Abstract
Background
Methamphetamine (METH) abuse continues to be a major illicit drug of abuse. Neuroimaging findings suggest that METH is neurotoxic and may alter various brain structures, but the effect of METH on the aging brain has not been studied.
Aim
The aim was to determine regional volumes of cortical grey matter in the brains of adult METH-users versus healthy control subjects, and their interaction with age and METH-usage variables.
Design
Cross-sectional study
Setting
Magnetic resonance imaging (MRI) Research Center located in a University-affiliated hospital.
Participants
Thirty-four METH-dependent subjects (21 men and 13 women; ages 33.1±8.9 years), diagnosed according to DSM-IV criteria, and 31 healthy non-METH user comparison subjects (23 men and 8 women ages 35.7±8.4 years).
Measurement
Regional grey matter volumes were segmented automatically in all subjects and evaluated in relation to age, using high-resolution MRIs at 3.0 Tesla.
Findings
After adjustment for the effects of cranium size, the METH-users showed enhanced cortical grey matter volume loss with age in the frontal (ANCOVA interaction-p=0.02), occipital (interaction-p=0.01), temporal (interaction-p<0.001), and the insular lobes (interaction-p=0.01) compared to controls, independently of METH-usage patterns. Additionally, METH-users showed smaller grey matter volumes than control subjects in several subregions (dorsolateral prefrontal: p=0.02; orbitofrontal: p=0.03; prefrontal: p=0.047; superior temporal: p=0.04).
Conclusions
Methamphetamine users appear to show increased cortical grey matter loss with age which raises the possibility of accelerated decline in mental functioning.
Keywords: neuroimaging, imaging, methamphetamine, stimulants, cortex, aging
INTRODUCTION
Abuse of methamphetamine (METH), an illicit addictive stimulant, is a growing problem worldwide (1, 2). It is estimated that there are between 15 and 16 million METH users worldwide, a figure similar to that for heroin or cocaine abuse at the global level (3). Often known as “ice” or “crystal meth”, METH is typically smoked, with subsequent euphoria and high energy that lasts for hours (4, 5). However, METH abuse can also lead to negative psychiatric symptoms, such as violence, increased risky sexual behavior, psychosis, suicide attempts, craving for the drug, and depression (6–11).
In preclinical studies, methamphetamine leads to high levels of cytoplasmic dopamine as well as disruption of vesicular storage that leads to accumulation of reactive oxygen species and severe oxidative stress (12, 13). Animal studies (14, 15) and human neuroimaging findings suggest that METH is neurotoxic and may lead to significant alterations in brain structures (16–22).
However, while other drugs of abuse have been shown to alter brain structures over time, little is known about how METH usage might affect the aging brain. For example, brain maturation may be arrested in adult chronic cocaine users (22, 23), and these cocaine addicts were shown to have greater decline in temporal lobe volumes with age (22). Furthermore, studies of alcoholism demonstrate exaggeration of cortical grey matter volume loss associated with alcohol consumption over time (24). However, whether greater than normal age-dependent grey matter volume loss occurs in METH users remains undocumented.
To address this issue, regional volumes of grey matter were assessed in adult METH-users and non-METH-user control subjects. We also determined whether grey matter volumes in METH-users and control subjects differ as a function of age and if and how these morphometric variables depend on METH-usage patterns.
METHODS
Research participants
Individuals were recruited through local treatment facilities, flyers, or word-of-mouth. The subjects were compensated with a nominal amount of cash for their participation. Inclusion criteria for all subjects included being 18–55 years old, able to provide informed consent, HIV seronegative, and demonstrating good health (as evidenced by normal physical examination, electrocardiogram, screening blood tests, and a negative urine pregnancy test in women). In addition, METH-users were included if they were currently or recently (within the past 5 years) METH-dependent and had no history of other drug-dependence (except for nicotine) according to the Diagnostic Statistical Manual of Mental Disorders (DSM)-IV criteria. The non-METH user subjects were required to have no history of any METH use and no history of drug-dependence (except nicotine) according to the DSM-IV criteria. Exclusion criteria for all subjects included any confounding neurological disorder, unstable or severe psychiatric illnesses (e.g. schizophrenia, bipolar disorder, acute METH-induced psychosis, or current major depressive disorder not related to drug withdrawal), any other significant medical illness, and ineligibility to perform MRI.
Detailed drug and psychiatric histories were obtained from all subjects during face-to-face interviews by trained research staff and verified by a board-certified Neurologist or Psychiatrist. Drug use history included last use, quantity, frequency, duration, and route of use for all licit or illicit drugs. If drug use patterns varied during different time periods in their lives, their history was detailed for each time period. The structured assessments included the self-administered Center for Epidemiologic Study-Depression (CES-D) questionnaire. Any subject whose self-report suggested an unclear psychiatric diagnosis, was on psychotropic medications, or scored greater than 16 on the CES-D was interviewed further by a board-certified psychiatrist or neurologist for an abbreviated psychiatric evaluation to ensure that the inclusion and exclusion criteria were met. Urine toxicology tests were performed using the Multi-Drug Six Panel Drug Test from Medimpex United (Bensalem, Pennsylvania).
Protocols were approved by the Investigational Review Board of The University of Hawaii. Written consent was obtained in all participants.
MRI acquisition and processing
MRI was performed on a 3T Siemens TIM Trio system (Erlangen, Germany), using an 8-channel head coil. Following a quick scout scan, a 3D axial T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) scan was acquired (TR/TE/TI=2200/4.91/1000 ms; 1 NEX; 256×208×144, 1mm isotropic resolution). FLAIR images (TR/TE=10000/85 ms; 1 NEX; 205×320×28; 0.9mm × 0.9mm × 3mm resolution) were also acquired. Structural MP-RAGE and FLAIR images were read by a trained physician to ensure the subjects did not show any significant structural brain abnormalities (e.g. brain tumors, cerebral infarcts, demyelinating diseases).
