Abstract
Objective
To examine whether the size of silicon nanovectors (SNV) inhibits their entrance into the fetal circulation.
Study Design
Pregnant rats were intravenously administered with SNV or saline. The SNVs were spherical particles with three escalating diameters: 519 nanometers (nm), 834 nm and 1000 nm. The maternal and fetal distribution of SNVs was assessed.
Results
In animals that received 1000 nm or 834 nm SNV, silicon (Si) levels were significantly higher in the maternal organs versus saline group, while silicon levels in fetal tissues were similar to control. However, in animals receiving 519 nm SNV, fetal silicon levels were significantly higher in the SNV group compared to saline group, (5.93 ± 0.67 μg Si per organ vs. 4.80 ± 0.33, p=0.01).
Conclusion
Larger SNVs do not cross the placenta to the fetus and, remaining within the maternal circulation, can serve as carriers for harmful medications in order to prevent fetal exposure.
Keywords: Silicon nanovector, Medications, Transplacental passage
Introduction
A longstanding problem in the field of obstetrics has been the limitations of medication and drug use in pregnant women. Medications are frequently used during pregnancy to treat maternal medical conditions or pregnancy complications.1–3 Up to 96.9% women report consuming at least one medication during their pregnancy.4 Multiple drug use occurs in 33.5% of women, with up to 13.6% consuming 4 or more medications. Although the safety of many medications has been proven in pregnancy, there are some that cause birth defects and are therefore contraindicated in pregnancy. Examples include warfarin, certain anti-epileptic medications and isotretinoin.5
Fetal exposure to potentially harmful medications is based on the ability of medications to cross the placenta from mother to fetus. The most common form of passage of medications across the placenta is by passive or simple diffusion.6 By this method, the amount of medications that does cross the placenta is dependent on the concentration of the medication in the maternal circulation. Although there are other important factors including physical and chemical properties of the drug, size is a strong predictor of successful transfer across the placenta. Medications that are less than 500 Dalton have varying levels of transfer whereas those greater than 6000 Dalton such as low molecular weight heparin rarely pass.7
Nanotechnology deals with materials and systems whose structure and components exhibit novel and significantly improved physical, chemical and biological properties, phenomena, and processes since their size ranges between 10−9 to 10−7 meters.8, 9 Nanovectors, serving as the “missing size link” between the biological objects of microns scale (e.g. living cells) to the therapeutic molecules (10−10–meter scale), have emerging biological behaviors that may be utilized to enhance therapeutic potential and to prevent side effects of currently used drugs.
As an example, nanovectors have the potential to target drug delivery to the specific location in the body, based on their physicochemical and geometrical features or specific ligands to the receptors over-expressed on the target tissue. Porous silicon-based nanovectors are widely studied for use in medicine since they can degrade in the body to naturally occurring silicic acid and can be readily modified to target specific organs.10–19 From an obstetrical perspective, such nano-devices can be developed to prohibit the transfer of medications from mother to fetus. If these nanoparticles can indeed remain within the maternal circulation, then teratogenic medications that were previously contraindicated in pregnancy could be used. This could lead to new opportunities in the medical treatment for pregnant women.
The objective of this study was to determine whether the diameter of silicon nanovectors (SNV) may restrict their distribution to only the maternal circulation and thereby inhibit their entrance into the fetal circulation.
Materials and Methods
Timed pregnant Sprague Dawley rats were obtained from Harlan Sprague Dawley (Indianapolis, Indiana) on gestational day (GD) 15. Rats were individually housed in polycarbonate cages in an environmentally controlled vivarium under 12 hour light and dark cycles. Animals were fed ad lib throughout the experiment. The animal care and experiments were approved by the University of Texas Health Science Center at Houston Animal Welfare Committee.
