Abstract
New treatments are needed for malignant pleural mesothelioma (MPM), which currently has a poor prognosis. Cellular immortalisation, one of the hallmarks of cancer, depends on the activity of a telomere length maintenance mechanism (TMM) – either telomerase or alternative lengthening of telomeres (ALT). The TMMs are widely regarded as potential targets for cancer therapies and telomerase inhibitors have entered clinical trials. The aim of this study was to determine what proportion of MPMs use ALT and/or telomerase. Forty-three MPMs from 42 patients were examined for telomerase and ALT activity. Telomerase activity was detected by immunoaffinity purification followed by the telomere repeat amplification protocol (TRAP), and ALT activity was determined by the C-circle assay and by assessing telomere lengths using terminal restriction fragment analyses. We found that 43 of 43 MPMs were telomerase-positive[+] and ALT-negative[−]. Therefore, to investigate whether pleural mesothelial cells are unusually susceptible to activation of telomerase, we examined activation of the TMMs in an in vitro model of cellular immortalisation, in which normal pleural mesothelial cells were transduced with simian virus 40 (SV40) oncogenes. We found that normal mesothelial cells were TMM-negative, and that expression of the SV40 oncogenes did not directly activate telomerase or ALT. Immortalisation, which in this experimental system results from additional genetic changes that have not yet been identified, was accompanied by activation of either TMM. Therefore, pleural mesothelial cells are capable of activating either TMM in vitro, and the observation that 100% of MPMs were telomerase[+] suggests that there are factors in vivo that select for telomerase activity during oncogenesis of this tumour type. We conclude that MPM is a tumour that could be considered for anti-telomerase therapy.
Keywords: Malignant mesothelioma, Telomerase, Alternative lengthening of telomeres, Immortalisation, Telomere maintenance mechanism, Pleura
1. Introduction
Activation of a telomere maintenance mechanism (TMM) is essential for tumour cells to overcome the normal limit on cell proliferation resulting from the telomere shortening that normally accompanies each cell division [1]. When telomeres become too short, a DNA damage response is activated resulting in senescence, which includes permanent cell cycle arrest [2]. Inactivation of key tumour suppressor genes, such as tumour protein 53 (TP53) and retinoblastoma protein (RB) can result in a temporary escape from senescence, but the telomeres continue to shorten and eventually become so short that the cell population enters a state referred to as crisis and ceases expansion [1]. Rarely, cells escape from crisis via epigenetic or genetic alterations that are currently unknown, and this is associated with activation of a TMM which prevents further telomere shortening, and results in acquisition of unlimited proliferative potential, i.e., immortalisation. Cellular immortalisation is one of the hallmarks of cancer [3].
There are two known TMMs: telomerase and alternative lengthening of telomeres (ALT). Telomerase is a ribonucleoprotein complex containing an RNA subunit that acts as the template for synthesis of telomeric DNA by the catalytic subunit, TERT [4]. Approximately 85–90% of all tumours are telomerase[+] [5], whereas most of the remaining tumours are ALT[+]. ALT is a recombination-dependent DNA replication mechanism that usually results in telomeres that are long on average and highly heterogeneous in length [6], and is most common in tumours of mesenchymal or neuroepithelial origin, such as soft tissue sarcomas, osteosarcomas, and glioblastoma multiforme [7]. It has recently been demonstrated that ALT[+] cell lines have elevated levels of extrachromosomal, partly single-stranded circles of telomeric DNA where the C-rich strand is essentially intact and the G-rich strand contains one or more gaps (C-circles) [8].