Image Analysis
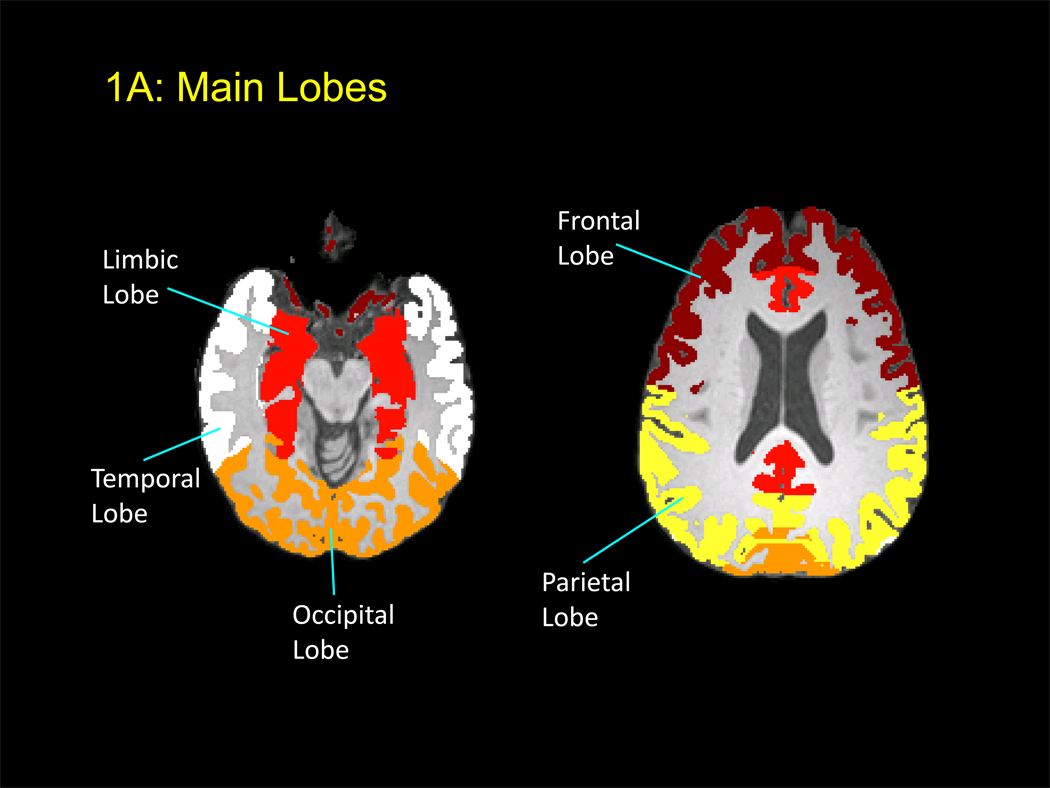
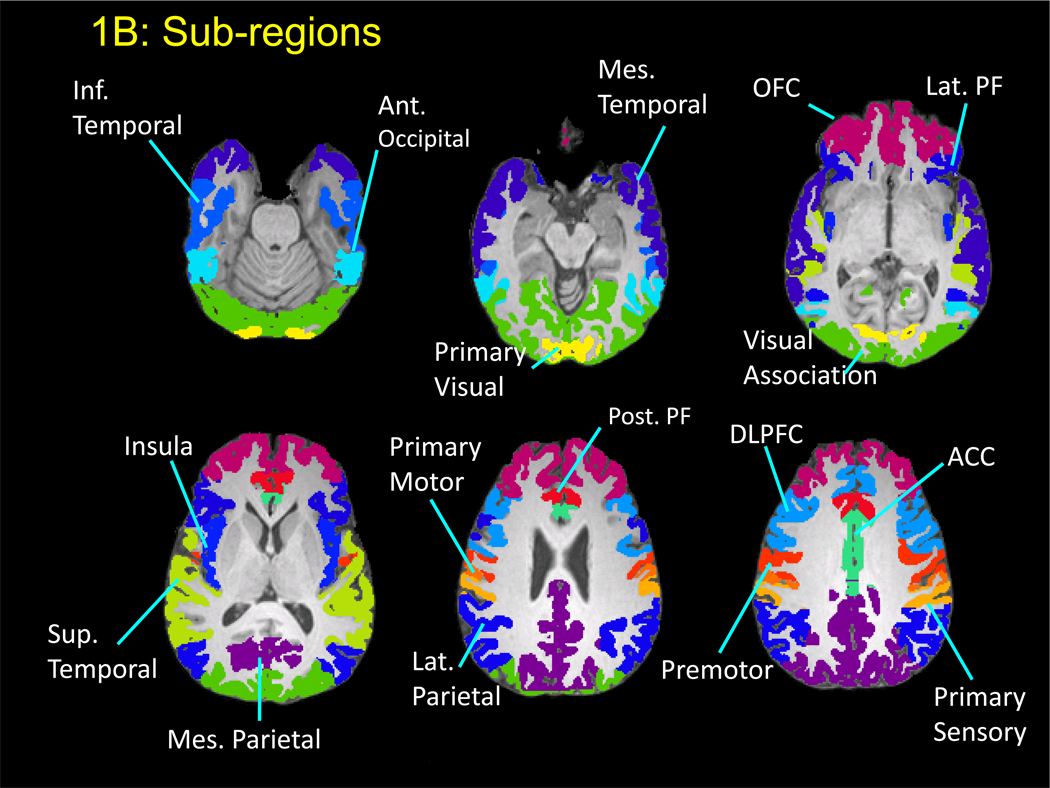
All MP-RAGE scans were assessed visually for the presence of motion artifacts, as well as quantitatively by calculating the ratio of background signal in phase encoding direction to that in frequency encoding direction. Scans that showed artifacts, or did not meet quality criteria, were repeated. Regional cortical grey matter volumes were determined from the T1-weighted MP-RAGE images in five steps: skull removal, registration, segmentation, correction of the segmentation for white-matter signal hyperintensities (WMSHs), and subject-specific aggregation of cortical grey matter volumes within each ROI. The details of these methods have been reported recently (25). Six main lobes and 17 subregions were defined (Figure 1A and B, Table 2). Of note, each of the main lobes was generally larger than the sum of the sub-regions. Skull removal, registration, and segmentation were carried out using tools within FMRIB Software Library (FSL), version 3.3 (Oxford, UK). Regions of interest (ROI) templates were created using Talairach atlas definitions as implemented in the Talairach Daemon resource, transformed to Montreal Neurological Institute (MNI) space using a nonlinear transform (26). The ROIs were then dilated in MNI space using nearest neighbor interpolation, classifying every voxel in MNI space as part of its nearest ROI. This made it possible to preserve the boundaries between adjacent ROIs while including areas near the pial and white matter surfaces of the cerebral cortex that were likely to be missed as a result of imperfect registrations. This resulted in an ROI template in MNI space that was then transformed to each individual’s T1-space. In addition, WMSHs were measured on FLAIR images using a method reported previously (27) for all subjects. Since WMSHs are often misclassified as grey rather than white matter, all such voxels were reclassified as white matter in the final analyses of the grey matter measurements.
Figure 1.
A: Axial MRI showing the main lobes that are automatically segmented using FSL.
B: Axial MRI showing the automatically segmented subregions within each of the main lobes.
Table 2.
Segmented gray matter volumes (mL) in the main lobes and subregions in METH users and Non-METH user controls
| Gray matter Region |
METH-users (n=34) |
Controls (n=31) |
t-test p-value |
ANCOVA drug by age interaction p-value |
Repeated measure ANCOVA for subregions p-value* |
|---|---|---|---|---|---|