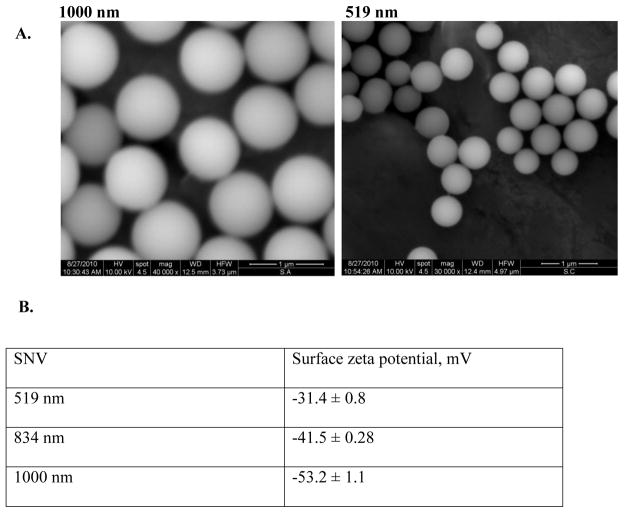
On GD 20, animals were administered either SNV dispersed in 1 ml saline or saline only via jugular vein injection. Three different sizes of spherical SNVs tagged with fluorescein were tested: 519 nanometers (nm), 834 nm or 1000 nm diameter. For characterization purposes, the particles were visualized under FEI Quanta 400 field emission scanning electron microscope (FEI Company, Hillsboro, OR) and the surface zeta potential of the particles was analyzed using a Zetasizer nano ZS (Malvern Instruments Ltd., Southborough, MA, USA). The particles as visualized under scanning electron microscope and their surface charges are shown in Figure 1. The concentration injected was 1.2 × 10−9 in each pregnant rat in 1 ml volume via jugular vein injection. Four hours after SNP administration, laparotomy was performed under isofluorane anesthesia and the maternal livers, uteri, placentas and fetuses were retrieved and weighed. Then, the maternal liver, uteri and placentas were randomly divided into two parts: one for elemental analysis of silicon (Si) and one for histological evaluation and identification of fluorescent SNVs distribution in the tissue following cryo-sectioning. For the fetus, 2 random whole fetuses were used for elemental analysis of the silicon, histologic evaluation and fluorescent SNV distribution each.
Figure 1.
Scanning electron micrographs (A) and surface charges (B) of spherical silicon nanovectors (SNV) used in this study.
Silicon levels were quantitatively measured in each organ using inductively coupled plasma (ICP) mass spectrometry. The parts of the organs intended for analysis of Si were weighed, digested in Proteinase K solution and left for up to 48 hours at 55° C for extraction of Si. Then the extracts were centrifuged at 4200 rpm for 25 min and 0.5 mL of the supernatant were withdrawn, diluted with 2.5 mL of DI water and analyzed for Si contents. Silicon contents were measured using a Varian Vista-Pro Inductively Coupled Plasma Atomic Emission Spectrometer (ICP–AES) housed in the Rice University Geochemistry Laboratory. Si was detected at the following wavelengths 250.69, 251. 43, 251.61 and 288.158 nm. Six standards were prepared using 1 ppm sodium silicate as a stock solution and 18-μΩ water as a diluent. Yttrium (1 ppm) was added to both standards and samples in order to correct for instrumental drift during the run. A calibration run including the internal control was made before each group of 15 samples. In addition, samples were analyzed in random order to avoid any bias in data acquisition. The detection limit of Si was 15 ppb. For measurement of the total silicon in each sample (100%), the original particles suspensions were diluted and dissolved in 1N NaOH for 24 hours at 37C. Further, all results were recalculated considering the dilutions performed.
Tissue distribution of SNVs was qualitatively assessed using fluorescence microscopy identifying the absence or presence of SNVs (tagged with fluorescein [FITC]) within each organ. Tissue was stained with DAPI (4′,6-diamidino-2-phenylindole) fluorescent stain to identify nuclear structures of cells. Images were taken with the BX51 fluorescent microscope (Olympus) using filters for DAPI and FITC at 100X magnification.
ANOVA was used to compare differences in silicon concentrations in each tissue based on the three different SNV size. Student’s t-test was used to compare differences in silicon concentrations between individual SNV sizes and control. Fetal silicon levels was measured in 2 random fetuses per litter obtained from a single pregnant rat within each SNV group. Silicon concentrations were calculated as micrograms of silicon per gram of tissue and are presented as mean ± standard deviation. A p–value of < 0.05 was considered significant.
