without proteinase K (PK) at 50 μg/ml at 37°C for 1 h. T1: PrPSc type 1 and T2: PrPSc type 2 from sporadic Creutzfeldt-Jakob disease. B: Less exposed film showing a better gel profile of PrP from the cerebellum (8) treated with or without PK.
The pathological hallmark of GSS is the presence of amyloid plaques which are easily visible on haematoxylin-eosin stained paraffin sections [1–3, 17–20], and the most significant ones are the multicentric amyloid plaques with a larger central deposit and smaller aggregates located at the periphery. Unicentric plaques are rounded, with a central mass surrounded by a pale halo. Kuru-type plaques are the third variety and present spikes at their periphery. In most GSS cases these plaques are observed in the molecular layer of the cerebellum and also in the cerebral cortex. Multicentric plaques, kuru-type plaques and the halo of unicentric plaques are strongly immunoreactive with PrP antibodies. These characteristic plaques were observed in at least one member of the four families with GSS due to OPRI. They were numerous in the case presented herein with six repeats and in another [10] which had also been reported with more histological details in a French paper [11]. Another French case with eight OPRI [12, 13], already published, presented several multicentric plaques visible on routine preparations, whereas PrP immunostaining was not available at that time. In a Dutch family with seven OPRI, multicentric plaques were present in the molecular layer of the cerebellum and showed characteristic birefringence under polarized light [14]. The major peptide extracted from amyloid fibrils in GSS is a ~7-kDa PrP fragment [17, 21, 22] as found in our patient's cerebellum where multicentric amyloid plaques are numerous. Such plaques may be present in the MV2 subtype of sporadic CJD but in moderate quantity [3, 23]. Concerning cases of inherited prion encephalopathies due to OPRI in the PRNP, two other immunostaining profiles for PrP have been described. The most characteristic are PrP patchy deposits, not visible on haematoxylin-eosin stained preparations and not stained by Congo red, but mainly visible in the molecular layer of the cerebellum where most of them are elongated and perpendicular to the surface of the cerebellar parenchyma (PPPP). This pattern has notably been observed in families with four [24], five [25], six [26] or seven [27, 28] OPRI. The last pattern has been observed in two remaining cases, first observed in 2005 in a case with six OPRI presenting numerous eosinophilic globular structures intensely marked by PrP-immunostaining, but without Congophilia and birefringence in polarized light [29]. This observation has adequately been compared to a Dutch case with “grape-like deposits” and eight OPRI [30].
In 1998, 18 cases from eight families and three individual cases had been reported and we stated that the number of inserted repeats determined the type of cerebellar deposits. PPPP were present in cases with four to seven OPRI, whereas genuine amyloid plaques were present in two other French cases from Brittany with eight OPRI [31]. This statement has been confirmed for cases with OPRI and whose cerebellar fragments have been studied by immunohistochemistry. The presence of characteristic PPPP has been evidenced in most of them, isolated or more commonly mixed with other ovoid or irregularly shaped deposits [3, 24–28]. Our statement [31] has to be revised for cases with PrP amyloid plaques since the GSS case reported herein has only six OPRI.
To conclude, the vast majority of GSS cases are due to a missense mutation in the PRNP gene but four families are concerned by OPRI, two with eight [10–13], one with seven [14] and our case with six OPRI. This pathogenesis deserves to be emphasized in spite of its rarity.
Acknowledgments
The authors are very grateful to Marie-Hélène Canron, Evelyne Doudnikoff and Amandine Berthet for expert technical assistance. This study was supported by the French Reference Center for Spongiform Encephalopathies, and in part by the National Institutes of Health (NIH) R01NS062787, the Creutzfeldt-Jakob-Disease Foundation, Alliance BioSecure, the University Center on Aging and Health, with the support of the McGregor Foundation and the President's discretionary Fund (Case Western Reserve University), as well as the National Institute on Aging AG-14359, the Centers for Disease Control and Prevention UR8/CCU515004 and the Britton Fund.
Footnotes
We have no conflict of interest.
