Abstract
Functional inhibitory synapses form in auditory cortex well before the onset of normal hearing. However, their properties change dramatically during normal development, and many of these maturational events are delayed by hearing loss. Here, we review recent findings on the developmental plasticity of inhibitory synapse strength, kinetics, and GABAA receptor localization in auditory cortex. Although hearing loss generally leads to a reduction of inhibitory strength, this depends on the type of presynaptic interneuron. Furthermore, plasticity of inhibitory synapses also depends on the postsynaptic target. Hearing loss leads reduced GABAA receptor localization to the membrane of excitatory, but not inhibitory neurons. A reduction in normal activity in development can also affect the use-dependent plasticity of inhibitory synapses. Even moderate hearing loss can disrupt inhibitory short- and long-term synaptic plasticity. Thus, the cortex did not compensate for the loss of inhibition in the brainstem, but rather exacerbated the response to hearing loss by further reducing inhibitory drive. Together, these results demonstrate that inhibitory synapses are exceptionally dynamic during development, and deafness-induced perturbation of inhibitory properties may have a profound impact on auditory processing.
Keywords: development, deafness, inhibitory interneuron, short-term depression, long-term potentiation, auditory cortex
Introduction
The regulation of synapse strength is a life-long process that permits neural circuits to adapt to an ever changing environment. This principle was initially established for excitatory connections, but inhibitory synapses are just as malleable, and many of the best examples emerge from studies on the central auditory system. During development, the strength of inhibitory synapses can decline or increase, as these connections become refined anatomically (for reviews, see Kandler et al., 2009; Sanes et al., 2009). At the other chronological extreme, during senescence, a decline in synaptic inhibition is associated with a diminished computational capacity of brainstem and cortical single neurons (Caspary et al., 2008). Perhaps, the most profound changes to inhibitory synapse function come about as a result of developmental hearing loss. This has been demonstrated at almost every level of the auditory neuraxis, and often includes a reduction in synaptic strength (for review, see Takesian et al., 2009).
In this review, we describe the maturational changes to inhibitory synapse function in auditory cortex (ACx), and present evidence that chronic deprivation elicits unique effects at inhibitory synapses that depend on the specific pre- and postsynaptic neurons. One clear reason for the focus on ACx is that it inherits and filters subcortical inputs and serves as the primary sensory representation to decoding centers (Budinger et al., 2000, 2006; Kaas and Hackett, 2000). Furthermore, changes to ACx processing that occur during normal development or following to environmental manipulations - including hearing loss - are thought to involve the modification of intrinsic inhibitory networks (Calford et al., 1993; Rajan, 1998; Kimura and Eggermont, 1999; Raggio and Schreiner, 1999; Kral et al., 2000; Qiu et al., 2000; Rajan, 2001; Noreña et al., 2003; Seki and Eggermont, 2003; Noreña et al., 2003; Chang et al., 2005; Razak and Fuzessery, 2007; Razak et al., 2008; Scholl and Wehr, 2008).
Despite being outnumbered by excitatory neurons by almost 4:1, inhibitory connections exert a profound influence on the pattern and magnitude of cortical network activity. This may reflect both the expansive architecture of inhibitory axonal arbors as well as the unique functional properties of interneurons (Chagnac-Amitai, Connors, 1989; Shuz and Palm, 1989; DeFelipe and Farinas, 1992; Bacci et al., 2003; Markram et al., 2004; Silberberg, 2008). Most neurons in auditory cortex receive synaptic input from a broad range of the audible spectrum (Wehr and Zador, 2003; Kaur et al., 2004; Lu et al., 2007; Wu et al., 2008). Furthermore, inhibitory interneurons may have even broader suprathreshold frequency tuning curves than excitatory cells (Atencio and Schreiner, 2008). A basic implication of this convergence is that activation of inhibitory circuits is often associated with the sharpening of excitatory receptive fields, while inactivation broadens receptive fields (Müller and Scheich, 1998; Chen and Jen, 2000; Wang et al., 2000; Foeller et al., 2001; Wang et al., 2002; Kaur et al., 2004). The overall goal of this review is to demonstrate that even moderate developmental hearing loss leads to a pervasive failure of ACx inhibitory synapses to mature properly, an outcome that is expected to compromise rate or temporal codes, thereby reducing stimulus selectivity.
Experimental Approaches
The amount of synaptic integration that occurs in auditory centers beneath the cortex is staggering. Therefore, to measure the function of cortical inhibitory synapses independent of the brain stem, one must stimulate inhibitory inputs selectively. This can be accomplished with a brain slice preparation of the auditory cortex that includes the projection from thalamus (Cruikshank et al., 2002). With this approach, it is possible to deliver electrical stimuli to a small region of tissue, pharmacologically isolate inhibitory synaptic currents under voltage-clamp conditions, and record from inhibitory interneuron-pyramidal cell pairs (Figure 1). These methods were used to record from gerbil ACx neurons, and are covered in detail elsewhere (Kotak et al., 2005; Xu et al., 2007; Kotak et a., 2008; Takesian et al., 2010). Neurons were recorded at postnatal (P) 8–11 (pre-hearing), or P17–22 (post-hearing, and age-matched hearing loss).
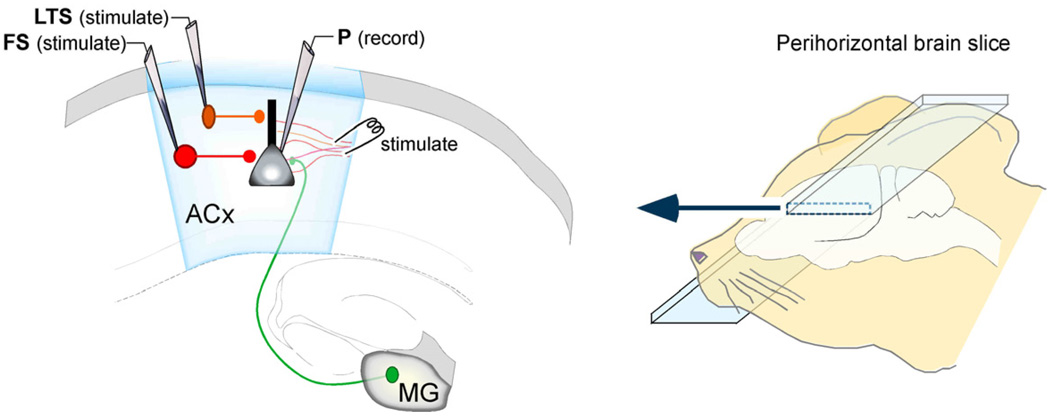
Figure 1.
Experimental preparation. A perihorizontal brain slice (right), contains the medial geniculate (MG) projection to auditory cortex (ACx). Whole cell voltage clamp recordings are obtained from supragranular pyramidal (P) neurons, and spontaneous inhibitory postsynaptic currents, or evoked currents (stimulating electrode) are acquired. In other experiments, supragranular interneurons are recorded spike-elicited IPSCs are recorded in the pyramidal neuron. Fast-spiking (FS) and low-threshold spiking (LTS) interneurons are identified based on soma shape visualized under IR-DIC, and by their discharge pattern in response to direct current injection into the cell body via the recording eletrode.