| Frontal lobe | 212.9 ± 21.5 | 222.2 ± 18.1 | t(63)=1.88, p=0.06 | F(1, 61)=6.11, p=0.02 | 2-way drug by age: F(1, 61)=6.86, p=0.01 |
| Dorsolateral prefrontal | 35.1 ± 3.3 | 36.9 ± 2.8 | t(63)=2.40, p=0.02 | F(1, 61)=5.18, p=0.03 | |
| Lateral prefrontal | 23.0 ± 2.4 | 23.7 ± 2.5 | t(63)=1.20, p=0.23 | F(1, 61)=2.58, p=0.11 | |
| Orbital frontal | 58.3 ± 5.9 | 61.3 ± 4.9 | t(63)=2.26, p=0.03 | F(1, 61)=5.67, p=0.02 | |
| Posterior prefrontal | 18.5 ± 2.2 | 19.0 ± 2.3 | t(63)=0.85, p=0.40 | F(1, 61)=3.31, p=0.07 | |
| Prefrontal | 139.3 ± 13.3 | 146 ± 11.5 | t(63)=2.02, p=0.047 | F(1, 61)=5.88, p=0.02 | |
| Premotor | 47.6 ± 6.3 | 50.0 ± 5.3 | t(63)=1.63, p=0.11 | F(1, 61)=6.75, p=0.01 | |
| Primary motor | 8.8 ± 1.4 | 8.9 ± 1.5 | t(63)=0.35, p=0.73 | F(1, 61)=1.11, p=0.30 | |
| Limbic lobe | 75.8 ± 6.9 | 76.7 ± 6.0 | t(63)=0.57, p=0.57 | F(1, 61)=2.40, p=0.13 | N/A |
| Anterior cingulate | 22.1 ± 1.9 | 22.4 ± 1.7 | t(63)=0.51, p=0.61 | F(1, 61)=0.29, p=0.59 | |
| Occipital lobe | 75.6 ± 7.1 | 78.5 ± 5.5 | t(63)=1.85, p=0.07 | F(1, 61)=6.60, p=0.01 | NS |
| Anterior occipital | 3.6 ± 0.5 | 3.8 ± 0.5 | t(63)=1.29, p=0.20 | F(1, 61)=0.15, p=0.70 | |
| Primary visual | 6.9 ± 1.1 | 7.0 ± 0.9 | t(63)=0.62, p=0.54 | F(1, 61)=0.03, p=0.87 | |
| Visual association | 55.5 ± 5.4 | 57.8 ± 4.2 | t(63)=1.93, p=0.06 | F(1, 61)=8.20, p=0.01 | |
| Parietal lobe | 98.2 ± 10.2 | 100.7 ± 8.9 | t(63)=1.03, p=0.30 | F(1, 61)=1.70, p=0.20 | 3-way drug by age by sub-region: F(2, 122)=2.81, p=0.06 |
| Lateral parietal | 33.3 ± 3.6 | 34.2 ± 2.7 | t(63)=1.23, p=0.22 | F(1, 61)=3.75, p=0.06 | |
| Primary sensory | 13.6 ± 1.9 | 14.2 ± 1.8 | t(63)=1.31, p=0.20 | F(1, 61)=3.15, p=0.08 | |
| Mesial parietal | 41.9 ± 4.4 | 42.6 ± 4.8 | t(63)=0.59, p=0.56 | F(1, 61)=0.15, p=0.70 | |
| Temporal lobe | 132.3 ± 11.9 | 134.2 ± 6.6 | t(63)=0.78, p=0.44 | F(1, 61)=20.22, p=<0.001 | 3-way drug by age by sub-region: F(2, 122)=3.45, p=0.04 |
| Inferior temporal | 23.8 ± 3.0 | 23.4 ± 2.6 | t(63)=−0.68, p=0.50 | F(1, 61)=12.88, p<0.001 | |
| Mesial temporal | 46.9 ± 4.7 | 47.7 ± 2.8 | t(63)=0.90, p=0.37 | F(1, 61)=11.23, p=0.001 | |
| Superior temporal | 25.4 ± 2.3 | 26.4 ± 1.5 | t(63)=2.15, p=0.04 | F(1, 61)=7.03, p=0.01 | |
| Insular lobe | 17.2 ± 2.1 | 17.6 ± 1.6 | t(63)=0.82, p=0.41 | F(1, 61)=8.23, p=0.01 | N/A |
| 6 main lobes | 2-way drug by age: F(1,61)=9.82, p=0.003 2-way sub-region by age: F(5, 310)=4.92, p<0.001 | ||||
| 17 sub-regions | 2-way drug by age: F(1,61)=7.10, p=0.01 2-way sub-region by age: F(16, 992)=2.29, p=0.003 |
2-way drug by age interactions are linear by linear.