Results
Six animals received 1000 nm SNV, six received 834 nm SNV, six received 519 nm SNV and 5 received saline. The mean weights for each organ are as follows: maternal liver 14.2 ± 7.5 grams, maternal uterus 6.0 ± 2.6 grams, placenta 0.9 ± 0.5 grams, and fetus 1.7 ± 0.9 grams. The number of pups in each group is as follows: 1000 nm SNV 11.7 ± 3.7 pups, 834 nm SNV 13.7 ± 1.6 pups, 519 nm SNV 14.5 ± 2.3 pups and the saline group 13.2 ± 1.3 pups. There were no maternal deaths or stillbirths.
Silicon levels in maternal organs and fetus are summarized in Table 1. In animals that received 1000 nm and 834 nm SNV, silicon levels were significantly higher in the livers and uteri of the SNV group versus saline (Figure 2). In contrast, placental and fetal silicon levels were similar in both groups. This demonstrates that in these large size groups, the SNV did not cross the placenta to the fetus, but remained within the maternal circulation.
Table 1.
Comparison of silicon concentrations (microgram silicon per gram of tissue) in the maternal liver, maternal uterus, placenta and fetus based on silicon nanovector (SNV) size.
| 1000 nm N=6 |
834 nm N=6 |
519 nm N=6 |
Control N=5 |
P values | |
|---|---|---|---|---|---|
| Maternal liver | 32.67 ± 3.55* | 24.86 ± 4.50* | 11.31 ± 2.30* | 6.20 ± 0.17 | <0.001 |
| Maternal uterus | 10.13 ± 2.01* | 8.06 ± 0.48* | 5.59 ± 0.81* | 2.14 ± 0.42 | <0.001 |
| Placenta | 5.27 ± 0.57 | 5.07 ± 0.30 | 4.64 ± 0.30 | 4.07 ± 1.35 | 0.034 |
| Fetus | 5.09 ± 0.75 | 4.97 ± 0.30 | 5.93 ± 0.67** | 4.80 ± 0.33 | 0.012 |
SNV is silicon nanovector. Silicon concentrations are in microgram per gram of tissue. N represents the number of pregnant rats in each SNV group. Fetal silicon levels was measured in 2 random fetuses per litter obtained from a single pregnant rat within each SNV group. ANOVA was used to compare differences in silicon levels (mean ± standard deviation) in each tissue based on SNV size. Student’s t-test was used to compare differences in silicon levels between individual SNV sizes and control.
p<0.001
p=0.01
Figure 2.
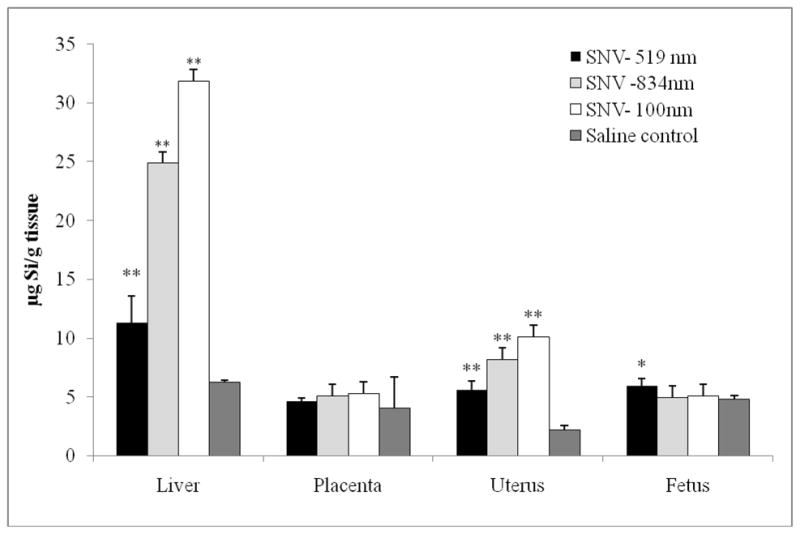
Comparison of silicon concentration (microgram silicon per gram tissue) in liver, uterus, placenta and fetal tissue based on size of silicon nanovector (SNV).
*p=0.01
**p<0.001
Animals that received the 519 nm SNV also demonstrated significantly increased silicon levels in the liver and uterus. Placental levels were similar between groups. However, fetal silicon levels were significantly higher in the 519 nm SNV group compared to saline. This demonstrates that smaller silicon particles lead to some passage across the placenta into the fetal circulation, particularly at a diameter size approaching 500 nm.