To measure the subcellular anatomical characteristics of single cortical inhibitory synapses, it is necessary to quantify images obtained from tissue processed for electron microscopic immunocytochemistry. Here, proteins localized to the presynaptic terminal (glutamic acid decarboxylase, GAD) or postsynaptic membrane (GABAA receptor β2/3 subunit) are bound with a specific antibody, visualized with a silver-intensified gold technique, and quantified at the ultrastructural level from photomicrographs. These methods were used in P17 gerbil ACx, and are covered in detail elsewhere (Sarro et al., 2008).
Developmental hearing loss was induced surgically in gerbils aged P10. This is just prior to ear canal opening, an age when anteroventral cochlear nucleus cell number is unaffected by cochlear ablation (Tierney and Moore, 1997). Sensorineural hearing loss (SNHL) was induced by removal of both cochleae, and conductive hearing loss (CHL) was induced by malleus extirpation on both sides, using procedures identical to those described previously (Vale and Sanes, 2002; Xu et al., 2007). All protocols were reviewed and approved by New York University Institutional Animal Care and Use Committee.
Results and Discussion
Unique pattern of inhibitory developmental plasticity in ACx
While studying the effect of hearing loss on synapse function in the auditory brainstem, we observed a significant loss of inhibition (Kotak et al., 1996; Vale et al., 2000). Therefore, we first sought to determine whether there was a similar outcome in thalamorecipient ACx. There were two broad possibilities: either the cortex compensated for the loss of inhibition in the brainstem, or it exacerbated the response to hearing loss by further reducing inhibitory drive. Our initial studies used 3 different approaches to assess inhibitory function. First, spontaneous inhibitory synaptic currents (sIPSC) were recorded from gerbil layers 2/3 pyramidal cells in the presence of ionotropic glutamate receptor antagonists. Although sIPSC amplitude is quite variable in each recorded pyramidal neuron, there is consistent 30% reduction following hearing loss (Kotak et al., 2008). Second, minimum-evoked IPSCs were obtained by delivering a minimally effective stimulus to a nearby region of cortex to elicit putative monosynaptic IPSCs from nearby GABAergic interneurons; the criterion for a minimum response is that half of the stimuli result in failures. This technique also reveals a >30% decline in the amplitude of putative unitary IPSCs following hearing loss (Kotak et al., 2008). Finally, inhibitory postsynaptic potentials (IPSP) were recorded in response to a maxiumum intracortical stimulus, once again ionotropic glutamate receptor antagonists. These maxiumum-evoked monosynaptic IPSPs were declined by >50% following hearing loss (Kotak et al., 2005). Each of these results were obtained with the more extreme form of hearing loss (SNHL), but similar reduction in inhibition can be observed with CHL (Takesian, Kotak, and Sanes, in submission).
Spontaneous and minimum-evoked inhibitory synaptic currents offer a global estimate of inhibitory function, and this sort of data also provides an efficient way to compare multiple age or treatment groups. However, it is important to bear in mind that there are many types of inhibitory neurons in cortex, and they display distinct patterns of connectivity and function (Markram et al., 2004). One must ultimately record the responses of individual interneurons to determine whether they are consistent with the conclusions drawn from less rigorous observations. Therefore, the synaptic properties were obtained for two major types of inhibitory interneurons, the fast-spiking (FS) and low-threshold spiking (LTS) cells. These cell types display characteristic intrinsic membrane and synaptic properties, and their terminals are targeted to different postsynaptic compartments on pyramidal cells (Gibson et al., 1999; Beierlein et al., 2000, 2003; Gupta et al., 2000; Di Cristo et al., 2004; Ma et al., 2010). In particular, mature FS-evoked IPSCs are much larger than those elicited by LTS interneurons.
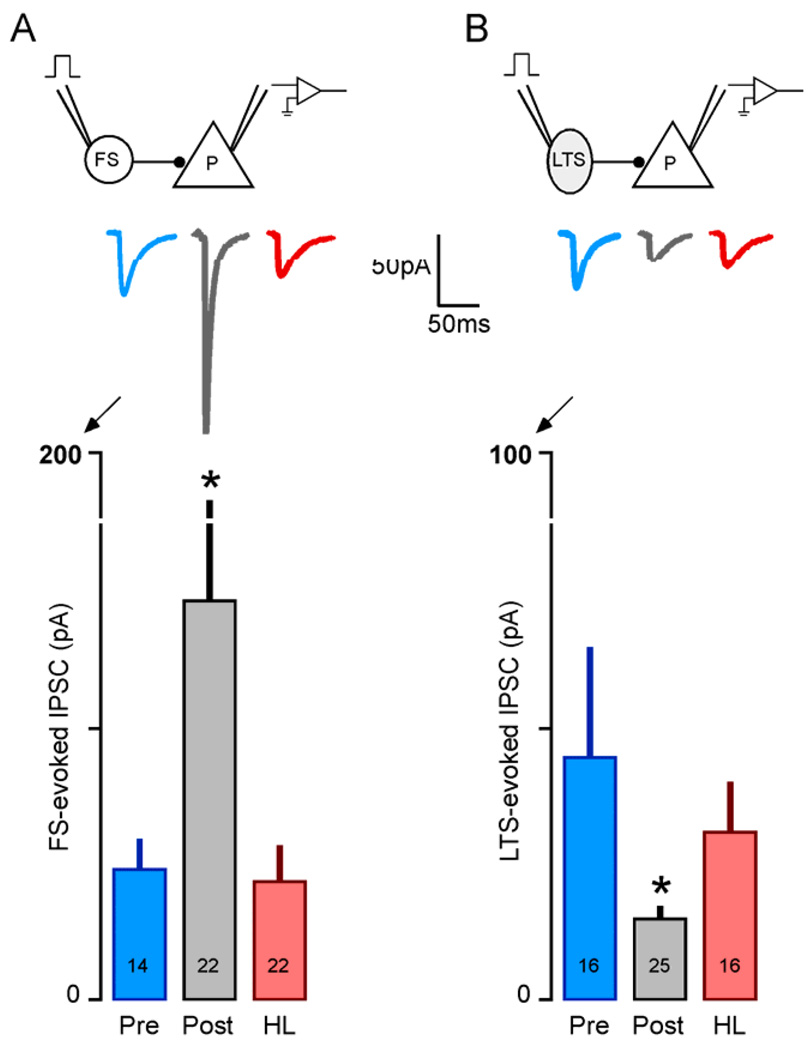
To assess the maturation of specific inhibitory connections in ACx, whole cell recordings were obtained pairs of neurons: either presyanaptic FS and postsynaptic pyramidal neurons (FS→P), or presynaptic LTS and postsynaptic pyramidal neurons (LTS→P). As shown in Figure 2A, the amplitude of FS→P IPSCs increases nearly 3-fold from the pre-to post-hearing period. In contrast, LTS→P connections display a larger IPSC in pre-hearing animals which then declines in amplitude (Figure 2B). For both types of interneurons, the normal developmental trajectory is disrupted by developmental sensorineural hearing loss. The IPSCs displayed by FS→P connections remain small, while those displayed by LTS→P connections remain relatively large (Figure 2, red bars).