The final image processing step used an in-house algorithm to aggregate all cortical grey matter voxels within each of the ROIs. This involved overlaying the ROIs on the grey matter segmentation and performing an automated sulcal and gyral correction algorithm separately in each hemisphere in order to correct for inaccuracies in the registration where an ROI either missed grey matter that was contained within and contiguous with the cortical surface captured by the ROI, typically due to sulcal infoldings, or where the ROI incorrectly captured grey matter of a nearby structure that was not directly connected to the majority of the ROI.
Statistical analysis
Student t-tests (unpaired two-tailed), chi-square or Fisher’s exact test, were used to compare demographic characteristics or cortical grey matter volumes between the two subject groups. For each region, analysis of covariance (ANCOVA) was used to assess whether the effect of age on grey matter volume differed between METH-users and controls, using between-subject variables drug status (METH-user vs. control) and age (continuous), and the drug status by age effect. Differences in age-related volume change were defined as differences between within-group regression coefficients (drug status by age, linear by linear interaction). Furthermore, a mixed models analysis with repeated measures ANCOVA was used to examine the effect of METH across the brain using: 1) the sub-regions within each of the frontal, occipital, parietal, and temporal lobe as the repeated measure, 2) the 17 individual sub-regions as the repeated measure and 3) the 6 main lobes as the repeated measure. Each of these models included two between-subject variables (drug status and age) and one within-subjects factor (sub-region). All 2- and 3-way interactions amongst drug status, age, and sub-region were included initially. The 3-way interaction was removed from the model if the term was not significant; the subsequent model included all 2-way interactions. For scaling purposes and to ensure normality, the grey matter volumes were log transformed prior to repeated measures analyses. Simple regression analyses were used to assess the correlation between the cortical grey matter volume and drug use or other clinical variables. Data were analyzed using Statistical Analysis Software (SAS) version 9.1. P-values less than 0.05 were considered statistically significant; trends were noted for p-values between 0.05 and 0.15. The Simes procedure was used to adjust for multiple comparisons (28). The Simes procedure uses a variable significance level p(j) to define significance rather than the fixed corrected significance level of Bonferroni. The null hypothesis is rejected when, for any j=1,…,m, p(j) ≤ jα/m where α is the desired overall significance level. To account for differences in premorbid brain size between subjects, we used a linear regression analysis to adjust all cortical grey matter volumes for cranium size, and the adjusted values were used in all subsequent analyses.
RESULTS
Research Participants and Their Drug Usage (see Table 1)
Table 1.
Demographic and Clinical Characteristics of Study Subjects (n=65)
| METH-users (n=34) | Controls (n=31) | p-value | |
|---|---|---|---|
| Age (range) | 33.1 ± 8.9 (18–50) | 35.7 ± 8.4 (20–55) | t(63)=1.24, p=0.22 |
| Education | 12.6 ± 1.1 | 14.6 ± 1.9 | t(63)=5.21, p<0.001 |
| Estimated VIQ | 99.0 ± 8.2 | 110.1 ± 10.4 | t(59)=4.68, p<0.001 |
| Sex, M/F | 21(62%)/13(38%) | 23(74%)/8(26%) | Χ2(1, n=65)=1.15, p=0.28 |
| CES-D | 15.7 ± 10.2 | 8.6 ± 6.7 | t(59)=−3.14, p=0.003 |
| BMI | 26.7 ± 5.1 | 25.6 ± 4.2 | t(63)=−0.96, p=0.34 |
| Systolic blood pressure | 123.5 ± 13.8 | 115.5 ± 9.1 | t(63)=−2.74, p=0.01 |
| Diastolic blood pressure | 78.4 ± 10.8 | 75.0 ± 8.2 | t(63)=−1.42, p=0.16 |
| Race (%) | Fisher’s exact test | ||
| Asian | 8 (24%) | 6 (19%) | p=0.01 |
| Mixed | 16 (47%) | 4 (13%) | |
| Pacific Islander/Hawaiian | 2 (6%) | 4 (13%) | |
| White | 8 (23%) | 17 (55%) | |
| Black | 0 | 0 | |
| Lifetime meth use (grams) | 787 (364, 2737) | Never used | n.a. |
| Length of meth abstinence (days) | 18 (0, 121) | n.a. | n.a. |
| Duration of meth use (months) | 120 (60, 180) | n.a. | n.a. |
| Frequency of meth use (days per week) | 5.7 (4.2, 7.0) | n.a. | n.a. |
| Lifetime log THC (Joints) | 2.4 ± 1.5 | 1.7 ± 1.5 | t(63)=−1.93, p=0.06 |
| Lifetime standard alcohol drinks | 8,464 ± 12,901 | 6,800 ± 12,139 | t(63)=−0.53, p=0.60 |
| Standard alcohol drinks per week (at time of heaviest use) | 8.4 ± 9.9 | 8.6 ± 12.9 | t(63)=0.07, p=0.95 |
| Lifetime Tobacco (pack year) | 8.3 ± 9.2 | 5.0 ± 8.9 | t(61)=−1.44, p=0.15 |
Data are presented as mean ± standard deviation, and percentage in parentheses. Meth usage variables are presented as median and interquartile range. Lifetime use was calculated by using the amount used times frequency times duration of use, and summed for each period of use if there were more than one period of use.