References
- 1.Hainfellner JA, Brantner-Inthaler S, Cervenakova L, Brown P, Kitamoto T, Tateishi J, Diringer H, Liberski PP, Regele H, Feucht M, Mayr N, Wessely P, Summer K, Seitelberger F, Budka H. The original Gerstmann-Sträussler-Scheinker family of Austria: divergent clinicopathological phenotypes but constant PrP genotype. Brain Pathol. 1995;5:201–211. doi: 10.1111/j.1750-3639.1995.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 2.Wadsworth JDF, Joiner S, Linehan JM, Cooper S, Powell C, Mallinson G, Buckell J, Gowland I, Asante EA, Budka H, Brandner S, Collinge J. Phenotypic heterogeneity in inherited prion disease (P102L) is associated with differential propagation of protease-resistant wild-type and mutant prion protein. Brain. 2006;129:1557–1569. doi: 10.1093/brain/awl076. [DOI] [PubMed] [Google Scholar]
- 3.Ironside JW, Ghetti B, Head MW, Piccardo P, Will RG. Prion diseases. In: Love S, Louis DN, Ellison DW, editors. Greenfield's Neuropathology. 8th edn. Hodder Arnold; London: 2008. pp. 1197–1273. [Google Scholar]
- 4.Webb TEF, Poulter M, Beck J, Uphill J, Adamson G, Campbell T, Linehan J, Powell C, Brandner S, Pal S, Siddique D, Wadsworth JD, Joiner S, Alner K, Petersen C, Hampson S, Rhymes C, Treacy C, Storey E, Geschwind MD, Nemeth AH, Wroe S, Collinge J, Mead S. Phenotypic heterogeneity and genetic modification of P102L inherited prion disease in an international series. Brain. 2008;131:2632–2646. doi: 10.1093/brain/awn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh Y, Yamada M, Hayakawa M, Shozawa T, Tanaka J, Matsushita M, Kitamoto T, Tateishi J, Otomo E. A variant of Gerstmann-Sträussler-Scheinker disease carrying codon 105 mutation with codon 129 polymorphism of the prion gene: a clinicopathological study. J Neurol Sci. 1994;127:77–86. doi: 10.1016/0022-510x(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 6.Tateishi J, Kitamoto T, Doh-ura K, Sakaki Y, Steinmetz G, Tranchant C, Warter JM, Heldt N. Immunochemical, molecular genetic, and transmission studies on a case of Gerstmann-Sträussler-Scheinker syndrome. Neurology. 1990;40:1578–1581. doi: 10.1212/wnl.40.10.1578. [DOI] [PubMed] [Google Scholar]
- 7.Mastrianni JA, Curtis MT, Oberholtzer JC, Da Costa MM, DeArmond S, Prusiner SB, Garbern JY. Prion disease (PrP-A117V) presenting with ataxia instead of dementia. Neurology. 1995;45:2042–2050. doi: 10.1212/wnl.45.11.2042. [DOI] [PubMed] [Google Scholar]
- 8.Mallucci GR, Campbell TA, Dickinson A, Beck J, Holt M, Plant G, de Pauw KW, Hakin RN, Clarke CE, Howell S, Davies-Jones GAB, Lawden M, Smith CML, Ince P, Ironside JW, Bridges LR, Dean A, Weeks I, Collinge J. Inherited prion disease with an alanine to valine mutation at codon 117 in the prion protein gene. Brain. 1999;122:1823–1837. doi: 10.1093/brain/122.10.1823. [DOI] [PubMed] [Google Scholar]
- 9.Ghetti B, Piccardo P, Frangione B, Bugiani O, Giaccone G, Young K, Prelli F, Farlow MR, Dlouhy SR, Tagliavini F. Prion protein amyloidosis. Brain Pathol. 1996;6:127–145. doi: 10.1111/j.1750-3639.1996.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb LG, Brown P, Vrbovska A, Baron H, McCombie WR, Cathala F, Gibbs CJ, Gajdusek DC. An insert mutation in the chromosome 20 amyloid precursor gene in a Gerstmann-Sträussler-Scheinker family. J Neurol Sci. 1992;111:189–194. doi: 10.1016/0022-510x(92)90067-u. [DOI] [PubMed] [Google Scholar]