Figure 2.
The development and plasticity of FS- and LTS-evoked inhibitory currents. (A) Paired recordings are obtained from fast-spiking (FS) interneurons and Pyramidal cells (P), and evoked IPSCs are measured (top). The bar graph illustrates the significant increase in FS-evoked IPSCs from pre- to post-hearing (asterisk, t-test, p<0.01). However, this change does not occur following developmental sensorineural hearing loss (HL) (p<0.01). Representative IPSCs are shown above each bar. (B) Paired recordings are obtained from low threshold-spiking (LTS) interneurons and Pyramidal cells (P), and evoked IPSCs are measured (top). The bar graph illustrates the significant decline in LTS-evoked IPSCs from pre- to post-hearing (asterisk, t-test, p<0.001). However, this change does not occur following HL (p<0.05). Representative IPSCs are shown above each bar. Note the difference between the y-axis scale (arrows). Number of recordings are shown within each bar. Adapted from Takesian et al., 2010.
A recent developmental study reports that IPSP amplitude at FS→P connections declines after about P18 in layer 4 of mouse ACx. This apparent decrease of inhibitory strength seems to contrast with our finding on FS-evoked inhibitory currents (Figure 2A). A possible explanation is that postsynaptic input resistance declines during this period, and would be expected to affect IPSPs recorded in current clamp mode (Oswald and Reyes, 2010). In addition, there are sub-types of fast-spiking interneurons, and it is conceivable that the recordings from gerbils and mice were obtained from a different subpopulation of cells (Markram et al., 2004). Thus, it is possible that our recordings from layer 2/3 pyramidal neurons sampled a different subpopulation of inhibitory synapses as compared to those recorded from mouse layer 4 neurons. In vivo whole-cell voltage-clamp recordings obtained from rat ACx neurons indicate that the strength of sound-evoked inhibitory synaptic currents responses change little between the onset of hearing and adulthood, although the tuning properties do differ with age (Sun et al., 2010; Dorrn et al., 2010; see Froemke et al, this issue). Although our data does suggest a dramatic increase in FS→P strength, it is possible that this could occur soon after the onset of hearing (Figure 2B). However, the strength of another type of inhibitory connection (LTS→P) declines, suggesting that tone-evoked responses may not reflect any single type of inhibitory connection (much as is the case for our sIPSC recordings).
The downregulation of ACx GABAergic transmission is consistent with findings from other sensory systems. For example, weakened connections between FS interneurons and pyramidal neurons are observed in the visual cortex of animals raised with monocular deprivation (Maffei et al., 2004). Similarly, in the somatosensory cortex, deprivation by whisker trimming during a developmental period leads to a downregulation of GABAA receptors (Fuchs and Salazar, 1998; Bartley et al., 2008). Since FS cells are endowed with fast membrane and spiking properties, they are likely to provide robust feed-forward inhibition to their targets to control spike timing (Swadlow et al., 1998; Gabernet et al., 2005, Cruikshank et al., 2007). Thus, reduced gain of the FS-evoked inhibition is a general outcome of sensory deprivation, and the resulting enhanced excitability may have deleterious consequences for time-critical features of sensory processing.
Developmental SNHL leads to comparable changes in the strength inhibitory synapses in the gerbil inferior colliculus (IC), along with increases excitatory strength (Vale and Sanes, 2000; Vale and Sanes, 2002). If compensatory mechanisms of this sort raise excitability in the IC, then one might have expected that sufficient activation would reach ACx synapses, resulting in their stability during hearing loss. An important mitigating factors to consider is that persistent hearing loss decreases the amount of sound-driven activity; only cellular changes that increase spontaneous activity should be capable of rescuing normal levels of activity. The existing evidence suggests that hearing loss tends to reduce both spontaneous and sound-driven activity in the CNS (Bock and Webster, 1974; Koerber et al., 1966; Born et al., 1991; Shepherd et al., 1999; Tucci et al., 1999; Tucci et al., 2001; Lee et al., 2001; Cook et al., 2002; Yu et al., 2005). Therefore, the changes observed in ACx probably reflect a net reduction in activity throughout the nervous system. The hearing loss-induced cellular changes in IC and ACx are expected to be cumulative, and may contribute to processing deficits (discussed below).
Developmental plasticity of inhibitory kinetics
Functional characteristics of GABAergic synaptic currents, such as their kinetic properties, are associated with the specific subunit composition of the heteropentameric GABAA receptor (Tia et al. 1996; Nusser et al. 1997, Vicini et al. 2001). Therefore, we examined the developmental trajectory of IPSC kinetics in ACx, and asked whether these properties were affected by hearing loss. When sIPSC are recorded from gerbil layers 2/3 pyramidal cells, they display a broad range of durations (Figure 3A, left). However, there is consistent transition with age, such that average duration declines by about 30% during the transition between pre- and post-hearing (Figure 3A, right). A similar observation can be made on minimum-evoked IPSCs (Kotak et al., 2008).
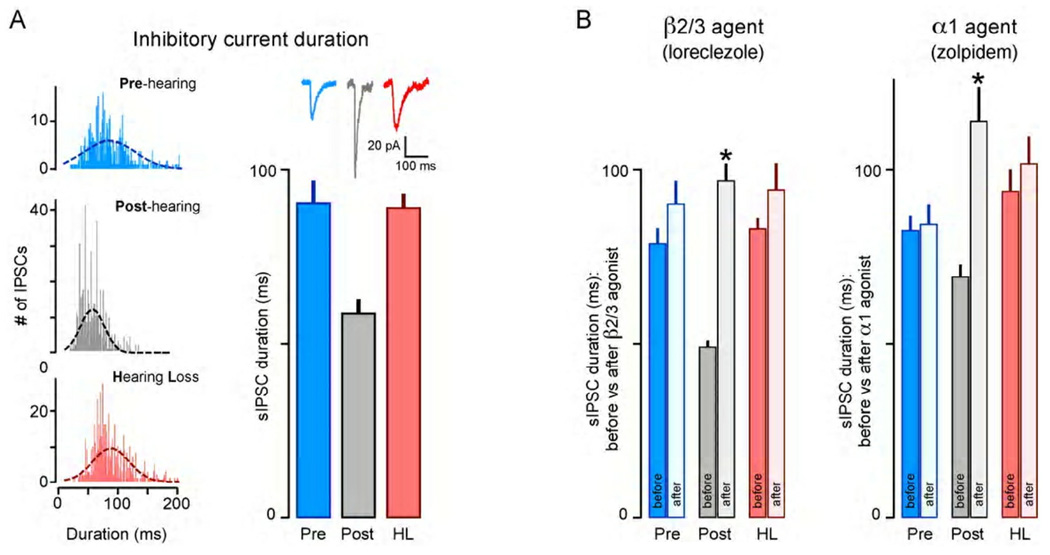
Figure 3.