Thirty-four METH-users and 31 non-METH-user comparison subjects fulfilled the study criteria and were studied. The subjects were in their fourth decade of life on average and were predominantly male. The two subject groups had similar ages but the METH subjects had fewer years of education (p<0.001) and had lower estimated premorbid verbal intelligence quotients (VIQ) (p<0.001) than the control subjects. Compared to control subjects, the METH-user subjects also had more depressive symptoms as assessed on the CES-D (p=0.003) and higher systolic blood pressure (SBP) (p=0.01) during their initial assessments.
The METH-users were generally heavy users with a cumulative median lifetime use of 787 grams, used for 10 years, at a frequency of greater than 5 times per week, and had been abstinent for 18 days. An overwhelming majority (94%) of METH-users smoked METH as their primary route, and only 6% primarily used METH intravenously. Eleven of the 34 (32%) METH-users tested positive for METH on their urine drug screen and all of the METH-users were negative for other drugs of abuse. Five of the 31 (16%) control subjects tested positive for marijuana on their urine drug screen and all of the control subjects were negative for other drugs of abuse. The 11 METH users who tested positive for METH showed a trend for higher SBP of 125.0±13.0 mmHg than the remaining 54 subjects who did not test positive for METH (SBP of 118.6±12.0, t(63)=1.60, p=0.12).
Diastolic blood pressure (DBP), body mass index (BMI), log lifetime tetrahydrocannibol (THC) joint usage, lifetime alcohol, and lifetime tobacco usage did not differ significantly between the two groups. The race distribution was reflective of the unique Hawaiian population and was different between the two groups (Fisher’s exact test of p=0.01), with self-described primarily mixed race (47%) in the METH-user group and whites (55%) in the control subjects.
Grey matter volumes in METH users and Controls (Table 2)
After adjustment for the effects of cranium size, METH-users showed slightly smaller cortical grey matter volumes than control subjects in all 6 main lobes and 17 subregions of the cortical grey matter. There were trends for smaller cortical grey matter volumes in the occipital (p=0.07) and frontal lobes (p=0.06). Among the 17 subregions of the cortex, significance was reached in the dorsolateral prefrontal (p=0.02), orbitofrontal (p=0.03), prefrontal (p=0.047), and superior temporal (p=0.04) cortices, whereas the premotor (p=0.11) and visual association (p=0.06) cortices showed trends for smaller cortical grey matter volumes compared to control subjects. When lifetime alcohol was included as a covariate in the analyses, it did not change the trends or significance of the above mentioned p-values, with the exception of the prefrontal (p=0.06) subregion of the cortex.
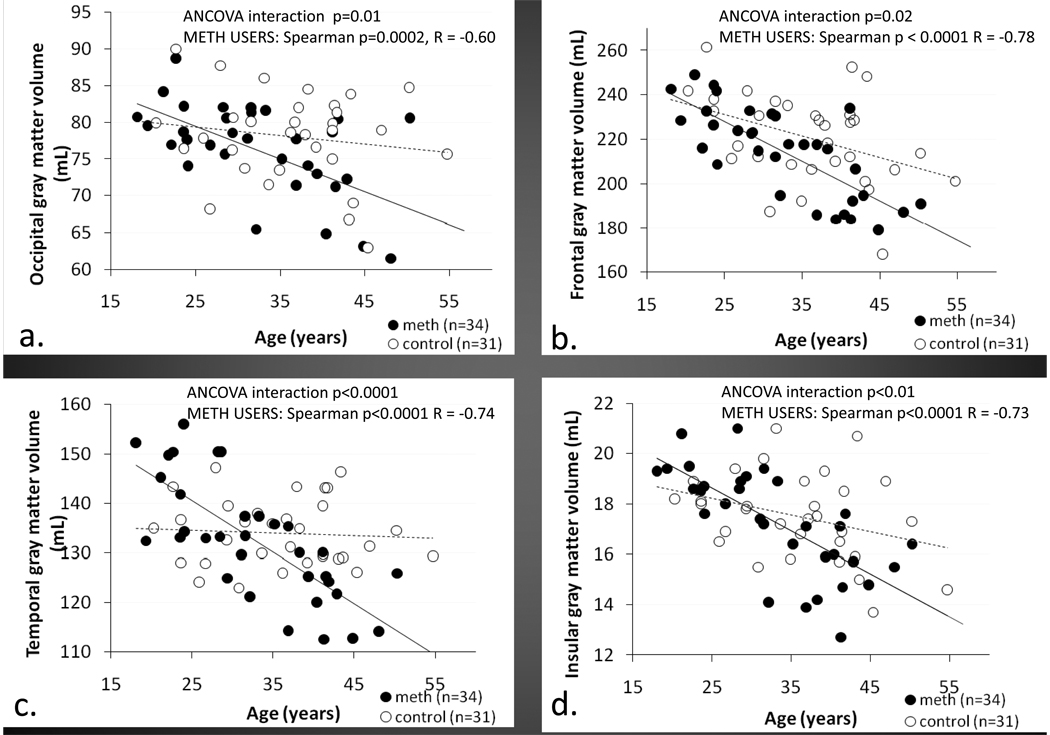
The effects of aging on cortical brain volumes were markedly different between the two groups. Among the six main lobes, the METH-users show greater than normal age-related cortical grey matter loss compared to control subjects in the frontal (ANCOVA interaction p=0.02), occipital (interaction p=0.01), and temporal (interaction p<0.001) cortices, and the insula (interaction p=0.01) (Figure 2). Our data extrapolate that if followed longitudinally, cortical grey matter would decline between 0.1 to 3.5% per decade in the control subjects, but at a greater rate of 6.4 to 8.5% in the METH users. All of these interactions remained significant after correction for multiple comparisons. No significant drug status by age interactions were observed in the limbic region and the parietal cortex.
