Abstract
Objectives
We investigated the accuracy of high-field proton magnetic resonance spectroscopy (1H-MRS) and fluorine-18 2-fluoro-deoxyglucose positron emission tomography (18F-FDG-PET) for diagnosis of glioma progression following tumor resection, stereotactic radiation and chemotherapy.
Methods
Twelve post-therapy patients with histology proven gliomas (6 grade II and 6 grade III) presented with Magnetic Resonance Imaging (MRI) and clinical symptoms suggestive but not conclusive of progression were entered into the study. 1H-MRS data were acquired and 3D volumetric maps of choline (Cho) over creatine (Cr) were generated. Intensity of 18F-FDG uptake was evaluated on a semiquantitative scale.
Results
The accuracy of 1H-MRS and 18F-FDG-PET imaging for diagnosis of glioma progression was 75% and 83% respectively. Classifying the tumors by grade improved accuracy of 18F-FDG-PET to 100% in high-grade gliomas and accuracy of 1H-MRS to 80% in low-grade tumors. Spearman's analysis demonstrated a trend between 18F-FDG uptake and tumor grading (ρ = 0.612, p-value = 0.272). The results of 18F-FDG-PET and 1H-MRS were concordant in 75% (9/12) of cases.
Conclusion
The combination of 1H-MRS data and 18F-FDG-PET imaging can enhance detection of glioma progression. 1H-MRS imaging was more accurate in low-grade gliomas and 18F-FDG-PET provided better accuracy in high-grade gliomas.
INTRODUCTION
The Central Brain Tumor Registry of the United States estimates that 62,930 new cases of primary non–malignant and malignant brain and central nervous system tumors are expected to be diagnosed in the United States in 2010. Ninety three percent of cases are individuals over 20 years old at the time of diagnosis and gliomas account for 80% of malignant brain tumors1, 2.
Most gliomas are characterized by diffuse infiltration of white matter tracts 3. Stereotactic biopsy studies have shown tumor infiltration in regions that appear normal on conventional contrast enhanced computed tomography (CT) and magnetic resonance imaging (MRI) 4. Eradication of the tumors, particularly higher grade neoplasms, is not usually possible 5. Thus, post-therapy imaging to detect the presence of residual or recurrent tumor is essential to optimize patient management. Assessments of tumor response to therapy are typically performed by visual interpretation of serial MRI examinations. Although such examinations provide useful morphologic information, they are not able to reliably distinguish active tumor from radiation necrosis. Anatomic distortion, scarring, altered vascular permeability and edema after therapy often impair interpretation of conventional CT and contrast enhanced MRI studies.
Metabolite and molecular imaging methods can help overcome this issue. Specific biologic information provided by 1H MRS such as cell membrane turnover 6, cellular density 7 and proliferation 8 as well as, glucose metabolism quantified by 18F-FDG PET imaging 9 may distinguish glioma progression from post-radiation changes.
Although numerous 1H MRS 10-20 and 18F-FDG PET 21-30 studies have been conducted in order to describe metabolite and metabolic patterns of brain tumors, (Table 1), only a few investigations focused on detecting tumor progression by evaluating both 1H MRS and 18F-FDG PET data in the same group of patients 31, 32. While these studies have provided valuable results, their methods could be further improved in several aspects. These studies did not attempt to co-register 18F-FDG PET images with MRI which has been shown to significantly improve sensitivity and specificity of distinguishing recurrent brain tumors from radionecrosis 33. The differences between grades of malignancy were not evaluated. Alger et al. focused on single voxel analysis of 1H MRS. They preferred to employ larger volumes rather than smaller, to maximize the probability of including the most active part of the tumor in the MR spectroscopy. Distinction between necrosis resulting from therapy and tumor regrowth was difficult in this study. Therefore, the statistical measures of the performance were not reported. In recent years, multispectral analysis of 1H MRS data that allows mapping the metabolite concentrations have significantly improved clinical application of this technique 34.
Table 1.