Malignant mesothelioma (MM) is a highly aggressive disease that arises from the mesothelial cells of the serosal membranes which line the pleura, peritoneum and pericardium. It can be classified into histological subtypes by epithelioid or sarcomatous morphology, or features of both (biphasic) [9]. The aetiology of pleural and peritoneal MMs has been linked to exposure to asbestos and in some studies has been associated with simian virus 40 (SV40) [10]; in vitro studies suggest that SV40 and asbestos can act as co-carcinogens [11,12]. The development of MM has a long latency period of between 20 and 40 years after exposure and the tumours are often unresectable and resistant to both radiotherapy and currently available chemotherapies. They account for approximately 1000 deaths per year in the UK and 2000–3000 in the US, with median survival of patients being approximately one year from diagnosis [13]. Despite recent improvements [14], there is an urgent need for much more effective therapy for MM. Because the presence of an activated TMM is an almost universal feature of the cancer phenotype, and non-malignant cells do not have sufficient levels of TMM activity to prevent telomere shortening, TMMs are attractive targets for anti-cancer therapies [15–18]. In this context, it is important to know whether MMs use telomerase or ALT to maintain telomere lengths. In previous studies, ALT and telomerase activity have both been detected in diffuse malignant peritoneal mesotheliomas (DMPM) – in 18% and 64% of tumours, respectively [19] – but a greater proportion (90%) of pleural MMs (MPMs) were reported to be telomerase[+] [20], and ALT activity has not been identified in any MPMs to date. In this study, we examined a set of 43 MPMs to determine which TMM was activated.
The results indicated that all of the MPMs were telomerase[+]. We therefore used an in vitro model system of immortalisation to determine whether pleural mesothelial cells are unusually susceptible to activation of telomerase. Activation of TMMs can be reproduced in vitro in human fibroblasts, and in mesothelial and epithelial cells following transduction with the oncogenes of DNA tumour viruses such as SV40 and human papillomaviruses (HPV) and also in rare circumstances through spontaneous immortalisation [1]. In these cells, escape from senescence and a temporarily extended proliferative life span results from inactivation of the TP53 and RB tumour suppressor pathways through the action of the viral oncoproteins, or through spontaneous genetic or epigenetic alterations. Inactivation of tumour suppressors by SV40 and HPV oncoproteins occurs through the direct binding and functional inactivation of p53 and RB family proteins by SV40 large T antigen, or by binding and degradation of p53 and pRb by HPV-16 E6 and E7 oncoproteins, respectively. Cultures of human cells transformed by these viral genes typically enter crisis, and rare cells may become immortalised by activating either telomerase or ALT through genetic or epigenetic changes that have not yet been identified.
However, in contrast to observations in other human cell culture models, pleural mesothelial cells infected with SV40 were reported to rapidly induce telomerase activity prior to immortalisation [21] suggesting that telomerase activation is not a critical factor for immortalisation of these cells; moreover, ALT was not activated in any of these cultures. We found here that like most other cell types, pleural mesothelial cells are TMM-negative prior to immortalisation, and that these cells are capable of activating either mechanism during immortalisation in vitro. Therefore, additional factors must select for telomerase activity in the genesis of MPM in vivo.
2. Methods
2.1. Tissue samples
MPMs were collected at the Karmanos Cancer Center, Detroit, Michigan, USA, with Institutional Review Board approval. These tumours had been surgically resected as part of the patients’ routine management and stored at −80°C until analysis. From 42 patients, 43 mesothelioma samples were obtained; one patient had two separate tumour samples taken at different surgeries (tumours 1a and b).
2.2. Cell culture
Normal mesothelial cells were obtained from individuals with non-malignant effusions, grown in culture and transduced with SV40 early region genes as described previously; the cells that gave rise to MeT-4 clones were from a pleural effusion and those that gave rise to MeT-5A cells were from ascites fluid [22]. ALT[+] GM847 cells and telomerase[+] HCT116 cells were grown in Dulbecco’s Modified Eagle Medium with 10% foetal bovine serum.
2.3. Total cell protein extractions
Fifty milligrams of tumour tissue or 1–5 × 106 cells were lysed in 200 μL Buffer A (20 mM 4-[2-hydroxyethyl]-1-piperazineethanesulphonic acid-KOH pH 7.9, 300 mM KCl, 2 mM MgCl2, 10% (v/v) glycerol, 1 mM ethylenediaminetetraacetic acid [EDTA] pH 8.0, 0.1% (v/v) Triton X-100, 1 mM dithiothreitol and phenylmethylsulphonyl fluoride) for 1 hour, rotating at 4°C. Samples were centrifuged for 20 min at 17,000 × g at 4°C, then protein concentration was measured using a BCA kit (Thermo Fisher Scientific, Waltham, USA).