Development and plasticity of inhibitory synaptic kinetics. (A) The duration of sIPSCs are plotted as histograms for 3 individual neurons from pre-hearing, post-hearing, and hearing loss animals (left). A normal fit of each distribution is shown on the histogram. The summary bar graph shows the average (±SEM) duration of sIPSCs recorded in each of the 3 groups, with representative sIPSCs above each bar (right). The sIPSC duration declines significantly from pre- to post-hearing, but this does not occur following developmental sensorineural hearing loss (HL). (B) The change in sIPSC duration in response to a β2/3-specific agent (loreclezole) is shown for neurons in each of the 3 groups (left). Filled bars indicate sIPSC duration before drug, and open bars indicate the duration after drug application. Only post-hearing neurons displayed a loreclezole-dependent increase in sIPSC duration (asterisk). A similar result is obtained for an α1-specific agent (zolpidem) (right). Adapted from Kotak et al., 2008.
The developmental transition in IPSC kinetics could be explained by changes to the GABAA receptor subunit composition. This was tested pharmacologically with agents that selectively bind to GABAA receptors that contain a specific subunit, and augment their response. Therefore, selective drugs were used to test for the presence of α1 or β2/3 subunits. As shown in Figure 3B (left), a β2/3-specific agent (loreclezole) has no effect on sIPSC duration in neurons from pre-hearing animals, but increases duration by almost 100% in post-hearing neurons. In a similar fashion, an α1-specific agent (zolpidem) is only effective in post-hearing animals in that it also prolongs sIPSC duration (Figure 3B, right).
Sensorineural hearing loss prevents the normal reduction in IPSC duration and the associated functional change in GABAA receptors. Figure 3A shows that the duration of sIPSCs recorded from animals with hearing loss, recorded at the same age as the control post-hearing group, is not significantly different than that displayed by the pre-hearing group. Similarly, the β2/3-and α1-specific agonists did not change the duration of sIPSCs recorded from animals with hearing loss (Figure 3B). Together, these results suggest that GABAA receptors fail to undergo normal maturation when deprived of activity. The maturation of IPSC kinetics also diverges for the two interneuron classes. FS-evoked IPSCs acquire faster rise times during development, and hearing loss prevents this change; in contrast, the LTS-evoked IPSCs do not become faster (Takesian et al., 2010). This data demonstrate a dissimilar pattern of development for the two types of interneurons. Furthermore, the results from paired recordings suggest that conclusions drawn from spontaneous IPSCs (Figure 3A) are likely to be due to the FS connections.
The latencies, rise, peak, and decay constant of FS-evoked IPSPs recorded in mouse layer 4 decline after about P18 (Oswald and Reyes, 2010), and this is generally consistent with our findings on spontaneous and evoked currents. Our results are also consistent with studies showing profound changes in developmental expression of specific subunits that correlate with the developmental modification in IPSC kinetics (Laurie et al., 1992; Vicini et al., 2001; Banks et al., 2002; Mody and Pearce, 2004; Bosman et al., 2005; Huntsman and Huguenard, 2006; Ing and Poulter, 2007). Therefore, these studies strongly imply that the decreased amplitude and prolonged kintetics of inhibition are partly due to an arrest in the normal development of GABAA receptor subunit expression.
Subcellular localization of GABAA subunit and GAD reflect functional changes
The electrophysiological results indicate that inhibition becomes weaker, particularly at FS→P connections, following developmental hearing loss. Moreover, the ineffectiveness of α1 or β2/3 subunit-specific agonists (Figure 3B) suggests that GABAA receptor expression or sub-cellular localization has been disrupted. Since both β2 and β3 play a role in agonist sensitivity by forming the binding sites for GABA and its potentiator, loreclezole (for review see, Möhler 2006), we examined the quantitative distribution of an antibody directed against the β2/3 subunit in ACx tissue from control and age-matched hearing loss animals. Using an electron microscopic immunocytochemical procedure, silver-intensified colloidal gold (SIG) particles were localized to specific membranous or intracellular compartments at symmetric synapses.
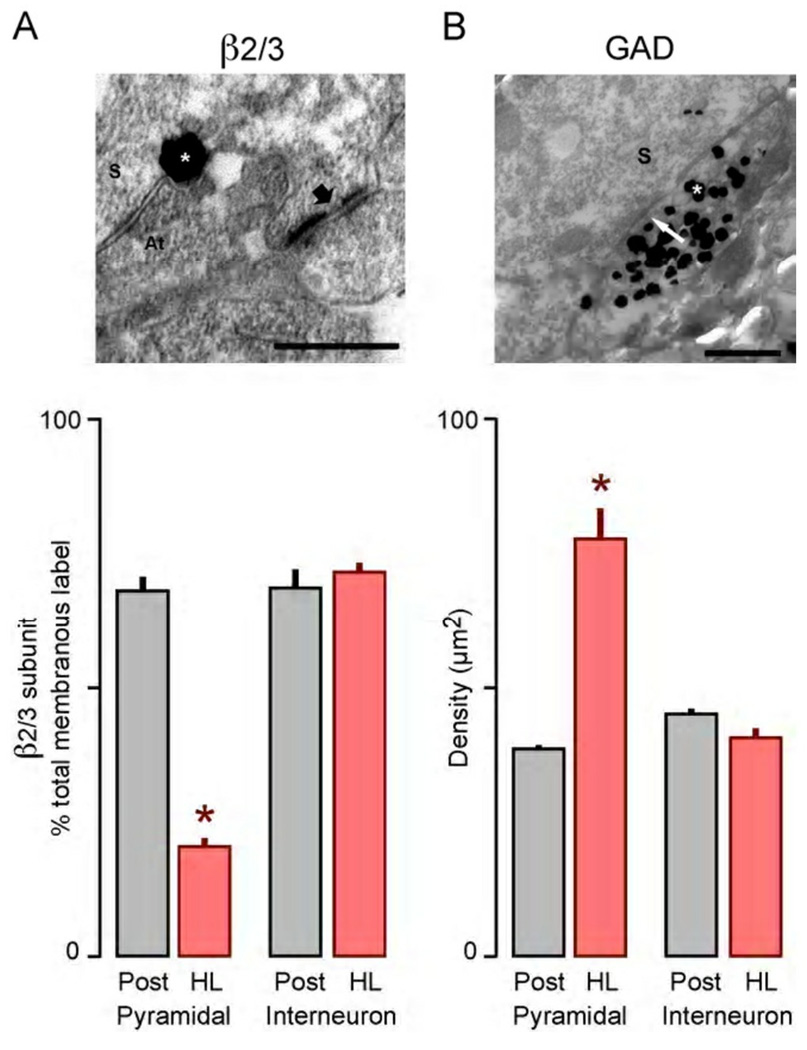
The β2/3 labeling of postsynaptic membrane was reduced by more than 50% following developmental hearing loss (Sarro et al., 2008). However, there was no significant difference in the total frequency of immunoreactivity encountered per unit area. Therefore, hearing loss apparently leads to failed trafficking of the β2/3 subunit to the synaptic membrane. When the analysis was restricted to neuronal somata that could be characterized as either pyramidal or interneuronal (presumed GABAeric interneurons), only the pyramidal neurons displayed a loss of membranous β2/3 (Figure 4A). This suggests that inhibitory synapses to the excitatory pyramidal cells become selectively weakened. Furthermore, the results lead to the prediction that inhibitory synapses onto inhibitory interneurons would not be weakened by hearing loss.