Microscopy was used to examine the liver, uterine, placental and fetal tissue. Figure 3 shows that DAPI was visualized in all tissues. FITC was visualized within the liver in those that received 834 and 519 nm SNV. FITC-SNV was seen sparsely within the uterine and placental tissue. Finally, FITC could only be detected within the fetal tissue in those that received 519 nm SNV.
Figure 3. Fluorescent microscopy of liver and fetal tissue.
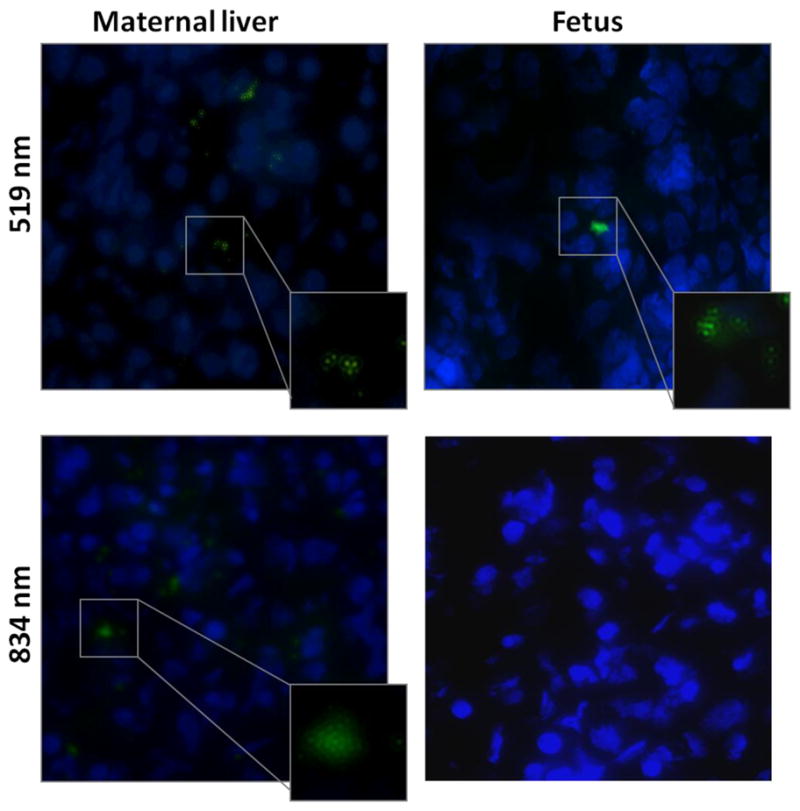
For each specimen, there is tissue fluorescing DAPI (blue) to denote nuclear material within the tissue, and tissue fluorescing FITC (green) which denotes the silicon nanovector (SNV) at 519 and 834 nm. Images at 100X magnification.
Discussion
Size of the SNV appears to be an important factor in its transplacental passage. The larger the size (>800 nm), the greater the concentration of SNV remained in the maternal circulation as demonstrated in the high levels of silicon in the maternal liver and uterus. However, as the SNV size became smaller (519 nm), fetal levels increased demonstrating its ability to cross the placenta into the fetal circulation (Figure 4). Thus, we have been able to determine a size of SNV that identifies its threshold for transplacental passage. Because this characteristic is important, a SNP greater than or equal to 800 nm could be used to load with medication. This device would ideally achieve the ability to prevent medications from reaching the fetal circulation by restricting its circulation only to the maternal circulation.