2.4. Immunoaffinity purification (IP) of telomerase enzyme
IP of telomerase was performed as described [23]. For each assay, 1 mg of total cell protein was diluted to 1 mL with Buffer A. Protein extracts were incubated with 20 μg anti-TERT antibody, rotating for 30 min at 4°C, then 40 μL 50% protein G-bead (Roche) slurry was added. After 1 h rotating at 4°C, protein-antibody-G-protein bead complexes were isolated on a microspin column (GE HealthCare, Buckinghamshire, UK) and washed with 5 mL Buffer A. Telomerase protein was eluted from the antibody-bead complex for 1 h in 200 μL Buffer A containing 15 nmoles of TERT peptide antigen at room temperature.
2.5. Telomere repeat amplification protocol polymerase chain reaction (PCR)
The PCR-based telomere repeat amplification protocol (TRAP) assay was performed as described [24] with the following modifications. For each reaction, 10, 3, 1, 0.3, 0.1, 0.03 and 0.01 μg of total cell protein or equivalent volumes of the immunopurified telomerase was used to add telomeric repeats onto a substrate primer M2 (TS), 5′-aatccgtcgagcagagtt-3′ for 30 min at 25°C. Telomeric DNA was then amplified using 1.8 ng M2 and 0.9 ng ACX primers, 5′-gcgcggcttacccttacccttaccctaacc-3′ in modified PCR conditions (45 mM Tris–HCl pH 8.8, 11 mM ammonium sulphate, 4.5 mM MgCl2, 6.7 mM 2-mercaptoethanol, 4.4 mM EDTA pH 8.0, 113 μg/mL bovine serum albumin [BSA], 1 mM each of dATP, dTTP, dGTP and dCTP and 10 units of Taq [Roche Diagnostics, Indianapolis, USA]). From 1 mg total tumour protein, 10 μg of total tumour protein was added to the TRAP PCR (a dilution factor of 100) and immunopurified telomerase was obtained from the remaining lysate in a final elution volume of 200 μL, of which 2 μL (a dilution factor of 100, equivalent to 10 μg of total tumour lysate) was used in the TRAP PCR. Protein from HCT116 cells was used as a positive control, and Buffer A alone and GM847 protein were used as negative controls. TRAP and IP-TRAP products were detected by SYBR Green I staining (Sigma–Aldrich, St. Louis, USA) after resolution in a 10% polyacrylamide gel with 1 × tris–borate–EDTA buffer. The specificity of all positive results was confirmed by heat-inactivation of the protein at 85°Cfor 15 min prior to its addition to the PCR. All negative results were confirmed with dilutions of the protein and by spiking with telomerase[+] protein to determine whether inhibitors were present in the lysates (data not shown).
2.6. C-circle (CC) assay
Using 30 ng of digested DNA, CC assays were performed as described by Henson et al. [8].
2.7. Terminal restriction fragment (TRF) analysis
Genomic DNA was extracted from 106 cells or 50 mg of tissue and digested overnight at 37°Cusing restriction enzymes Hinf1 and Rsa1 (40 units each/10 μg DNA). TRF analyses were performed as described by Perrem et al. [25] using 1–2 μg digested DNA. Mean TRF lengths were calculated using the formula [Σ Si]/[ Σ ( Si/Li)] where Si is TRF signal at a given distance after background subtraction and Li is the corresponding distance at i [26].