Accuracy of PET and MRS in differentiation of radiation injury from tumor progression
| Author | Year | Histology | N | Sensitivity | Specificity | Accuracy | Parameters | |
|---|---|---|---|---|---|---|---|---|
| MRS | Law | 2003 | High-grade | 43 | 76% | 48% | 69% | Cho/Cr |
| Law | High grade | 160 | 74% | 63% | 71% | Cho/NAA | ||
| Plotkin | 2004 | Glioma | 25 | 89% | 83% | 88% | Cho/Cr | |
| Lichy | 2005 | Glioma | 34 | 81% | 71% | Cho/Cr and Cho/NAA | ||
| Zeng | 2007 | High-grade | 28 | 94% | 100% | 96% | Cho/Cr and Cho/NAA | |
| Prat | 2010 | Glioma | 9 | 100% | 100% | 100% | Cho and NAA | |
| PET | Patronas | 1982 | Low-grade | 5 | 100% | 100% | 100% | |
| Doyle | 1987 | High-grade | 9 | 100% | 100% | 100% | ||
| Di Chiro | 1988 | Glioma | 95 | 100% | 100% | 100% | ||
| Valk | 1988 | Glioma | 38 | 81% | 88% | 86% | ||
| Kim | 1992 | Glioma | 33 | 80% | 94% | 88% | ||
| Janus | 1993 | Glioma | 20 | 83% | 63% | 75% | ||
| Kahn | 1994 | Glioma | 17 | 81% | 40% | |||
| Ricci | 1998 | Glioma | 31 | 86% | 22% | 71% | ||
| Van Laere | 2004 | Glioma | 30 | 80% | 95% | 50% | ||
| Wang | 2006 | Glioma | 129 | 80% | 96% | 87% | ||
| Prat | 2010 | Glioma | 9 | 50% | 75% | 63% |
The main aim of our study was to assess and compare the contribution of 1H MRS and 18F-FDG PET to non-invasively differential grades II and III glioma progression from necrosis. The specific focus of this study was to detect lesions by visual analysis of 1H MRS data presented as 3D maps of metabolites and 18F-FDG PET images co-registered with MRI.
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
Subjects for this study were drawn from a total of 193 patients who were referred from our neurooncology group for a conventional clinical brain MRI during the period from 3/2007 to 3/2009. From this group 53 patients had a history of grade II or grade III glioma resection, stereotactic radiation and chemotherapy. Patients with no evidence of progression (N = 24) and cases demonstrating significant tumor growth were excluded (N = 3) from further consideration, since their clinical status was considered stable or definite progression from the clinical MRI scan and/or symptoms. The remaining patients (n= 26), had clinical symptoms and radiographic findings suspicious but not conclusive for glioma progression, and were referred for an 18F-FDG PET scan. All patients in this group were adult male or female patients older than 20 years.
In western Pennsylvania, third party payer constraints on coverage for MRS limit the use of MRS for brain tumor indications. UPMC Health Plan has followed the recommendations of the Neurooncology Program faculty and provides coverage for patients with primary brain tumors undergoing MRS as part of clinical care. The seventeen patients who had UPMC health insurance were also evaluated by 3T 1H MRS. In most cases the MRS and PET scans were ordered at approximately the same time, and therefore either 1H MRS or 18F-FDG PET could have been performed first and in all cases no patient was excluded on the basis of 1H MRS and 18F-FDG PET findings. Of the total of 17 1H MRS scans, five cases were excluded from data analysis because the time interval between the two studies was longer than one month. Twelve cases (5 men, 7 women; median age at surgery 39; range, 25–70 years) were selected for the study.
A combination of clinical follow-up and multiple sequential MR studies were used for clinical outcome validation. This retrospective study was approved by our Institutional Review Board which did not require signed informed consent from the patients.
Magnetic Resonance Spectroscopy Imaging
MRI and MRS data were acquired on a Siemens (Erlangen, Germany) 3T Magnetom TIM Trio whole-body high-performance scanner. The patients were positioned in the standard radiofrequency (RF) 12-channel head coil. A fast 3D gradient echo sequence (MP-RAGE) was performed using a TE of 2.63ms, TR of 2110ms, inversion time of 1100ms, flip angle of 8 degrees, image matrix 2562, 128 slices 1.5mm thick and a 240×192 field of view, resulting in a scan time of 3.8 min. Parallel acquisition techniques (PAT) factor of 2 was used to decrease the imaging time. These parameters resulted in images optimized for the best contrast among gray matter, white matter, and CSF and to provide high-resolution delineation of cortical and sub-cortical structures.
The 1H MRS data were acquired using a hybrid chemical shift imaging (CSI) sequence with TE of 135ms, TR of 1700 ms, slice thickness of 10mm and three averages over a 160×160mm field of view, resulting in a scan time of 6.8 min. Outer volume suppression pulses were used to suppress subcutaneous lipid signals. The positioning of the CSI slice was dependent on the location of the lesion. An automatic 3D shimming was used to maximize spectral resolution and homogeneity over the volume of interest. Water suppression was performed using the vendor-supplied adjustment employing a special pulse train.