Figure 4.
Sensorineural hearing loss-induced changes to the localization of GABAA receptors and GAD in ACx. (A) Electron micrograph of GABAA β2/3 immunolabeling on the plasma membrane, as revealed by the SIG procedure (top). In this example from control tissue, a cluster of SIG particles (white asterisk) appears at the junction between 2 plasma membranes forming a symmetric synapse between an axon terminal (At) and a soma (S). A nearby asymmetric synapse, presumed to be excitatory, is unlabeled (arrow). The histogram (bottom), illustrates that the percentage of membranous β2/3 subunits was significantly lower (asterisk) on pyramidal neuron somata following hearing loss (HL), but this change did not occur at interneurons. (B) Electron micrograph of GAD65/67 immunolabeling, as revealed by the SIG procedure (top). In this example from HL tissue, SIG particles (white asterisk) are found within inhibitory axon profiles forming symmetric synapses, presumed to be inhibitory, onto adjacent somata (S). White arrow denotes the active zone. The histogram (bottom), illustrates that the density of GAD was significantly higher in axons targeting pyramidal neuron somata (asterisk) following hearing loss (HL), but this change did not occur at interneuronal somata. Scale bars are 500 nm. Adapted from Sarro et al., 2008.
Electrophysiological findings suggest that changes to the presynaptic terminal also occur following hearing loss (Kotak et al., 2008; Takesian et al., 2010). Therefore, the electron microscopic immunocytochemical procedure was used to localize the rate-limiting enzyme for GABA synthesis, glutamic acid decarboxylase (GAD). There was a net increase in GAD localized at ACx inhibitory terminals (Sarro et al., 2008). When the analysis was restricted to terminals on pyramidal versus interneuronal somata, the change was once again found to be restricted to excitatory pyramidal cells (Figure 4B). This up-regulation may reflect a compensatory response to decreased postsynaptic efficacy due to loss of postsynaptic GABAA receptors. This finding is correlated with an increased frequency of sIPSCs following hearing loss, which also indicates a compensatory increase in GABA release (Kotak et al., 2008). In summary, whereas hearing loss disrupts the pre- and postsynaptic GABAergic proteins at excitatory pyramidal neurons, this does not occur at interneurons.
Metaplasticity at ACx inhibitory synapses
Each synapse has a history of activity which can establish its current capacity to change with use, a phenomenon referred to as metaplasticity (Abraham and Bear, 1996). While this principle has received broad support from studies on excitatory connections (Ehlers, 2003; Slutsky et al., 2004; Thiagarajan et al., 2007; Mockett and Hulme, 2008), it is clearly operational for inhibitory connections in the auditory cortex. Here we describe two examples of inhibitory synapse metaplasticity: the first considers alterations to short-term depression, and the second considers long-term potentiation.
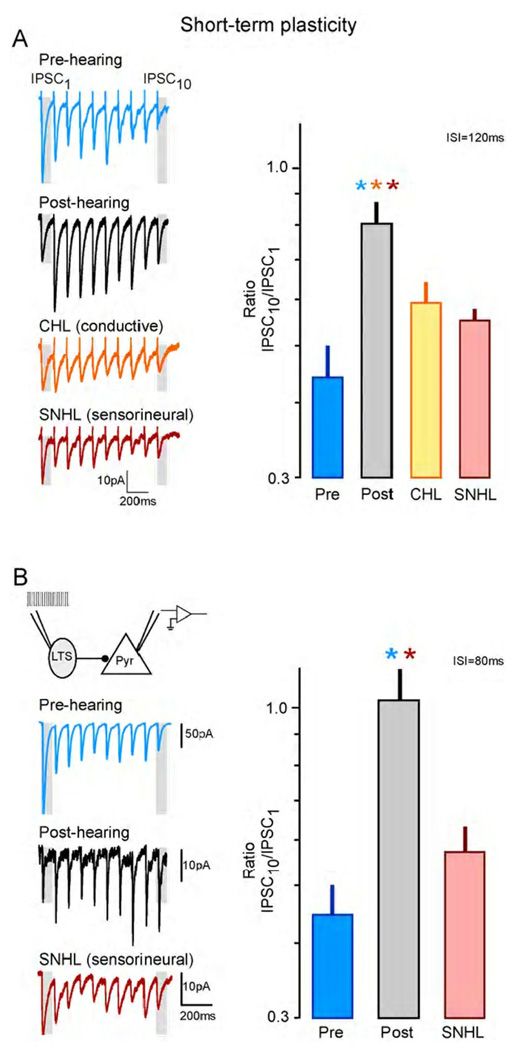
The short-term plasticity of inhibitory connections was initially examined by recording from layer 2/3 pyramidal neurons while stimulating local inhibitory afferents in the presence of ionotropic glutamate receptor antagonists (Takesian et al., 2010). As shown in Figure 5A, trains of stimuli elicit IPSCs that display short-term depression (STD) in pre-hearing neurons, but little depression is observed post-hearing. However, both conductive (CHL) and sensorineural (SNHL) hearing loss prevent this form of short-term plasticity from emerging. To clarify whether these characteristics apply to all ACx inhibitory synapses impinging upon L2/3 pyramidal cells, paired recordings were examined. Here, we found that the LTS→P synapses were responsible for the phenotype. LTS connections display a reduction of STD during normal development, but fail to undergo this transition following hearing loss (Figure 5B). The cellular basis for this short-term plasticity is mediated primarily by presynaptic GABAB receptors. These receptors decline during normal development, leading to the decline in depression, but remain following hearing loss (Takesian et al., 2010)
Figure 5.
Both conductive (CHL) and sensorineural (SNHL) hearing loss disrupt inhibitory short-term plasticity. (A) Representative recordings obtained from pyramidal neurons in response to brief trains of stimuli delivered locally (left), show that normal pre-hearing neurons display short-term depression which is reduced in post-hearing neurons. However, the short-term depression remains following developmental CHL or SNHL. The bar graph (right) plots the ratio of the 10th to the 1st IPSC in the train (highlighted with gray bars in each example trace), as a measure of short-term depression. There is a significant increase in synaptic depression (i.e., reduction in the ratio) for neurons from animals with either form of developmental hearing loss. The interstimulus interval for this data is 120ms. (B) Representative responses from paired LTS-pyramidal cell recordings in pre-hearing, post-hearing, and SNHL neurons (left). The bar graph (right) plots the ratio of the 10th to the 1st IPSC in the train (highlighted with gray bars in each example trace), as a measure of short-term depression. There is a significant decrease of inhibitory short-term depression (i.e., increase in the ratio) at LTS synapses during normal development (pre- to post-hearing), but this change is not observed following developmental SNHL. The interstimulus interval for this data is 80ms. Adapted from Takesian et al., 2010.