3. Results
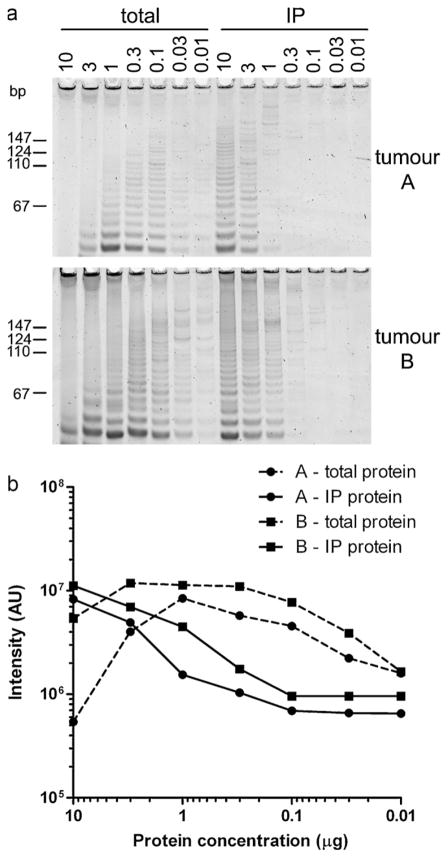
Tumour protein lysates can often contain PCR inhibitors that affect the detection of telomerase activity in the standard TRAP assay. Using 10 μg total protein lysate in the TRAP assay, tumours A and B were found to be negative for telomerase activity, but dilution of protein lysates through to 0.01 μg resulted in detection of telomerase activity in both tumours (Fig. 1a). This demonstrates that the standard TRAP assay can be susceptible to giving false negative results due to TRAP reaction inhibitors that are present in some tumour lysates. To remove these inhibitors, telomerase was immunoaffinity purified (IP) from each total tumour lysate using the TERT HTCS2 antibody. The IP-TRAP assay was positive for telomerase activity, and diluting the protein did not increase telomerase activity demonstrating there were no TRAP inhibitors in the IP protein (Fig. 1a and b). Therefore the IP-TRAP assay was used to determine telomerase activity in MPMs.
Fig. 1.
Telomerase assays. A comparison of conventional TRAP and IP-TRAP assays at 10, 3, 1, 0.3, 0.1, 0.03 and 0.01 μg total protein lysate or equivalent immunopurified (IP) protein for tumours A and B (a). Quantitation of band intensity in AUs (arbitrary units) is shown in (b).
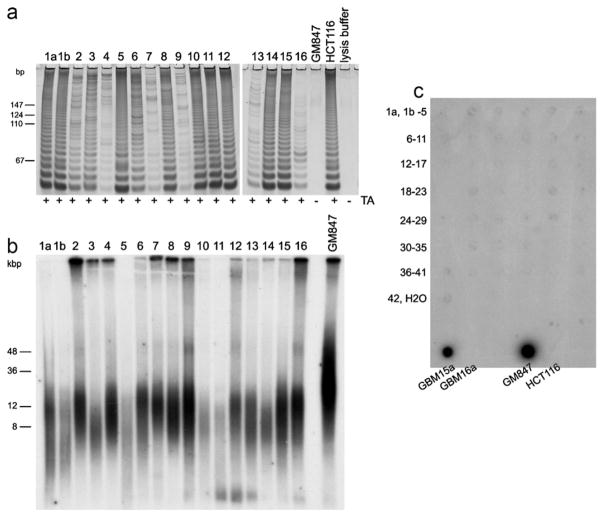
We found that all 43 MPMs were positive for telomerase activity using the IP-TRAP assay with representative results shown in Fig. 2a. Varying levels of telomerase activity were observed, with 24 tumours showing high levels of telomerase activity while moderate activity could be detected in nine tumours and 10 tumours had low levels of telomerase (Table 1). The mean TRF lengths for the 43 tumours ranged from 7.4 to 17.5 kb (Table 1) and for each tumour there was only moderate TRF length heterogeneity, which is characteristic of telomeres maintained by telomerase; representative TRF profiles are shown in Fig. 2b. No tumours displayed long, heterogeneous telomere lengths or had elevated C-circle levels which are characteristic of the ALT phenotype (Fig. 2c).
Fig. 2.