Integrals of resonance signals (areas under the peaks) were obtained after baseline correction and phase correction were applied to all the spectra. This allowed for visualization of the voxel-dependent intensities of metabolites within the defined region of interest. Metabolite images were generated on the scanner console using routines provided by the manufacturer. The CSI slice graphic was displayed as an overlay on the MP-RAGE sequence.
Positron Emission Tomography Method
The patients were instructed to fast for 4–6 hours before the injection of 353–532 MBq (9.5–14.4 mCi) of 18F-FDG. The PET data were acquired on a Siemens/CTI ECAT HR+ scanner in 3D imaging mode (63 parallel planes); axial field-of-view: 15.2 cm; in-plane resolution: 4.1 mm full-width at half-maximum; slice width: 2.4 mm). The scanner gantry was equipped with a Neuro-insert (CTI PET Systems, Knoxville, TN) to reduce the contribution of scattered photon events35. PET data were reconstructed using filtered back-projection (Fourier rebinning and 2D back projection with Hann filter: kernel FWHM = 3 mm). Data were corrected for photon attenuation, scatter, and radioactive decay. A windowed transmission scan (10–15 min) was obtained for attenuation correction using rotating 68Ge/68Ga rods and a model-based correction was applied to account for a 3D scatter fraction36.
Interpretation of MRS and PET Studies
All 1H MRS and 18F-FDG PET images were interpreted by two examiners. One examiner was board certified in radiology and nuclear medicine and the other examiner was board certified in nuclear medicine. The images were reviewed using image analysis and fusion software (MIMvista Corp. Cleveland, OH). The interpreters were unaware of the patients' clinical history and prior imaging studies.
Quantitative analysis of 1H MRS data was performed by placing a region of interest composed of 64 voxels over the tumor visually. Metabolite levels of each voxel (1.6 • 1.6 • 1.6 cm3) were obtained by integration of the area under peaks. The voxel with the highest choline to creatine ratio (Cho/Cr) was selected and compared to the normal contralateral hemisphere. Three-dimensional maps of Cho/Cr were generated on a semiquantitative scale and co-registered with MR images for visual interpretation as illustrated in figure 1. The observers favored tumor progression versus necrosis with use of the following criteria: (a) Presence of spectra with interpretable signal intensities. (b) Cho/Cr signal ratio of the tumor substantially higher than that of the normal contralateral hemisphere. (c) A decrease in the NAA signal intensity. (d) Visible lactate signal in the spectrum.
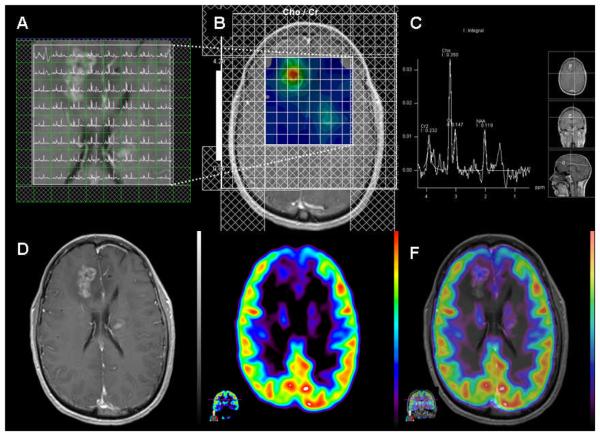
Figure 1.
Anaplastic Astrocytoma, WHO grade III (case 5). A. Multiple-voxel spectra co-registered with post-contrast T1-weighted MRI B. Map of Cho/Cr demonstrates a focus of signal intensity in the right frontal lobe. MRSI signal intensity is presented on a rainbow color scale where blue-green is normal background and bright red corresponds to greatly elevated signal intensity. C. Spectral analysis of the voxel demonstrating maximal Cho/Cr ratio. D. T1-weighted MRI (post-contrast) demonstrating enhancing lesion in the right frontal lobe. E. 18F-FDG PET scan shows a focus of increased tracer activity greater than white matter in the right frontal lobe. F. 18F-FDG PET image co-registered with post-contrast T1-weighted MRI.
PET images were co-registered with MRI to better localize the lesions (Figure 1). 18F-FDG PET studies were considered either positive or negative semi-quantitatively by comparing metabolic activity of the lesions with the normal white and gray matters. Based on visual inspection, the examiners graded the highest activity level of the tumor using the metabolic grading; 0, no discernible uptake; 1, uptake less or equal to normal white matter; 2, uptake greater than normal white matter and less than gray matter; 3, uptake equal to or greater than gray matter37, 38. The examiners favored tumor progression for lesions with metabolic activity of grade 2 and 3.