Figure 2.
Scatterplots showing greater than normal age-related cortical grey matter loss in METH user than controls in the occipital cortex (A),frontal cortex (B), temporal cortex (C), and the insular cortex (D). The cortical volumes are the sum of both hemispheres.
When the sub-regions were analyzed for each lobe, using repeated-measures ANCOVA, significant 3-way interactions (drug status by age by sub-region) were only seen in the temporal lobe (p=0.04, 3 sub-regions) and parietal lobe (trend p=0.06, 3 sub-regions). A significant 2-way interaction of drug status by age was observed in the frontal cortex (p=0.01, 7 sub-regions). No significant 2- or 3-way interactions were seen in the occipital cortices. Of note, each of the main lobes was generally larger than the sum of the sub-regions.
When the analysis was performed using the six main lobes as a repeated measures, the 3-way interaction (p=0.18) was not significant, but 2-way interactions of drug status by age (p=0.003) and sub-region by age (p<0.001) were significant. Similarly, the 3-way interaction was not significant when all 17 sub-regions were included as the repeated measure (p=0.16), but 2-way interactions of drug status by age (p=0.01) and sub-region by age (p=0.003) were significant.
Race, education, estimated VIQ, CES-D, SBP, lifetime tobacco, lifetime THC, and lifetime alcohol use did not significantly affect the drug status by age results when they were included individually as covariates in the analyses. Similarly, all associations between the cortical grey matter volumes and age among METH users remained significant when the METH-usage variables (including lifetime grams used, frequency, days abstinent, and duration of use) were included as covariates.
Regarding the METH-usage variables, the frequency of METH use per week correlated positively with occipital cortical grey matter volume (r=0.44, p=0.01), while Log10 total lifetime usage (in grams) correlated positively with the parietal cortical grey matter volume (r=0.36, p=0.04). However, these correlations were no longer significant after correction for multiple comparisons.
DISCUSSION
We found greater than normal age-related loss in cortical grey-matter volumes of the temporal, occipital, frontal, and insular lobes of a group of individuals with a history of METH-dependence compared to non-METH users. Longitudinally, although white matter volume loss appears to have a U-shaped trajectory, grey matter neocortical volume loss generally occur in a linear relationship with aging in adulthood (29, 30); but our cross-sectional study findings show that the loss may be more rapid in METH-users. Our data extrapolate that if followed longitudinally, cortical grey matter volume would decline between 0.1 to 3.5% per decade in the control subjects, but at a greater rate of 6.4 to 8.5% in the METH users. This marked difference suggests that METH-users may be at a higher risk to develop cognitive or degenerative disorders at an earlier age compared to healthy non-METH users.
The pathophysiology underlying grey matter loss during normal aging is still poorly understood, but shrinkage of large neurons, rather than a reduction in the number of neurons, appears to play an important role (31). This normal age-related grey matter loss occurs in multiple areas of the brain, primarily in the frontal cortex, parietal, temporal, and occipital cortices (32, 33). Studies in mild cognitive impairment (MCI) and brain alterations prior to the development of MCI demonstrate that cortical thickness is decreased in the frontal, temporal, and parietal lobes (34, 35). Furthermore, measurements of regional cerebral blood flow suggest that the occipito-parietal visual pathway is sensitive to the effects of aging (36, 37). Importantly, a large body of literature show that Alzheimer’s Disease is associated with a reduction of grey matter volume predominantly in the hippocampal formation and enterorhinal cortices (38–40). However, we did not specifically evaluate the hippocampal or the enterohinal cortex. Nevertheless, our findings of more rapid grey matter loss in the frontal, temporal, occipital, and insular lobes resemble a pattern of accelerated aging. Our findings support the notion that more grey matter loss associated with METH-use may render them more vulnerable to cognitive decline by reducing their brain reserve capacity (41).
Enhanced loss of the occipital and temporal lobe volumes may be related to other co-morbid conditions common in METH users. For example, smaller occipital grey matter volumes in the visual association area was associated with chronic schizophrenia and possibly the manifestation of visual hallucinations (42). Temporal cortical grey matter reductions also were more marked in patients with a long duration of psychosis (43), and progressive reduction of the superior temporal gyrus volume preceded the first expression of florid psychosis (44). Therefore, significantly greater than normal age-related loss in all subregions of the temporal cortex and in the visual association area of the occipital cortex in our METH-users may contribute to psychiatric symptoms, such as psychosis or hallucinations, that are often reported by METH users (8, 45). Future studies should evaluate whether psychosis is associated with regional brain volume changes in METH-users.