Telomere length maintenance status of MPMs. Representative IP-TRAP assays (a) and terminal restriction fragment (TRF) profiles (b), and CC assays of all MPMs (c) are shown. ALT[+] and ALT[−] cell lines (GM847 and HCT116, respectively), and ALT[+] and ALT[−] glioblastoma multiforme (GBM) tumours (15a and 16a, respectively) were used as positive and negative controls. Telomerase activity (TA) results are indicated as positive (+) or negative (−) (a) and sizes of molecular weight markers (in base pairs [bp] and kilobase pairs [kbp]) are indicated to the left of panels A and B.
Table 1.
Telomerase activity levels and mean terminal restriction fragment (TRF) lengths in malignant pleural mesotheliomas.
| Sample # | TRF lengthsa | Telomerase activityb | Sample # | TRF lengthsa | Telomerase activityb |
|---|---|---|---|---|---|
| 1a | 12.5 | +++ | 22 | 17.5 | +++ |
| 1b | 10.1 | +++ | 23 | 10.5 | + |
| 2 | 12.1 | ++ | 24 | 12.1 | +++ |
| 3 | 9.3 | ++ | 25 | 9.5 | +++ |
| 4 | 15.3 | + | 26 | 8.3 | +++ |
| 5 | 10.0 | +++ | 27 | 9.7 | +++ |
| 6 | 13.4 | ++ | 28 | 9.8 | +++ |
| 7 | 14.5 | + | 29 | 13.6 | +++ |
| 8 | 13.2 | +++ | 30 | 9.0 | +++ |
| 9 | 14.1 | + | 31 | 7.8 | ++ |
| 10 | 8.7 | +++ | 32 | 11.3 | + |
| 11 | 7.4 | +++ | 33 | 8.8 | + |
| 12 | 12.4 | +++ | 34 | 8.2 | +++ |
| 13 | 9.5 | + | 35 | 7.8 | +++ |
| 14 | 11.5 | +++ | 36 | 13.2 | + |
| 15 | 12.5 | +++ | 37 | 9.4 | +++ |
| 16 | 10.9 | + | 38 | 8.5 | ++ |
| 17 | 10.8 | ++ | 39 | 10.5 | + |
| 18 | 12.6 | +++ | 40 | 7.6 | +++ |
| 19 | 10.5 | +++ | 41 | 9.3 | ++ |
| 20 | 14.7 | ++ | 42 | 8.1 | +++ |
| 21 | 16.2 | + |
Mean TRF lengths in kilobases.
“+++” high, “++” moderate and “+” low.
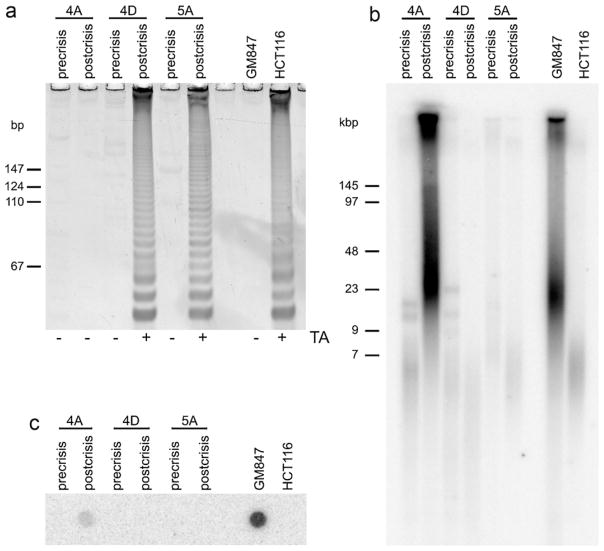
We examined whether TMMs were activated in normal mesothelial cells that had been transduced with an origin of replication (ori)-defective SV40 plasmid containing early region DNA. SV40 transfection of mesothelial cells from two donors (designated 4 and 5) produced three immortalised clones: two clones from donor 4 (MeT-4A and MeT-4D) and one clone from donor 5 (MeT-5A) [22]. During the phase of lifespan extension following SV40 transformation and prior to crisis, these clones did not exhibit telomerase activity, had short, homogeneous telomeres and no C-circles (precrisis lanes, Fig. 3a–c). Six SV40-transformed mesothelial cell clones from four other donors were also negative for telomerase activity (data not shown). Once cells had become immortalised (postcrisis), MeT-4D and MeT-5A cells exhibited telomerase activity and retained telomeres that were homogeneously short. TRFs after immortalisation were slightly shorter than in the precrisis cultures, which is consistent with telomere shortening during crisis phase, followed by telomerase-mediated telomere length maintenance. In contrast, MeT-4A cells had long, heterogeneous TRFs after immortalisation, did not exhibit telomerase activity and produced elevated levels of C-circles, which are all characteristics of the ALT phenotype (postcrisis lanes, Fig. 3a–c). These data support the conclusion that SV40-induced transformation of mesothelial cells does not directly induce either ALT or telomerase activity, and when immortalisation of pleural mesothelial cells occurs, these cells can activate either telomerase or ALT to maintain telomere lengths.