Both examiners evaluated all 1H MRS and 18F-FDG PET studies independently in the same session. Selected voxels for quantitative analysis were visually examined to represent the same lesion. The examiners agreed on all cases except one MRS study (case 9), which was subsequently discussed and reached consensus.
Composite Reference
Histological verification, which would have been the most accurate reference standard, was not feasible in all cases. Therefore, a combination of follow-up clinical evaluations and multiple sequential MR studies were used as the reference standard to determine the accuracy of 1H MRS and 18F-FDG PET in the diagnosis of progression.
Statistical Analysis
Data were divided into two groups of high-grade and low-grade gliomas. Sensitivity, specificity, and accuracy with 95% confidence interval (CI) were calculated 39-42 for each group as well as the combined data using the composite reference standard. Concordance between 1H MRS and 18F-FDG PET grading was assessed by the McNemar's Exact test. For correlation between 18F-FDG uptake and tumor grading, the Spearman nonparametric correlation coefficient was calculated. The 18F-FDG uptake and Cho/Cr ratio of low-grade and high-grade tumors were compared using the Mann–Whitney nonparametric analysis. A 2-sided p-value of less than 0.05 was considered significant.
RESULTS
Twelve cases were selected for the study. Six patients had glioma grade II and six patients had glioma grade III on the basis of initial histological diagnosis (Table 2). All suspicious cases demonstrated post-contrast enhancement on MRI studies expect one (case 6), who showed progressive increase in fluid-attenuated inversion recovery (FLAIR) signal at the margins of the resection cavity on two consecutive MRI studies. Metabolites and metabolic evaluation of the lesions were performed for all patients within one month. MRS preceded PET in five cases. In another five cases PET preceded MRS and in two cases both studies were performed on the same day (mean and median time intervals from radiation therapy to scan were 18 months and 13 months respectively, range, 42 days–9 years).
Table 2.
Demographic data and history
| No. | Age (years) at Surgery |
Sex | Histology | WHO Grade |
Interval Since Radiation (months) |
PET-MRS Interval (days) * |
Necrosis versus Progression |
|---|---|---|---|---|---|---|---|
| 1 | 49 | M | Anaplastic Astrocytoma | 3 | 10 | 3 | Necrosis |
| 2 | 29 | F | Anaplastic Oligodendroglioma | 3 | 7 | 0 | Necrosis |
| 3 | 45 | M | Anaplastic Astrocytoma | 3 | 111 | 22 | Progression |
| 4 | 29 | M | Anaplastic Astrocytoma | 3 | 1 | 0 | Progression |
| 5 | 30 | F | Oligodendroglioma | 2 | 8 | 19 | Progression |
| 6 | 54 | F | Astrocytoma | 2 | 19 | −14 | Progression |
| 7 | 60 | F | Oligodendroglioma | 2 | 1 | −15 | Progression |
| 8 | 70 | F | Oligodendroglioma | 2 | 2 | 5 | Necrosis |
| 9 | 30 | M | Oligodendroglioma | 2 | 17 | −1 | Necrosis |
| 10 | 25 | F | Astrocytoma | 2 | 14 | −1 | Necrosis |
| 11 | 60 | F | Anaplastic Astrocytoma | 3 | 17 | −7 | Necrosis |
| 12 | 33 | M | Anaplastic Astrocytoma | 3 | 13 | 11 | Necrosis |
Positive numbers demonstrate MRS preceded PET and negative numbers show MRS was performed after PET.
Two patients (cases 1 and 5) had subsequent brain biopsies which revealed glioma progression. The remaining nine patients had a minimum of 12 months follow-up. Sequential imaging studies and clinical assessments demonstrated glioma progression in three of these patients. Seven patients did not show any clinical or radiographic evidence of progression during the observation period.
The sensitivity of 1H MRS for diagnosis of glioma progression was 80% (95% CI, 30% to 99%). 18F-FDG PET demonstrated no false negative findings. Therefore, sensitivity of 18F-FDG PET was 100% (95% CI, 46% to 100%). The specificity of 1H MRS and 18F-FDG PET was 71% (95% CI, 30% to 95%) for both studies. The accuracy of 1H MRS and 18F-FDG PET studies was 75% (95% CI, 43% to 93%) and 83% (95% CI, 51% to 97%), respectively. Classifying the tumors into high and low grade gliomas improved accuracy of 1H MRS studies to 80% (95% CI, 30% to 95%) in low-grade gliomas and accuracy of 18F-FDG PET to 100% (95% CI, 56% to 100%) in high-grade gliomas (Table 3).