The two major categories of interneurons display seemingly different responses to auditory deprivation: FS-evoked inhibition displays a reduction in basal synaptic strength while basal LTS-evoked inhibition is unaltered, yet displays greater short-term depression (i.e., becomes weaker with use). Such dissimilar reactions may be related to the roles played by each interneuron type during stimulus processing. As discussed above, FS cells provide fast feed-forward inhibition to their targets at stimulus onset. Therefore, in the deprived brain, it would be necessary to decrease basal FS-evoked IPSCs to maximize the likelihood that an excitatory input would activate a postsynaptic pyramidal neuron at stimulus onset. In contrast, LTS cells respond weakly to initial thalamic activity, but provide slow, temporally integrated suppression of postsynaptic spiking during sustained stimulation (Beierlein et al., 2003, Tan et al., 2008). In the deprived brain, it would be necessary to decrease LTS-evoked IPSCs after stimulus onset to preserve a postsynaptic response to sustained stimuli. Thus, each system undergoes an appropriate homeostatic change to increase stimulus driven activity.
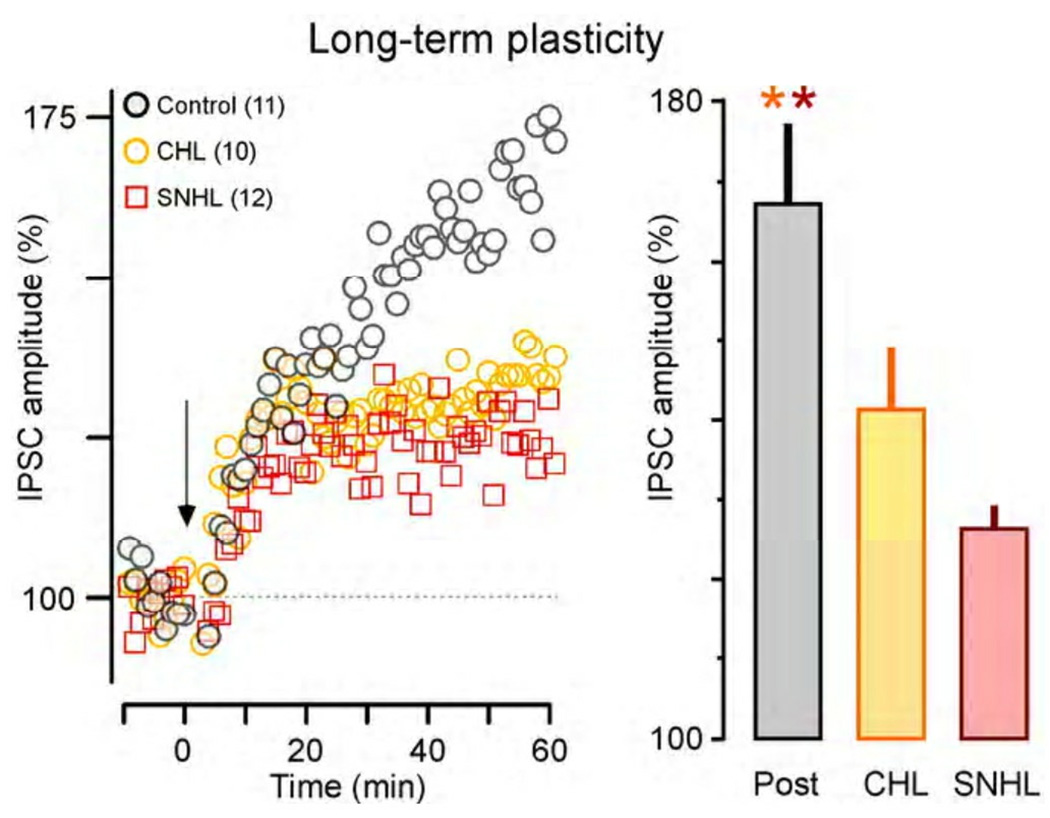
Inhibitory synapses, including those in ACx, can also display long-term adjustment in their gain. As shown in Figure 6, the IPSCs recorded in normal post-hearing pyramidal neurons display long-term potentiation (LTP) following the application of conditioning stimuli (Xu et al., 2010). This form of plasticity depends on the brain-derived neurotrophic factor (BDNF)-TrkB signaling pathway which has been shown to influence the maturation of cortical inhibitory synapses (Huang et al., 1999; Kohara et al., 2007; Abidin et al., 2008; Hong et al., 2008). When spontaneous and/or sound-evoked activity is reduced during development, through either SNHL or CHL, there is a significant reduction of inhibitory LTP (Figure 6). This appears to be due to a loss of available BDNF (Xu et al., 2010), indicating that deprivation may affect function and plasticity via the same pathway. Together, these findings indicate that a history of activity is required for the normal emergence of both short- and long-term inhibitory synaptic plasticity.
Figure 6.
Both conductive (CHL) and sensorineural (SNHL) hearing loss disrupt inhibitory long-term plasticity in the ACx. Recordings were obtained from layers 2/3 pyramidal neurons in response to stimuli delivered in layer 4, and the change in IPSC amplitude was monitored over 70 mins (left). Following a series of conditioning stimuli (arrow), control neurons from post-hearing animals displayed long-term potentiation (black circles). In contrast, neurons from CHL and SNHL animals displayed less potentiation. The bar graph (right) plots the final percent increase in IPSC amplitude recorded at 50–60 mins post conditioning, and shows that there was significantly less potentiation for both forms of developmental hearing loss (asterisks). Adapted from Xu et al., 2010.
Comparison to other systems
The profound changes in synaptic function associated with developmental hearing loss are consistent with a compensatory homeostatic response, similar to that observed in many other systems (Marder and Prinz, 2002; Turrigiano, 2007). In this regard, we have previously discussed the relationship between homeostatic mechanisms observed in auditory cortex and those observed in other cortices or the auditory brainstem (Takesian et al., 2009; Sanes et al., 2009). While many of these studies suggest that neuron excitability is increased by hearing loss, due in part to reduced inhibition, it must be emphasized that there is a great diversity of mechanisms. For example, the efficacy of neuromodulators can be effected. In control animals, serotonin decreases layer 2/3 pyramidal neuron excitability, but this effect is lost following developmental hearing loss (Rao et al., 2010).
Several in vivo findings from animals with hearing can be explained as resulting from a decline in synaptic inhibition, including those that show a significant increase in excitability (Qiu et al., 2000; Kral et al., 2000; Noreña et al., 2003; Seki and Eggermont, 2003). For example, when the cochlea is activated directly with stimulating electrodes in vivo, the threshold current is reported to be lower (i.e. better) in animals that have hearing loss as compared to controls (Raggio and Schreiner, 1999: Fallon et al., 2008). While these findings will certainly not apply universally to all inhibitory cell types along the auditory neuraxis, let alone the auditory cortex, they do provide an explicit, plausible hypothesis that can be tested in vivo in future experiments.