Fig. 3.
Telomere length maintenance mechanisms in SV40-transduced mesothelial cells. IP-TRAP (a), terminal restriction fragment (b) and C-circle (c) assays of precrisis and postcrisis SV40-transduced pleural mesothelial cells. ALT[+] and telomerase[+] cell lines (GM847 and HCT116, respectively) were used as positive and negative controls. Telomerase activity (TA) results are indicated as positive (+) or negative (−) (a) and sizes of molecular weight markers are indicated to the left of panels A and B.
4. Discussion
We found that all 43 MPMs were positive for telomerase activity, had short TRF lengths and an absence of C-circle activity indicating that no tumours were ALT[+]. This result is consistent with the study of Dhaene et al. [20] showing that 20/22 MPMs had telomerase activity. Previous studies have shown that there is a greater prevalence of ALT in tumours derived from the mesenchyme [7] and although ALT has not been detected in MPMs, Villa et al. [19] found that 18% of peritoneal MMs had ALT. This suggests that there may be differences between peritoneal and pleural mesothelial cells that result in a different pattern of TMM activation during tumorigenesis. In this regard, it is of interest to note that MMs of pleural and peritoneal origins have different clinical features and gene expression patterns [27].
The observation that peritoneal MMs may utilise either telomerase or ALT indicates that subsets of peritoneal MMs can undergo different pathways of tumorigenesis that result in activation of either TMM. In support of this hypothesis, it has been shown that distinct gene expression signatures can distinguish tumours and cell lines that are ALT[+] from those that are telomerase[+] [28].
The observation that peritoneal MMs may be either telomerase[+] or ALT[+] is consistent with in vitro data showing when cells of the same cell type and from the same individual were immortalised following transformation with SV40 early region genes, immortalisation was associated with activation of either ALT or telomerase [29]. We demonstrate here that pleural mesothelial cells in vitro behave similarly to other cell types during the immortalisation process: MeT-4A and MeT-4D cells, which are separately immortalised clones derived from SV40-transformed pleural mesothelial cells, were ALT[+] and telomerase[+], respectively. Interestingly, this was not reflected in MPMs in vivo where, in this and the previous study [20] 43/43 and 20/22, respectively, MPMs were found to be telomerase[+] and none were found to be ALT[+]. Presumably, either the genetic events involved in SV40-induced immortalisation differ from those involved in TMM activation during the oncogenesis of MPMs, or there are selection pressures in vivo that overwhelmingly favour survival and progression of telomerase[+] MPMs. It remains a possibility that activation of ALT in MPMs is a rare occurrence; investigation of a larger panel of MPMs would be required to confirm this.
We have previously reported that normal mesothelial cells from pleura or peritoneum have an extended, but finite, proliferative capacity following transformation with either the SV40 early region genes, the whole SV40 genome, or the HPV-16 E6 and E7 genes, and most cultures did not escape from the population crisis that ensued [22,30]. Moreover, SV40- and HPV-transformed cells had no detectable ALT or telomerase activity during the extended phase of proliferation prior to crisis, but activated one or other of these TMMs after escape from crisis [29]. This indicates that neither the SV40 nor the HPV-16 oncoproteins are able to induce ALT or telomerase activity. We have extended these results by demonstrating that telomerase was not activated in a total of nine SV40-transformed, non-immortalised mesothelial cell clones from six individual donors (data not shown), and that pleural mesothelial cells from a single individual were able to activate either ALT (MeT-4A cell line) or telomerase (MeT-4D cell line), but only after escape from crisis. These data reinforce the evidence that the SV40 oncoproteins are unable to directly activate either TMM.