Table 3.
Statistical measures of the performance of 18F-FDG PET and 1H MRS
| Visual Analysis | Parameter (%) | All Cases | Low–Grade | High–Grade |
|---|---|---|---|---|
| 18F-FDG PET | Sensitivity | 80 (30–99) | 100 (20–100) | 67 (13–98) |
| Specificity | 71 (30–95) | 67 (13–98) | 75 (22–99) | |
| PPV | 83 (36–99) | 100 (20–100) | 75 (22–99) | |
| NPV | 67 (24–94) | 67 (13–98) | 67 (13–98) | |
| Accuracy | 75 (43–93) | 80 (30–99) | 71 (30–95) | |
| 1H MRS | Sensitivity | 100 (46–100) | 100 (20–100) | 100 (31–100) |
| Specificity | 71 (30–95) | 33 (2–87) | 100 (40–100) | |
| PPV | 100 (46–100) | 100 (5–100) | 100 (40–100) | |
| NPV | 71 (30–95) | 50 (9–91) | 100 (31–100) | |
| Accuracy | 83 (51–97) | 60 (17–93) | 100 (56–100) |
Numbers in parentheses are 95% confidence intervals.
Concordance analysis using McNemar's Exact test did not show statistically significant difference between the two methods (p-value = 0.625). The results of 1H MRS and 18F-FDG PET were concordant in 75% (9/12) of cases (Table 4).
Table 4.
Concordance between PET and MRSI
| MRSI | ||||
|---|---|---|---|---|
| Concordance | Progression | No progression | ||
| PET | Progression | 5 | 2 | Concordance = 75% (9/12) p-value = 0.625 |
| No progression | 1 | 4 | ||
The concordance results are based on the experts' analyses of MRSI and PET studies.
Prior studies reported a positive correlation between metabolic rate of glucose and histological grade of cerebral gliomas before initiation of treatment 42, 43. We obtained similar results in post-therapy patients with recurrent gliomas. There was a trend toward positive correlation between tumor grading and 18F-FDG uptake; however it did not reach statistical significance level (Spearman ρ = 0.612, p = 0.272). No correlation was identified between tumor grading and Cho/Cr.
DISCUSSION
To our knowledge this is the first clinical study to compare 1H MRS data presented in semiquantitative 3D maps of Cho/Cr and 18F-FDG PET imaging to detect glioma progression from necrosis. Radiation necrosis following radiation therapy for treatment of gliomas is not uncommon and has been well described in autopsy and imaging studies 44, 45. Similarities with conventional imaging characteristics of glioma progression, including contrast enhancement, mass effect, and vasogenic edema have confounded differential diagnostic evaluation. Advanced imaging techniques including proton magnetic resonance spectroscopy and positron emission tomography have increased diagnostic accuracy. As illustrated in the figure 1, 1H MRS and 18F-FDG PET offer different information about the tumors. 1H MRS primarily provides a measure of the tissue concentrations of various metabolites, including choline, creatine, N-acetylaspartate and lactate. Choline is the main metabolite that has been assessed in gliomas. Increased choline levels are associated with higher cell membrane turnover and higher cell density 7, arising from the proliferation of tumor cells 8. Creatine plays a role in maintaining energy-dependent systems in cells. It is the most stable cerebral metabolite 46 and is used as internal reference value. An increase in Cho/Cr ratio is suggestive of neoplastic process. In order to facilitate simultaneous visual analysis of multiple-voxel spectra, Cho/Cr maps of the regions of interest were generated as shown in figure 1. The examiners favored tumor progression for the lesions with increased Cho/Cr compared with the contralateral hemisphere. This approach, in contrast to the prior studies that focused on spectrum analysis of a single voxel, can expedite the diagnostic process and provide a more practical approach in the clinical setting. The examiners also evaluated the NAA and lactate resonances. NAA is a marker of neuronal viability and density. It is localized in mature neurons and is not found in glial cells 47, 48. Lactate peak represents anaerobic glycolysis in tumors 34 which has been shown to increase in neoplastic cells 49. A decrease in NAA and a visible lactate peak were also considered as evidence of tumor progression.
18F-FDG PET can measure the rate at which the glucose is consumed. Most cancer cells including gliomas produce energy by a high rate of glycolysis 49, quantifiable by 18F-FDG PET imaging. We co-registered all 18F-FDG PET studies with MRI images which have been shown to significantly improve diagnostic accuracy of distinguishing recurrent brain tumors from radionecrosis 33.