The few morphometric studies in METH-users have found variable alterations in different brain regions. For example, two studies reported larger grey matter volumes in the striatum (20, 46) and bilateral parietal cortices (46), while other studies demonstrated less grey matter volumes in the mid-frontal, cingulate, limbic, and temporal cortices of METH users (18, 22, 47). Although we did not evaluate the subcortical volumes, we also found smaller volumes in all six main cortical regions and 17 sub-regions of the cortical grey matter in our METH-users compared to our control subjects. This consistent reduction in grey matter volumes across multiple brain regions suggests that METH-users either had smaller grey matter as a premorbid condition, METH use causes grey matter volume shrinkage due to its known neurotoxic effects, or there might be other confounding variables that we were unable to document. Because systematic evaluations of human subjects before and after starting METH may be feasibly difficult, administration of METH in animal models may provide further insights to these possible etiologies.
Because we purposely excluded enrollment of subjects with a history of any drug or alcohol dependence (other than nicotine or METH dependence), unlike a recent study (48) that found that amphetamine usage was associated with reduced cortical thickness in patients co-morbid for heavy alcohol use, our subjects’ lifetime alcohol use did not affect the significant grey matter differences found between METH users and controls, nor did alcohol affect the significant drug status by age interactions in any of the main or subregion grey matter analyses (listed in table 2). Therefore, our results indicate that in non-dependent alcohol users, alcohol does not play a significant role in grey matter differences in METH users or control subjects.
If the cortical volume loss is caused by chronic METH usage, we would expect correlations between the METH usage variables and the grey matter volume loss. The lack of association suggests that there might be a threshold effect for METH-mediated neurotoxicity, at least in terms of regional brain volumes. Another possibility for the lack of association might be due to the moderate sample size in our study. Furthermore, whether the smaller cortical grey matter volumes in METH-users would recover with prolonged abstinence remains unknown and should be evaluated in future longitudinal research. Such brain volume recovery has been observed in alcoholics who remained abstinent over time (49).
Our METH users had higher SBP, which could be secondary to recent METH use since those who tested positive for METH had higher SBP compared to those who did not test positive for METH. Although stress was not evaluated, it is plausible that our METH-users had higher stress levels, which also could have contributed to their higher SBP (50) and depression scores (11, 51). The combination of METH-use and depression (11) could further potentiate the risk for dementia (52) as the METH-using population ages.
Several limitations of the current study warrant consideration. First, this study was cross-sectional, and only longitudinal studies can provide direct evidence of accelerated brain shrinkage with age. Second, the moderate sample size limited our ability to further evaluate subgroup differences, for instance, between male and female users, or current users versus abstinent users. Because of the moderate sample size, we consider this investigation as preliminary. The results from our population of heavy, METH-users who smoked the drug, may not generalize to those with light use or a different route of METH-use. Third, substance users can be poor historians, and hence some of their usage data may not be accurate, which might have led to the lack of associations with the cortical volume loss. Last, future research should include additional measures of stress markers, such as cortisol levels, which would allow us to evaluate the possible contributions of stress on brain atrophy in chronic METH users.
As the population ages, the growing abuse of METH worldwide imposes an urgent need to understand how the effects of METH are manifested in senescence. Our findings of greater than normal age-related cortical grey matter loss in METH users may also have important implications in understanding the reinforcing effects of METH, as well as psychotic and cognitive disorders in METH users. These findings also emphasize the importance of prevention, abstinence, and intervention programs for METH abuse.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health [R01-DA12734; HHSN271200688514C; K24-DA16170; K02-DA16991; G12-RR003061; the Specialized Neuroscience Research Program (1U54NS56883), University of Hawaii Clinical Research Center (CRC, 5P20-RR11091)] and the Office of National Drug Control Policy. We especially thank our research participants and MR staff at our Research Center for their assistance in subject recruitment, assessments, and data collection.
Footnotes
The authors listed above have no competing interests.
Finanancial disclosure: All the authors reported no financial interests or potential conflicts of interest.
Contributor Information
Helenna Nakama, Assistant Professor of Psychiatry, University of Hawaii, John A. Burns School of Medicine, Department of Psychiatry, Honolulu, Hawaii
Linda Chang, University of Hawaii, John A. Burns School of Medicine, Department of Medicine, Honolulu, Hawaii
George Fein, Neurobehavioral Research Inc., Honolulu, Hawaii
Ryan Shimotsu, Neurobehavioral Research Inc., Honolulu, Hawaii
Caroline S. Jiang, University of Hawaii, John A. Burns School of Medicine, Department of Medicine, Honolulu, Hawaii
Thomas Ernst, University of Hawaii, John A. Burns School of Medicine, Department of Medicine, Honolulu, Hawaii
REFERENCES
- 1.Rawson RA, Condon TP. Why do we need an Addiction supplement focused on methamphetamine? Addiction. 2007;102 Suppl 1:1–4. doi: 10.1111/j.1360-0443.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- 2.(SAMHSA) SAaMHSA. The National Survey on Drug Use and Health Report, Office of Applied Studies. 2005
- 3.World Drug Report. United Nations Office of Drug and Crime. 2008:153.
- 4.SERVICES USDOHAH, Administration SAaMHS, Studies OoA, editor. Report DaASISD: Smoked Methamphetamine/Amphetamines: 1992–2002. 2005 January 7; www.samhsa.gov.