- 11.Genthon R, Gray F, Salama J, Duyckaerts C, Belin C, Brucher JM, Baron H, Delaporte P. Maladie de Gerstmann-Sträussler-Scheinker. Etude pathologique et généalogique. Rev Neurol (Paris) 1992;148:335–342. [PubMed] [Google Scholar]
- 12.Foncin JF, Cardot JL, Martinet Y, Arnott G. Maladie de Gerstmann-Sträussler-Scheinker. Etude anatomoclinique et généalogique. Rev Neurol (Paris) 1982;138:123–135. [PubMed] [Google Scholar]
- 13.Laplanche JL, El Hachimi KH, Durieux I, Thuillet P, Defebvre L, Delasnerie-Lauprêtre N, Peoc'h K, Foncin JF, Destée A. Prominent psychiatric features and early onset in an inherited prion disease with a new insertional mutation in the prion protein gene. Brain. 1999;122:2375–2386. doi: 10.1093/brain/122.12.2375. [DOI] [PubMed] [Google Scholar]
- 14.Jansen C, Voet W, Head MW, Parchi P, Yull H, Verrips A, Wesseling P, Meulstee J, Baas F, van Gool WA, Ironside JW, Rozemuller AJM. A novel seven-octapeptide repeat insertion in the prion protein gene (PRNP) in a Dutch pedigree with Gerstmann-Sträussler-Scheinker disease phenotype: comparison with similar cases from the literature. Acta Neuropathol. 2011;121:59–68. doi: 10.1007/s00401-010-0656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan J, Xiao X, McGeehan J, Dong Z, Cali I, Fujioka H, Kong Q, Kneale G, Gambetti P, Zou WQ. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J Biol Chem. 2006;281:34848–34858. doi: 10.1074/jbc.M602238200. [DOI] [PubMed] [Google Scholar]
- 16.Zou WQ, Langeveld J, Xiao X, Chen S, McGeer PL, Yuan J, Payne MC, Kang HE, McGeehan J, Sy MS, Greenspan NS, Kaplan D, Wang GX, Parchi P, Hoover E, Kneale G, Telling G, Surewicz WK, Kong Q, Guo JP. PrP conformational transitions alter species preference of a PrP-specific antibody. J Biol Chem. 2010;285:13874–13884. doi: 10.1074/jbc.M109.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccardo P, Dlouhy SR, Lievens PMJ, Young K, Bird TD, Nochlin D, Dickson DW, Vinters HV, Zimmerman TR, Mackenzie IRA, Kish SJ, Ang LC, de Carli C, Pocchiari M, Brown P, Gibbs CJ, Gajdusek DC, Bugiani O, Ironside J, Tagliavini F, Ghetti B. Phenotypic variability of Gerstmann-Sträussler-Scheinker disease is associated with prion protein heterogeneity. J Neuropath Exp Neurol. 1998;57:979–988. doi: 10.1097/00005072-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Mohr M, Tranchant C, Steinmetz G, Floquet J, Grignon Y, Warter JM. Gerstmann-Sträussler-Scheinker disease and the French-Alsatian A117V variant. Clin Exp Path. 1999;47:161–175. [PubMed] [Google Scholar]
- 19.Boellaard JW, Brown P, Tateishi J. Gerstmann-Sträussler-Scheinker disease – The dilemma of molecular and clinical correlations. Clin Neuropathol. 1999;18:271–285. [PubMed] [Google Scholar]