Our results demonstrate that 1H MRS imaging is more accurate in detecting low-grade glioma progression and high-grade gliomas are better detected by 18F-FDG PET. Classifying the tumors by grade improved the accuracy of 1H MRS from 75% to 80% in low-grade gliomas. In addition, accuracy of 18F-FDG PET increased from 83% to 100% in high-grade gliomas (Table 3). While the sensitivity of 18F-FDG PET in detecting glioma progression was very high (100%), its specificity in differentiating post-therapy inflammation from true tumor progression was low (71%), leading to high false positive rate (29%) in post radiation therapy patients.
In our study, 1H MRS and 18F-FDG PET were concordant in 75% (9/12) of patients. In the three discordant cases, 18F-FDG PET imaging confirmed tumor progression at the margins of the resection cavity in a patient with a history of high grade glioma (case 3). PET also correctly showed an absence of abnormal metabolic activity in the region of prior tumor resection in a patient with a history of high-grade glioma. This patient did not show any evidence of progression during more than a two-year follow-up (case 2). In the third discordant case, 1H MRS correctly diagnosed tumor progression in a previously treated low-grade glioma (case 8). Failure in detecting viable tumors on 18F-FDG PET imaging could be due to several factors, including high rate of glucose metabolism by the normal brain tissue, small size of the lesions, and modest increase in glucose metabolism in low-grade gliomas (relative to the background metabolic activity) 28, 43.
This study has several limitations. Histopathologic confirmation of tumor progression was not performed for all cases. Because of the inherent risks and invasiveness of a stereotactic biopsy, it was not always reasonable to obtain histological proof. Only two cases had subsequent tissue biopsy (cases 1 and 5). Sequential morphologic imaging and prolonged clinical follow-up had to be used as surrogate markers for tissue identification in the remainder of patients. Second, we were not able to confirm possibility of high-grade transformation. All data were analyzed based on the patients' last histopathology report. Third limitation is the relatively small number of patients in this study. Finally, the current study did not attempt a comparison with other biomarkers, such as 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) and 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine (18F-FDOPA).
In conclusion, the combination of 1H MRS data and 18F-FDG PET imaging improves the accuracy of distinguishing glioma progression from radionecrosis. 18F-FDG PET was more accurate in identifying tumor progression in high-grade gliomas and Cho/Cr 1H MRS provided better accuracy in low-grade gliomas. Proton MR spectroscopy and 18F-FDG PET appear to be useful complementary tools to conventional MR imaging in detecting brain tumor progression.
ACKNOWLEDGMENTS
This work was support by the US National Institutes of Health research grants R01 CA106840 and U01 CA140230.
The authors thank the technology staff of the University of Pittsburgh Medical Center PET-Cyclotron facility and MR Research Center for expert adherence to PET and MRI scan acquisition protocols.
REFERENCES
- 1.CBTRUS Central Brain Tumor Registry of the United States Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. 2010 [Google Scholar]
- 2.kKleihues P, Cavenee WK, International Agency for Research on Cancer . Pathology and genetics of tumours of the nervous system. IARC Press; Lyon: 2000. [Google Scholar]
- 3.Kelly PJ, Daumas-Duport C, Scheithauer BW, Kall BA, Kispert DB. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987 Jun;62(6):450–459. doi: 10.1016/s0025-6196(12)65470-6. [DOI] [PubMed] [Google Scholar]
- 4.Earnest Ft, Kelly PJ, Scheithauer BW, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology. 1988 Mar;166(3):823–827. doi: 10.1148/radiology.166.3.2829270. [DOI] [PubMed] [Google Scholar]
- 5.Buckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007 Oct;82(10):1271–1286. doi: 10.4065/82.10.1271. [DOI] [PubMed] [Google Scholar]
- 6.Danielsen ER, Ross B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. CRC Press; 1999. [Google Scholar]