Theoretical implications for auditory processing
Deafness-induced changes to inhibitory synaptic physiology raise a set of practical questions: Do these cellular changes impact auditory perception and, if so, do they result in diminished performance. Although purely speculative, subjects with hearing loss display certain perceptual characteristics that are consistent with a loss of inhibitory synaptic strength. For example, when tested at low sound levels, humans with certain forms of hearing loss display greater sensitivity to amplitude modulation as compared to control subjects (Formby, 1987; Glasberg and Moore, 1990; Bacon and Gleitman, 1992). In a similar fashion, the subjective response to the intensity of a sound (i.e., loudness) near threshold is apparently greater for subjects with hearing loss (Buus and Florentine, 2002). Behavioral findings from an avian species with hereditary high frequency hearing loss also suggest that intensity discrimination is somewhat better near threshold (Lauer et al. 2007). Temporal integration time is another characteristic that differs being significantly smaller in subjects with hearing loss as compared to and controls (Florentine et al., 1988; Lauer et al. 2007). While many explanations for these results are possible (e.g., Neubauer and Heil, 2004), one plausible idea is that the weak inhibitory connections result in a relatively large response to small changes in level near threshold. Furthermore, the greater amount of short-term depression of excitatory synapses that attends CHL (Xu et al., 2007) may limit the sustained response to longer and louder sounds. Ultimately, an uncontrolled neural response at stimulus onset would be particularly disadvantageous in listening environments that include multiple sources and reverberation. These ideas have not yet been evaluated experimentally for cases of developmental hearing loss, but it is worth noting that gap detection thresholds are elevated in aging gerbils, but normal performance can be rescued with a drug that elevates GABA levels (Gleich et al., 2003).
Summary
The decrease of inhibitory function that attends sensory deprivation is now a well-established principle. In the auditory brainstem, this has been demonstrated with anatomical, molecular, and elecrophysiological measures (Takesian et al., 2009). In this review, we have summarized the evidence showing that changes ACx inhibitory transmission exacerbate this outcome, rather than compensating for reduced inhibition in the brainstem. This is not surprising for experimental SNHL because the system receives no cochlear drive; thus, adjustments to inhibitory synaptic strength can not amplify an input signal. In contrast, experimental CHL leaves the cochlea intact, spontaneously active, and able to respond to sound, albeit at a higher threshold. In this case, the dramatic reduction of brainstem inhibition might be expected to increase the gain of excitatory afferent drive to the ACx. Nonetheless, our findings indicate that ACx inhibitory synapses respond in a similar fashion to CHL and SNHL (Figures 5 and 6), and probably contribute to elevating ACx excitability (Kotak et al., 2005).
The normal development and plasticity of ACx inhibition can not be described with a single rule. Rather, the dynamic nature of inhibitory synapse function depends both on the specific type of pre- and postsynaptic neuron. Paired recordings demonstrate that the strength of FS-evoked IPSCs display a dramatic increase during development that is largely absent following hearing loss, whereas LTS cells exhibit the opposite phenotype (Figure 2). With respect to short-term plasticity, it is the LTS neurons that display the greatest plasticity (Figure 5). While these conclusions are valid for postsynaptic pyramidal neurons, quantitative EM results indicate that the rules are different for postsynaptic interneurons, where hearing loss does not lead to a reduction of membranous GABAA receptors. Finally, since our analyses have been restricted to the supragranular region, it remains possible that an entirely different set of rules operate amongst infragranular neurons.
Developmental hearing loss generally originates in the periphery, yet causes a pervasive impairment of CNS synapses and membrane properties. In particular, it has been suggested that inhibitory synapse dysfunction is a causative factor in many disorders, including hearing loss during early development or aging (for reviews, see Caspary et al., 2008; Sanes et al., 2009). These central changes could explain, in part, why children who experience even a transient loss of hearing can suffer long-term behavioral deficits, and perhaps why aging listeners with relatively normal thresholds can experience diminished speech comprehension. In this context, it is interesting to note that improvements in central processing and perception are correlated with stronger GABAergic transmission (Gleich et al., 2003; Leventhal et al., 2003; Edden et al., 2009). Thus, two fundamental predictions that emerge from the findings discussed above are that specific perceptual deficits will, eventually, be explained by ACx inhibitory deficits, and that rescuing normal inhibitory transmission will restore at least some degree of normal perceptual performance.
Acknowledgments
Supported by DC006864 and DC011284 (DHS and VCK)
Abbreviations
- ACx
auditory cortex
- BDNF
brain-derived neurotrophic factor
- CHL
conductive hearing loss
- FS
fast-spiking
- GABA
gamma-aminobutyric acid
- GAD
glutamic acid decarboxylase
- IC
inferior colliculus
- IPSC
inhibitory postsynaptic current
- IPSP
inhibitory postsynaptic potential
- LTP
long-term potentiation
- LTS
low threshold spiking
- P
pyramidal neuron
- SIG
silver-intensified colloidal gold
- sIPSC
spontaneous inhibitory synaptic currents
- SNHL
sensorineural hearing loss
- STD
short-term depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Abidin I, Eysel UT, Lessmann V, Mittmann T. Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. J Physiol. 2008;586:1885–1901. doi: 10.1113/jphysiol.2007.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE. Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons. J Neurosci. 2008;28:3897–3910. doi: 10.1523/JNEUROSCI.5366-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SP, Gleitman RM. Modulation detection in subjects with relatively flat hearing losses. J Speech Hear Res. 1992;35:642–653. doi: 10.1044/jshr.3503.642. [DOI] [PubMed] [Google Scholar]
- Banks MI, Hardie JB, Pearce RA. Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- Bartley AF, Huang ZJ, Huber KM, Gibson JR. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J Neurophysiol. 2008;100:1983–1994. doi: 10.1152/jn.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Bock GR, Webster WR. Spontaneous activity of single units in the inferior colliculus of anesthetized and unanesthetized cats. Brain Res. 1974;76:150–154. doi: 10.1016/0006-8993(74)90521-6. [DOI] [PubMed] [Google Scholar]
- Born DE, Durham D, Rubel EW. Afferent influences on brainstem auditory nuclei of the chick: nucleus magnocellularis neuronal activity following cochlea removal. Brain Res. 1991;557:37–47. doi: 10.1016/0006-8993(91)90113-a. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Heinen K, Spijker S, Brussaard AB. Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J Neurophysiol. 2005;94:338–346. doi: 10.1152/jn.00084.2005. [DOI] [PubMed] [Google Scholar]
- Budinger E, Heil P, Scheich H. Functional organization of auditory cortex in the Mongolian gerbil (Meriones unguiculatus). III. Anatomical subdivisions and corticocortical connections. Eur J Neurosci. 2000;12:2425–2451. doi: 10.1046/j.1460-9568.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- Budinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: connections of the primary auditory cortical field with other sensory systems. Neuroscience. 2006;143:1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Buus S, Florentine M. ‘Growth of loudness in listeners with cochlear hearing losses: Recruitment reconsidered. J Assoc Res Otolaryngol. 2000;3:120–139. doi: 10.1007/s101620010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Rajan R, Irvine DR. Rapid changes in the frequency tuning of neurons in cat auditory cortex resulting from pure-tone-induced temporary threshold shift. Neuroscience. 1993;55:953–964. doi: 10.1016/0306-4522(93)90310-c. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol. 1989;61:747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U.S.A. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QC, Jen PH. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear. Res. 2000;150:161–174. doi: 10.1016/s0378-5955(00)00197-0. [DOI] [PubMed] [Google Scholar]