These results were in contrast to a study in which infection of pleural mesothelial cells with SV40 virus resulted in the induction of telomerase activity [21]. Moreover, these clones did not have a defined senescence or crisis period and there was a high rate of immortalisation. It is possible that these different outcomes reflect the different experimental approaches, i.e. infection with whole SV40 virus [21] versus transfection with the viral oncogenes, or possibly differences in oncogene expression levels.
In our studies, mesothelial cell immortalisation by transfected SV40 DNA was accompanied by activation of ALT in one cell line, which was not observed with SV40 infection [11,21]. The mechanism of ALT activation is largely unknown, however it is often associated with chromosomal instability such as chromosomal deletions and amplification in liposarcomas [31] and chromosomal breakage/fusion/bridge cycles causing neoacrocentric and minute chromosomes in ALT cell lines [32]. Somatic cell hybridisation analyses indicate that immortalisation via activation of ALT results from loss of putative ALT repressors [33], which may be due to chromosomal instability.
In conclusion, we have confirmed that MPMs use telomerase to maintain their telomeres and found no evidence of ALT activity in this tumour type. Anti-telomerase drugs may therefore be a useful treatment for these tumours. We found that transformation of pleural mesothelial cells in vitro by the SV40 oncogenes did not activate telomerase or ALT activity directly, but that pleural mesothelial cells are capable of activating either ALT or telomerase when they escape crisis due to as yet unknown genetic or epigenetic events. It will therefore need to be determined whether treatment of telomerase[+] MPMs with effective anti-telomerase drugs would result in activation of ALT in MPMs in vivo.
5. Conclusions
Pleural mesothelial cells are capable of activating either TMM in vitro, but 43/43 MPMs examined were telomerase[+], suggesting that there are factors in vivo that select for telomerase activity during genesis of this malignancy. Pleural MM is a tumour that may be considered for telomerase-targeted therapies.
Acknowledgments
This research was supported by a Cancer Institute NSW Early Career Development Award and Cancer Council NSW Program Grant, and in part by the Intramural Research Program of the NIH.
Footnotes
Conflict of interest
None. The funding agencies had no role in the study or its publication.
References
- 1.Reddel RR. The role of senescence and immortalization in carcinogenesis. Carcinogenesis. 2000;21:477–84. doi: 10.1093/carcin/21.3.477. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–40. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn EH. Telomerases. Annu Rev Biochem. 1992;61:113–29. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- 5.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 6.Henson JD, Reddel RR. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett. 2010;584:3800–11. doi: 10.1016/j.febslet.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–30. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 8.Henson JD, Cao Y, Huschtscha LI, Chang AC, Au AY, Pickett HA, et al. DNA C-circles are specific and quantifable markers of alternative-lengthening-of-telomeres activity. Nat Biotechnol. 2009;27:1181–5. doi: 10.1038/nbt.1587. [DOI] [PubMed] [Google Scholar]