- 7.Michaelis T, Merboldt KD, Bruhn H, Hanicke W, Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology. 1993 Apr;187(1):219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- 8.Herminghaus S, Pilatus U, Moller-Hartmann W, et al. Increased choline levels coincide with enhanced proliferative activity of human neuroepithelial brain tumors. NMR Biomed. 2002 Oct;15(6):385–392. doi: 10.1002/nbm.793. [DOI] [PubMed] [Google Scholar]
- 9.Tewson TJ, Welch MJ, Raichle ME. [18F]-labeled 3-deoxy-3-fluoro-D-glucose: synthesis and preliminary biodistribution data. J Nucl Med. 1978 Dec;19(12):1339–1345. [PubMed] [Google Scholar]
- 10.Senft C, Hattingen E, Pilatus U, et al. Diagnostic value of proton magnetic resonance spectroscopy in the noninvasive grading of solid gliomas: comparison of maximum and mean choline values. Neurosurgery. 2009 Nov;65(5):908–913. doi: 10.1227/01.NEU.0000356982.82378.BA. discussion 913. [DOI] [PubMed] [Google Scholar]
- 11.Fayed N, Davila J, Medrano J, Olmos S. Malignancy assessment of brain tumours with magnetic resonance spectroscopy and dynamic susceptibility contrast MRI. Eur J Radiol. 2008 Sep;67(3):427–433. doi: 10.1016/j.ejrad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Zonari P, Baraldi P, Crisi G. Multimodal MRI in the characterization of glial neoplasms: the combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology. 2007 Oct;49(10):795–803. doi: 10.1007/s00234-007-0253-x. [DOI] [PubMed] [Google Scholar]
- 13.Zeng QS, Li CF, Zhang K, Liu H, Kang XS, Zhen JH. Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neurooncol. 2007 Aug;84(1):63–69. doi: 10.1007/s11060-007-9341-3. [DOI] [PubMed] [Google Scholar]
- 14.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003 Nov-Dec;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 15.Reijneveld JC, van der Grond J, Ramos LM, Bromberg JE, Taphoorn MJ. Proton MRS imaging in the follow-up of patients with suspected low-grade gliomas. Neuroradiology. 2005 Dec;47(12):887–891. doi: 10.1007/s00234-005-1435-z. [DOI] [PubMed] [Google Scholar]
- 16.Lichy MP, Plathow C, Schulz-Ertner D, Kauczor HU, Schlemmer HP. Follow-up gliomas after radiotherapy: 1H MR spectroscopic imaging for increasing diagnostic accuracy. Neuroradiology. 2005 Nov;47(11):826–834. doi: 10.1007/s00234-005-1434-0. [DOI] [PubMed] [Google Scholar]
- 17.Floeth FW, Pauleit D, Wittsack HJ, et al. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J Neurosurg. 2005 Feb;102(2):318–327. doi: 10.3171/jns.2005.102.2.0318. [DOI] [PubMed] [Google Scholar]
- 18.Fayed N, Modrego PJ. The contribution of magnetic resonance spectroscopy and echoplanar perfusion-weighted MRI in the initial assessment of brain tumours. J Neurooncol. 2005 May;72(3):261–265. doi: 10.1007/s11060-004-2180-6. [DOI] [PubMed] [Google Scholar]
- 19.Plotkin M, Eisenacher J, Bruhn H, et al. 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol. 2004 Oct;70(1):49–58. doi: 10.1023/b:neon.0000040810.77270.68. [DOI] [PubMed] [Google Scholar]
- 20.McKnight TR, von dem Bussche MH, Vigneron DB, et al. Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg. 2002 Oct;97(4):794–802. doi: 10.3171/jns.2002.97.4.0794. [DOI] [PubMed] [Google Scholar]
- 21.Patronas NJ, Di Chiro G, Brooks RA, et al. Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology. 1982 Sep;144(4):885–889. doi: 10.1148/radiology.144.4.6981123. [DOI] [PubMed] [Google Scholar]
- 22.Doyle WK, Budinger TF, Valk PE, Levin VA, Gutin PH. Differentiation of cerebral radiation necrosis from tumor recurrence by [18F]FDG and 82Rb positron emission tomography. J Comput Assist Tomogr. 1987 Jul-Aug;11(4):563–570. doi: 10.1097/00004728-198707000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Di Chiro G, Oldfield E, Wright DC, et al. Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Roentgenol. 1988 Jan;150(1):189–197. doi: 10.2214/ajr.150.1.189. [DOI] [PubMed] [Google Scholar]
- 24.Valk PE, Budinger TF, Levin VA, Silver P, Gutin PH, Doyle WK. PET of malignant cerebral tumors after interstitial brachytherapy. Demonstration of metabolic activity and correlation with clinical outcome. J Neurosurg. 1988 Dec;69(6):830–838. doi: 10.3171/jns.1988.69.6.0830. [DOI] [PubMed] [Google Scholar]
- 25.Kim EE, Chung SK, Haynie TP, et al. Differentiation of residual or recurrent tumors from post-treatment changes with F-18 FDG PET. Radiographics. 1992 Mar;12(2):269–279. doi: 10.1148/radiographics.12.2.1561416. [DOI] [PubMed] [Google Scholar]