Porous silicon nanoparticles are nanovectors that have the potential to enhance drug delivery.13 This system is attractive in the field of medicine due to its biocompatibility, biodegradable and resorption characteristics. These porous particles range in diameter from 400 nm to several μm and approximately 50–80% of the particle is free volume that can be loaded with molecular entities, usually a drug. The pores of the silicon particle can vary to accommodate a variety of medications.11 Due to the silicon’s electronic, chemical and optical properties, these nanovectors can be customized to target specific tissues. In oncology, delivering high concentration of agents/medications to specific tumor sites is a primary focus for these nanovectors. Anti-cancer therapeutics have utilized porous silicon for the delivery of cisplatin and doxorubicin in vitro.13 In cardiovascular medicine, various nanovectors, e.g. liposomes, have been used as carriers for contrast media for diagnostic studies such as MRI and computed tomography to enhance the imaging.16
From an obstetrical perspective, nano-scale devices have been studied to determine their effects in pregnancy. Liposomes have been investigated to try to reduce placental transfer of medications such as valproic acid and thyroxine.20, 21 Liposomes are comprised of lipids than can encapsulate particles, proteins and medications. Both in vitro and in vivo studies demonstrate that large, positively charged, cationic liposomes tend to not allow medications to cross the placenta.20–26 Despite these characteristics, liposomes have not entered the clinical practice of obstetrics. More recently, dendrimers have been studied to improve medication delivery via a transvaginal route, in particular for intra-amniotic infection.27, 28
Our study is the first to explore silicon-based nanovectors in the field of obstetrics. The ultimate goal of this delivery system is to tailor physicochemical characteristics to improve medical therapy in pregnancy by inhibiting the transplacental passage of low-molecular weight medications harmful to the fetus. This initial study focused on determining the size threshold of the spherical silicon nanovectors that inhibits its passage across the placenta. The pregnant rat model was used due to its simplicity to examine placental passage of medications during pregnancy. There are some differences between the rat and human placenta. The rat placenta has a triepithelial placental barrier whereas the human has a hemomonochoriial placenta.29 The pore size for the rat is approximately 10–20 nm which is larger than the 6 nm size in the human. Moreover, the electrical potential across the rat placenta is + 15 mV and in the human placenta it is −3 mV. Thus, it is important to consider the differences between the rat and human placenta before simply extrapolating these results to the human.
Here we have demonstrated that a SNV greater than 800 nm does not cross the placenta and remains within the maternal circulation. Smaller SNPs with size approaching 500 nm do cross the placental and reach the fetal circulation. Thus, the SNP represents an ideal delivery that effectively addresses a fundamental problem in obstetrical care-adverse fetal exposures to maternal medications. Future studies will be focused on evaluation of the effect of different shapes of the vectors on transplacental passage and on loading the SNP vehicle with medications and measuring its targeting ability.
Acknowledgments
This work was supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 RR024148 [TL1 RR024147 for the T32 program; KL2 RR0224149 for the K12 program] from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
We would also like to acknowledge Matthew G. Landry for the illustrations (Department of Nanomedicine at the University of Texas Health Science Center at Houston).
Financial Support: Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 RR024148 [TL1 RR024147 for the T32 program; KL2 RR0224149 for the K12 program] from the National Center for Research Resources
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aviv RI, Chubb K, Lindow SW. The prevalence of maternal medication ingestion in the antenatal period. S Afr Med J. 1993;83:657–60. [PubMed] [Google Scholar]
- 2.Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188:1039–45. doi: 10.1067/mob.2003.223. [DOI] [PubMed] [Google Scholar]
- 3.Glover DD, Rybeck BF, Tracy TS. Medication use in a rural gynecologic population: prescription, over-the-counter, and herbal medicines. Am J Obstet Gynecol. 2004;190:351–7. doi: 10.1016/j.ajog.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Refuerzo JS, Blackwell SC, Sokol RJ, et al. Use of over-the-counter medications and herbal remedies in pregnancy. Am J Perinatol. 2005;22:321–4. doi: 10.1055/s-2005-873235. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. ACOG Educational Bulletin. Washington DC: 1997. Teratology; p. 236. [PubMed] [Google Scholar]