- 5.Newton TF, De La Garza R, 2nd, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005;82(1):90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13(2):181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]
- 7.Semple SJ, Zians J, Grant I, Patterson TL. Methamphetamine use, impulsivity, and sexual risk behavior among HIV-positive men who have sex with men. J Addict Dis. 2006;25(4):105–114. doi: 10.1300/J069v25n04_10. [DOI] [PubMed] [Google Scholar]
- 8.McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 9.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100(9):1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 10.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R. Clinical course and outcomes of methamphetamine-dependent adults with psychosis. J Subst Abuse Treat. 2008;35(4):445–450. doi: 10.1016/j.jsat.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Nakama H, Chang L, Cloak C, Jiang C, Alicata D, Haning W. Association between psychiatric symptoms and craving in methamphetamine users. Am J Addict. 2008;17(5):441–446. doi: 10.1080/10550490802268462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tata DA, Yamamoto BK. Interactions between methamphetamine and environmental stress: role of oxidative stress, glutamate and mitochondrial dysfunction. Addiction. 2007;102 Suppl 1:49–60. doi: 10.1111/j.1360-0443.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 13.Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Xu W, Angulo J. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006;140(2):607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey DC, Lacan G, Melegan WP. Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp Brain Res. 2000;133(3):349–358. doi: 10.1007/s002210000386. [DOI] [PubMed] [Google Scholar]
- 16.Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008;13(9):897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkow N, Chang L, Wang G, Fowler J, Ding Y, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine d(2) receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 18.Thompson P, Hayashi K, Simon S, Geaga J, Hong M, Sui Y, Lee J, Toga A, Ling W, London E. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10(6):765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 20.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biological Psychiatry. 2005;57(9):967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 22.Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98(2):93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 23.Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51(8):605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- 24.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical grey matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55(10):905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 25.Fein G, Shimotsu R, Chu R, Barakos J. Parietal grey matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcohol Clin Exp Res. 2009;33(10):1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brett M. The MNI brain and the Talairach atlas. 1999 [Google Scholar]
- 27.Fein G, Shimotsu R, Di Sclafani V, Barakos J, Harper C. Increased white matter signal hyperintensities in long-term abstinent alcoholics compared with nonalcoholic controls. Alcohol Clin Exp Res. 2009;33(1):70–78. doi: 10.1111/j.1530-0277.2008.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751–754. [Google Scholar]
- 29.Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total grey matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23(8):1327–1333. [PMC free article] [PubMed] [Google Scholar]
- 30.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 31.Terry R, DeTeresa R, Hansen L. Neocortical cell counts in normal human adult aging. Annal of Neurology. 1987;21(6):530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- 32.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009 doi: 10.1212/WNL.0b013e3181c3f293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal grey matter. Cereb Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 34.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, Markesbery WR. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68(16):1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H. Alterations in Cortical Thickness and White Matter Integrity in Mild Cognitive Impairment Measured by Whole-Brain Cortical Thickness Mapping and Diffusion Tensor Imaging. AJNR Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- 37.Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14(3 Pt 2):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohnishi T, Matsuda H, Tabira T, Asada T, Uno M. Changes in brain morphology in Alzheimer disease and normal aging: is Alzheimer disease an exaggerated aging process? AJNR Am J Neuroradiol. 2001;22(9):1680–1685. [PMC free article] [PubMed] [Google Scholar]
- 39.Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63(5):693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 40.Du AT, Schuff N, Zhu XP, Jagust WJ, Miller BL, Reed BR, Kramer JH, Mungas D, Yaffe K, Chui HC, Weiner MW. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60(3):481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fein G, Di Sclafani V. Cerebral reserve capacity: implications for alcohol and drug abuse. Alcohol. 2004;32(1):63–67. doi: 10.1016/j.alcohol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Onitsuka T, McCarley RW, Kuroki N, Dickey CC, Kubicki M, Demeo SS, Frumin M, Kikinis R, Jolesz FA, Shenton ME. Occipital lobe grey matter volume in male patients with chronic schizophrenia: A quantitative MRI study. Schizophr Res. 2007;92(1–3):197–206. doi: 10.1016/j.schres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lappin JM, Morgan K, Morgan C, Hutchison G, Chitnis X, Suckling J, Fearon P, McGuire PK, Jones PB, Leff J, Murray RM, Dazzan P. Grey matter abnormalities associated with duration of untreated psychosis. Schizophr Res. 2006;83(2–3):145–153. doi: 10.1016/j.schres.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive grey matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66(4):366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 45.Mahoney JJ, 3rd, Kalechstein AD, De La Garza R, 2nd, Newton TF. Presence and persistence of psychotic symptoms in cocaine- versus methamphetamine-dependent participants. Am J Addict. 2008;17(2):83–98. doi: 10.1080/10550490701861201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jernigan T, Gamst A, Archibald S, Fennema-Notestine C, Mindt M, Marcotte T, Heaton R, Ellis R, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162(8):1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9(2):221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- 48.Lawyer G, Bjerkan PS, Hammarberg A, Jayaram-Lindstrom N, Franck J, Agartz I. Amphetamine dependence and co-morbid alcohol abuse: associations to brain cortical thickness. BMC Pharmacol. 10:5. doi: 10.1186/1471-2210-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston DW, Gold A, Kentish J, Smith D, Vallance P, Shah D, Leach G, Robinson B. Effect of stress management on blood pressure in mild primary hypertension. Bmj. 1993;306(6883):963–966. doi: 10.1136/bmj.306.6883.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67(2):247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- 52.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]