- 26.Janus TJ, Kim EE, Tilbury R, Bruner JM, Yung WK. Use of [18F]fluorodeoxyglucose positron emission tomography in patients with primary malignant brain tumors. Ann Neurol. 1993 May;33(5):540–548. doi: 10.1002/ana.410330520. [DOI] [PubMed] [Google Scholar]
- 27.Kahn D, Follett KA, Bushnell DL, et al. Diagnosis of recurrent brain tumor: value of 201Tl SPECT vs 18F-fluorodeoxyglucose PET. AJR Am J Roentgenol. 1994 Dec;163(6):1459–1465. doi: 10.2214/ajr.163.6.7992747. [DOI] [PubMed] [Google Scholar]
- 28.Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol. 1998 Mar;19(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- 29.Van Laere K, Ceyssens S, Van Calenbergh F, et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging. 2005 Jan;32(1):39–51. doi: 10.1007/s00259-004-1564-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang SX, Boethius J, Ericson K. FDG-PET on irradiated brain tumor: ten years' summary. Acta Radiol. 2006 Feb;47(1):85–90. doi: 10.1080/02841850500335101. [DOI] [PubMed] [Google Scholar]
- 31.Alger JR, Frank JA, Bizzi A, et al. Metabolism of human gliomas: assessment with H-1 MR spectroscopy and F-18 fluorodeoxyglucose PET. Radiology. 1990 Dec;177(3):633–641. doi: 10.1148/radiology.177.3.2243962. [DOI] [PubMed] [Google Scholar]
- 32.Prat R, Galeano I, Lucas A, et al. Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-((18)F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J Clin Neurosci. 2009 Dec 1; doi: 10.1016/j.jocn.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001 Jun 20;96(3):191–197. doi: 10.1002/ijc.1016. [DOI] [PubMed] [Google Scholar]
- 34.Castillo M, Kwock L, Mukherji SK. Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol. 1996 Jan;17(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- 35.Weinhard K. The theory and practice of 3D PET. Kluwer Academic; Dordrecht ; Boston: 1998. Applications of 3D PET. In: Bendriem B, Townsend DW, eds; pp. 133–167. [Google Scholar]
- 36.Watson CC. New, faster, image-based scatter correction for 3D PET. Nuclear Science, IEEE Transactions on. 2000;47(4):1587–1594. [Google Scholar]
- 37.Padma MV, Said S, Jacobs M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003 Sep;64(3):227–237. doi: 10.1023/a:1025665820001. [DOI] [PubMed] [Google Scholar]
- 38.Meyer PT, Schreckenberger M, Spetzger U, et al. Comparison of visual and ROI-based brain tumour grading using 18F-FDG PET: ROC analyses. Eur J Nucl Med. 2001 Feb;28(2):165–174. doi: 10.1007/s002590000428. [DOI] [PubMed] [Google Scholar]
- 39.Julious SA. Two-sided confidence intervals for the single proportion: comparison of seven methods by Robert G. Newcombe, Statistics in Medicine 1998; 17:857-872. Stat Med. 2005 Nov 15;24(21):3383–3384. doi: 10.1002/sim.2164. [DOI] [PubMed] [Google Scholar]
- 40.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998 Apr 30;17(8):857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 41.Wilson EB. Probable Inference, the Law of Succession, and Statistical Inference. Journal of the American Statistical Association, 1927 Jun.22(158):209–212. 1927. [Google Scholar]
- 42.Vollset SE. Confidence intervals for a binomial proportion. Stat Med. 1993 May 15;12(9):809–824. doi: 10.1002/sim.4780120902. [DOI] [PubMed] [Google Scholar]
- 43.Olivero WC, Dulebohn SC, Lister JR. The use of PET in evaluating patients with primary brain tumours: is it useful? J Neurol Neurosurg Psychiatry. 1995 Feb;58(2):250–252. doi: 10.1136/jnnp.58.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger PC, Mahley MS, Jr., Dudka L, Vogel FS. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979 Oct;44(4):1256–1272. doi: 10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 45.Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981 Feb;7(2):243–252. doi: 10.1016/0360-3016(81)90443-0. [DOI] [PubMed] [Google Scholar]
- 46.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993 Oct;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 47.Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991 Apr;4(2):47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- 48.Castillo M, Kwock L, Scatliff J, Mukherji SK. Proton MR spectroscopy in neoplastic and non-neoplastic brain disorders. Magn Reson Imaging Clin N Am. 1998 Feb;6(1):1–20. [PubMed] [Google Scholar]
- 49.Warburg O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]