- 20.Bugiani O, Giaccone G, Piccardo P, Morbin M, Tagliavini F, Ghetti B. Neuropathology of Gerstmann-Sträussler-Scheinker disease. Microsc Res Tech. 2000;50:10–15. doi: 10.1002/1097-0029(20000701)50:1<10::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Parchi P, Chen SG, Brown P, Zou W, Capellari S, Budka H, Hainfellner J, Reyes PF, Golden GT, Hauw JJ, Gajdusek DC, Gambetti P. Different patterns of truncated prion protein fragments correlate with distinct phenotypes in P102L Gerstmann-Sträussler-Scheinker disease. Proc Natl Acad Sci USA. 1998;95:8322–8327. doi: 10.1073/pnas.95.14.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagliavini F, Lievens PMJ, Tranchant C, Warter JM, Mohr M, Giaccone G, Perini F, Rossi G, Salmona M, Piccardo P, Ghetti B, Beavis RC, Bugiani O, Frangione B, Prelli F. A 7-kDa prion protein (PrP) fragment, an integral component of the PrP region required for infectivity, is the major amyloid protein in Gerstmann-Sträussler-Scheinker disease A117V. J Biol Chem. 2001;276:6009–6015. doi: 10.1074/jbc.M007062200. [DOI] [PubMed] [Google Scholar]
- 23.Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, Poser S, Rojiani A, Streichemberger N, Julien J, Vital C, Ghetti B, Gambetti P, Kretzschmar H. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46:224–233. [PubMed] [Google Scholar]
- 24.Campbell TA, Palmer MS, Will RG, Gibb WRG, Luthert PJ, Collinge J. A prion disease with a novel 96-base pair insertional mutation in the prion protein gene. Neurology. 1996;46:761–766. doi: 10.1212/wnl.46.3.761. [DOI] [PubMed] [Google Scholar]
- 25.Jansen C, van Swieten JC, Capellari S, Strammiello R, Parchi P, Rozemuller AJM. Inherited Creutzfeldt-Jakob disease in a Dutch patient with a novel five octapeptide repeat insertion and unusual cerebellar morphology. J Neurol Neurosurg Psychiatry. 2009;80:1386–1389. doi: 10.1136/jnnp.2008.169359. [DOI] [PubMed] [Google Scholar]
- 26.King A, Doey L, Rossor M, Mead S, Collinge J, Lantos P. Phenotypic variability in the brains of a family with a prion disease characterized by a 144-base pair insertion in the prion protein gene. Neuropathol Appl Neurobiol. 2003;29:98–105. doi: 10.1046/j.1365-2990.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- 27.Kitamoto T, Doh-ura K, Muramoto T, Miyazono M, Tateishi J. The primary structure of the prion protein influences the distribution of abnormal prion protein in the central nervous system. Am J Pathol. 1992;141:271–277. [PMC free article] [PubMed] [Google Scholar]
- 28.Dermaut B, Cruts M, Backhovens H, Lübke U, Everbroeck BV, Sciot R, Dom R, Martin JJ, Van Broeckhoven C, Cras P. Familial Creutzfeldt-Jakob disease in a patient carrying both a presenilin 1 missense substitution and a prion protein gene insertion. J Neurol. 2000;247:364–368. doi: 10.1007/s004150050603. [DOI] [PubMed] [Google Scholar]
- 29.Gelpi E, Kovacs GG, Ströbel T, Koperek O, Voigtländer T, Liberski PP, Budka H. Prion disease with a 144 base pair insertion; unusual cerebellar prion protein immunoreactivity. Acta Neuropathol. 2005;110:513–519. doi: 10.1007/s00401-005-1073-x. [DOI] [PubMed] [Google Scholar]
- 30.Van Gool WA, Hensels GW, Hoogerwaard EM, Wiezer JHA, Wesseling P, Bolhuis PA. Hypokinesia and presenile dementia in a Dutch family with a novel insertion in the prion protein gene. Brain. 1995;118:1565–1571. doi: 10.1093/brain/118.6.1565. [DOI] [PubMed] [Google Scholar]
- 31.Vital C, Gray F, Vital A, Parchi P, Capellari S, Petersen RB, Ferrer X, Jarnier D, Julien J, Gambetti P. Prion encephalopathy with insertion of octapeptide repeats: the number of repeats determines the type of cerebellar deposits. Neuropathol Appl Neurobiol. 1998;24:125–130. doi: 10.1046/j.1365-2990.1998.00098.x. [DOI] [PubMed] [Google Scholar]