- 6.van der Aa EM, Peereboom-Stegeman JH, Noordhoek J, Gribnau FW, Russel FG. Mechanisms of drug transfer across the human placenta. Pharm World Sci. 1998;20:139–48. doi: 10.1023/a:1008656928861. [DOI] [PubMed] [Google Scholar]
- 7.Forestier F, Daffos F, Capella-Pavlovsky M. Low molecular weight heparin (PK 10169) does not cross the placenta during the second trimester of pregnancy study by direct fetal blood sampling under ultrasound. Thromb Res. 1984;34:557–60. doi: 10.1016/0049-3848(84)90260-3. [DOI] [PubMed] [Google Scholar]
- 8.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine--challenge and perspectives. Angew Chem Int Ed Engl. 2009;48:872–97. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theis T, Parr D, Binks P, et al. nan’o.tech.nol’o gy n. Nat Nanotechnol. 2006;1:8–10. doi: 10.1038/nnano.2006.77. [DOI] [PubMed] [Google Scholar]
- 10.Cerami A. Inflammatory cytokines. Clin Immunol Immunopathol. 1992;62:S3–10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 11.Salonen J, Kaukonen AM, Hirvonen J, Lehto VP. Mesoporous silicon in drug delivery applications. J Pharm Sci. 2008;97:632–53. doi: 10.1002/jps.20999. [DOI] [PubMed] [Google Scholar]
- 12.Salonen J, Laitinen L, Kaukonen AM, et al. Mesoporous silicon microparticles for oral drug delivery: loading and release of five model drugs. J Control Release. 2005;108:362–74. doi: 10.1016/j.jconrel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Anglin EJ, Cheng L, Freeman WR, Sailor MJ. Porous silicon in drug delivery devices and materials. Adv Drug Deliv Rev. 2008;60:1266–77. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YY, Cunin F, Link JR, et al. Polymer replicas of photonic porous silicon for sensing and drug delivery applications. Science. 2003;299:2045–7. doi: 10.1126/science.1081298. [DOI] [PubMed] [Google Scholar]
- 15.Tasciotti E, Liu X, Bhavane R, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3:151–7. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 16.Godin B, Gu J, Serda RE, et al. Tailoring the degradation kinetics of mesoporous silicon structures through PEGylation. J Biomed Mater Res A. 2010;94:1236–43. doi: 10.1002/jbm.a.32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serda RE, Gu J, Bhavane RC, et al. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009;30:2440–8. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Serda RE, Ferrati S, Godin B, Tasciotti E, Liu X, Ferrari M. Mitotic trafficking of silicon microparticles. Nanoscale. 2009;1:250–9. doi: 10.1039/b9nr00138g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SY, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20:495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 20.Bajoria R, Fisk NM, Contractor SF. Liposomal thyroxine: a noninvasive model for transplacental fetal therapy. J Clin Endocrinol Metab. 1997;82:3271–7. doi: 10.1210/jcem.82.10.4301. [DOI] [PubMed] [Google Scholar]
- 21.Barzago MM, Bortolotti A, Stellari FF, et al. Placental transfer of valproic acid after liposome encapsulation during in vitro human placenta perfusion. J Pharmacol Exp Ther. 1996;277:79–86. [PubMed] [Google Scholar]
- 22.Bajoria R, Contractor SF. Effect of the size of liposomes on the transfer and uptake of carboxyfluorescein by the perfused human term placenta. J Pharm Pharmacol. 1997;49:675–81. doi: 10.1111/j.2042-7158.1997.tb06091.x. [DOI] [PubMed] [Google Scholar]
- 23.Bajoria R, Contractor SF. Effect of surface charge of small unilamellar liposomes on uptake and transfer of carboxyfluorescein across the perfused human term placenta. Pediatr Res. 1997;42:520–7. doi: 10.1203/00006450-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Bajoria R, Sooranna SR, Contractor SF. Endocytotic uptake of small unilamellar liposomes by human trophoblast cells in culture. Hum Reprod. 1997;12:1343–8. doi: 10.1093/humrep/12.6.1343. [DOI] [PubMed] [Google Scholar]
- 25.Tuzel N, Patel HM, Ryman BE. The fate in vivo of liposomally entrapped drugs in pregnant rats. Biochem Soc Trans. 1980;8:559–60. doi: 10.1042/bst0080559. [DOI] [PubMed] [Google Scholar]
- 26.Tuzel-Kox SN, Patel HM, Kox WJ. Uptake of drug-carrier liposomes by placenta: transplacental delivery of drugs and nutrients. J Pharmacol Exp Ther. 1995;274:104–9. [PubMed] [Google Scholar]
- 27.Menjoge AR, Navath RS, Asad A, et al. Transport and biodistribution of dendrimers across human fetal membranes: implications for intravaginal administration of dendrimer-drug conjugates. Biomaterials. 2010;31:5007–21. doi: 10.1016/j.biomaterials.2010.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Navath RS, Menjoge AR, et al. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int J Pharm. 2010;395:298–308. doi: 10.1016/j.ijpharm.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stulc J. Placental transfer of inorganic ions and water. Physiol Rev. 1997;77(3):805–836. doi: 10.1152/physrev.1997.77.3.805. [DOI] [PubMed] [Google Scholar]