- Cruikshank SI, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- Cook RD, Hung TY, Miller RL, Smith DW, Tucci DL. Effects of conductive hearing loss on auditory nerve activity in gerbil. Hear Res. 2002;164:127–137. doi: 10.1016/s0378-5955(01)00424-5. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10(4):462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: Morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang J. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DR, Shepherd RK. Cochlear implants and brain plasticity. Hear Res. 2008;238:110–117. doi: 10.1016/j.heares.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentine M, Fastl H, Buus S. Temporal integration in normal hearing, cochlear impairment, and impairment simulated by masking. J Acoust Soc Am. 1988;84:195–203. doi: 10.1121/1.396964. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kössl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J. Assoc. Res. Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formby C. Modulation threshold functions for chronically impaired Meniere patients. Audiology. 1987;26:89–102. doi: 10.3109/00206098709078410. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Salazar E. Effects of whisker trimming on GABA(A) receptor binding in the barrel cortex of developing and adult rats. J Comp Neurol. 1998;395:209–216. [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48(2):165–166. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ. Psychoacoustic abilities of subjects with unilateral and bilateral cochlear hearing impairments and their relationship to the ability to understand speech. Scand Audiol. 1990 Suppl 32 [PubMed] [Google Scholar]
- Gleich O, Hamann I, Klump GM, Kittel M, Strutz J. Boosting GABA improves impaired auditory temporal resolution in the gerbil. Neuroreport. 2003;14:1877–1880. doi: 10.1097/00001756-200310060-00024. [DOI] [PubMed] [Google Scholar]
- Gleich O, Hamann I, Kittel MC, Klump GM, Strutz J. A quantitative analysis of psychometric functions for different auditory tasks in gerbils. Hear Res. 2006;220:27–37. doi: 10.1016/j.heares.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of BDNF transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Fast IPSCs in rat thalamic reticular nucleus require the GABAA receptor beta1 subunit. J Physiol. 2006;572:459–475. doi: 10.1113/jphysiol.2006.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing T, Poulter MO. Diversity of GABA(A) receptor synaptic currents on individual pyramidal cortical neurons. Eur J Neurosci. 2007;25:723–734. doi: 10.1111/j.1460-9568.2007.05331.x. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J. Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2000;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Eggermont JJ. Effects of acute pure tone induced hearing loss on response properties in three auditory cortical fields in cat. Hear Res. 1999;135:146–162. doi: 10.1016/s0378-5955(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Koerber KC, Pfeiffer RR, Warr WB, Kiang NY. Spontaneous spike discharges from single units in the cochlear nucleus after destruction of the cochlea. Exp Neurol. 1966;16:119–130. doi: 10.1016/0014-4886(66)90091-4. [DOI] [PubMed] [Google Scholar]
- Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer specific manner. Cereb Cortex. 2000;10:714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Dooling RJ, Leek MR, Poling KP. Detection and discrimination of simple and complex sounds by Belgian Waterslager canaries. J Acoust Soc Am. 2008;122:3615–3627. doi: 10.1121/1.2799482. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci. 2007;10:1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WP, Liu BH, Li YT, Huang ZJ, Zhang LI, Tao HW. Visual representations by cortical somatostatin inhibitory neurons--selective but with weak and delayed responses. J Neurosci. 2010;30:14371–14379. doi: 10.1523/JNEUROSCI.3248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. BioEssays. 2002;24:1145. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Hulme SR. Metaplasticity: new insights through electrophysiological investigations. J Integr Neurosci. 2008;7:315–336. doi: 10.1142/s0219635208001782. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. TINS. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABAA receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Müller CM, Scheich H. Contribution of GABAergic inhibition to the response characteristics of auditory units in the avian forebrain. J. Neurophysiol. 1988;59:1673–1689. doi: 10.1152/jn.1988.59.6.1673. [DOI] [PubMed] [Google Scholar]
- Neubauer H, Heil P. Towards a unifying basis of auditory thresholds: The effects of hearing loss on temporal integration reconsidered. J Assoc Res Otolaryngol. 2004;5:436–458. doi: 10.1007/s10162-004-5031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res. 2000;139:153–171. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation III Activation patterns in short- and long-term deafness. J Neurophysiol. 1999;82:3506–3526. doi: 10.1152/jn.1999.82.6.3506. [DOI] [PubMed] [Google Scholar]
- Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat. Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- Rajan R. Plasticity of excitation and inhibition in the receptive field of primary auditory cortical neurons after limited receptor organ damage. Cereb Cortex. 2001;11:171–182. doi: 10.1093/cercor/11.2.171. [DOI] [PubMed] [Google Scholar]
- Rao D, Basura GJ, Roche J, Daniels S, Mancilla JG, Manis PB. Hearing loss alters serotonergic modulation of intrinsic excitability in auditory cortex. J Neurophysiol. 2010;104:2693–2703. doi: 10.1152/jn.01092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of inhibitory mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurosci. 2007;27:1769–1781. doi: 10.1523/JNEUROSCI.3851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci U.S.A. 2008;105:4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Sarro EC, Takesian AE, Aoki C, Kotak VC. Regulation of inhibitory synapse function in the developing auditory CNS. In: Pallas SL, editor. Developmental Plasticity of Inhibitory Circuitry. New York: Springer-Verlag; 2009. [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABA-A receptors in the auditory cortex. Cereb Cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Wehr M. Disruption of balanced cortical excitation and inhibition by acoustic trauma. J Neurophysiol. 2008;100:646–656. doi: 10.1152/jn.90406.2008. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Baxi JH, Hardie NA. Response of inferior colliculus neurons to electrical stimulation of the auditory nerve in neonatally deafened cats. J Neurophysiol. 1999;82:1363–1380. doi: 10.1152/jn.1999.82.3.1363. [DOI] [PubMed] [Google Scholar]
- Shuz A, Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurol. 1989;286:442–455. doi: 10.1002/cne.902860404. [DOI] [PubMed] [Google Scholar]
- Silberberg G. Polysynaptic subcircuits in the neocortex: spatial and temporal diversity. Curr Opin Neurobiol. 2008;18:332–337. doi: 10.1016/j.conb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron. 2004;44:835–849. doi: 10.1016/j.neuron.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Beloozerova IN, Sirota MG. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J Neurophysiol. 1998;79(2):567–582. doi: 10.1152/jn.1998.79.2.567. [DOI] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu B-H, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc Nat Acad Sci. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VK, Sanes Hearing loss disrupts synaptic inhibition: Implications for auditory processing. Future Neurol. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Tierney TS, Moore DR. Naturally occurring neuron death during postnatal development of the gerbil ventral cochlear nucleus begins at the onset of hearing. J Comp Neurol. 1997;387:421–429. [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D. Conductive hearing loss results in a decrease in central auditory system activity in the young gerbil. Laryngoscope. 1999;109:1359–1371. doi: 10.1097/00005537-199909000-00001. [DOI] [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D. Effects of conductive hearing loss on gerbil central auditory system activity in silence. Hear Res. 2001;155:124–132. doi: 10.1016/s0378-5955(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11(5):1137–1140. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;19:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2003;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. Lateral sharpening of cortical frequency tuning by approximately balance inhibition. Neuron. 2008;58:132–143. doi: 10.1016/j.neuron.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wadghiri YZ, Sanes DH, Turnbull DH. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci. 2005;8:961–968. doi: 10.1038/nn1477. [DOI] [PMC free article] [PubMed] [Google Scholar]