- 9.Husain AN, Colby TV, Ordonez NG, Krausz T, Borczuk A, Cagle PT, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–31. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol. 2008;9:147–57. doi: 10.1007/s11864-008-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, et al. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci USA. 2000;97:10214–9. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, et al. Crocidolite asbestos and SV40 are cocarcinogens in human mesothe-lial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci USA. 2006;103:14128–33. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002–2050. Br J Cancer. 2005;92:587–93. doi: 10.1038/sj.bjc.6602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weder W, Kestenholz P, Taverna C, Bodis S, Lardinois D, Jerman M, et al. Neoad-juvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol. 2004;22:3451–7. doi: 10.1200/JCO.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 15.Shay JW, Keith WN. Targeting telomerase for cancer therapeutics. Br J Cancer. 2008;98:677–83. doi: 10.1038/sj.bjc.6604209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folini M, Brambilla C, Villa R, Gandellini P, Vignati S, Paduano F, et al. Antisense oligonucleotide-mediated inhibition of hTERT, but not hTERC, induces rapid cell growth decline and apoptosis in the absence of telomere shortening in human prostate cancer cells. Eur J Cancer. 2005;41:624–34. doi: 10.1016/j.ejca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov. 2006;5:577–84. doi: 10.1038/nrd2081. [DOI] [PubMed] [Google Scholar]
- 18.Beisner J, Dong M, Taetz S, Nafee N, Griese EU, Schaefer U, et al. Nanoparticle mediated delivery of 2′-O-methyl-RNA leads to efficient telomerase inhibition and telomere shortening in human lung cancer cells. Lung Cancer. 2010;68:346–54. doi: 10.1016/j.lungcan.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Villa R, Daidone MG, Motta R, Venturini L, De Marco C, Vannelli A, et al. Multiple mechanisms of telomere maintenance exist and differentially affect clinical outcome in diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2008;14:4134–40. doi: 10.1158/1078-0432.CCR-08-0099. [DOI] [PubMed] [Google Scholar]
- 20.Dhaene K, Hubner R, Kumar-Singh S, Weyn B, Van Marck E. Telomerase activity in human pleural mesothelioma. Thorax. 1998;53:915–8. doi: 10.1136/thx.53.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foddis R, De Rienzo A, Broccoli D, Bocchetta M, Stekala E, Rizzo P, et al. SV40 infection induces telomerase activity in human mesothelial cells. Oncogene. 2002;21:1434–42. doi: 10.1038/sj.onc.1205203. [DOI] [PubMed] [Google Scholar]
- 22.Ke Y, Reddel RR, Gerwin BI, Reddel HK, Somers AN, McMenamin MG, et al. Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos-induced mesothelioma. Am J Pathol. 1989;134:979–91. [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen SB, Reddel RR. A sensitive direct human telomerase activity assay. Nat Methods. 2008;5:355–60. doi: 10.1038/nmeth.f.209. [DOI] [PubMed] [Google Scholar]
- 24.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 25.Perrem K, Colgin LM, Neumann AA, Yeager TR, Reddel RR. Coexistence of alternative lengthening of telomeres and telomerase in hTERT-transfected GM847 cells. Mol Cell Biol. 2001;21:3862–75. doi: 10.1128/MCB.21.12.3862-3875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 27.Trupiano JK, Geisinger KR, Willingham MC, Manders P, Zbieranski N, Case D, et al. Diffuse malignant mesothelioma of the peritoneum and pleura, analysis of markers. Mod Pathol. 2004;17:476–81. doi: 10.1038/modpathol.3800067. [DOI] [PubMed] [Google Scholar]
- 28.Lafferty-Whyte K, Cairney CJ, Will MB, Serakinci N, Daidone MG, Zaffaroni N, et al. A gene expression signature classifying telomerase and ALT immortalization reveals an hTERT regulatory network and suggests a mesenchymal stem cell origin for ALT. Oncogene. 2009;28:3765–74. doi: 10.1038/onc.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–8. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Silva R, Whitaker NJ, Rogan EM, Reddel RR. HPV-16 E6 and E7 genes, like SV40 early region genes, are insufficient for immortalization of human mesothelial and bronchial epithelial cells. Exp Cell Res. 1994;213:418–27. doi: 10.1006/excr.1994.1218. [DOI] [PubMed] [Google Scholar]
- 31.Costa A, Daidone MG, Daprai L, Villa R, Cantu S, Pilotti S, et al. Telomere maintenance mechanisms in liposarcomas: association with histologic subtypes and disease progression. Cancer Res. 2006;66:8918–24. doi: 10.1158/0008-5472.CAN-06-0273. [DOI] [PubMed] [Google Scholar]
- 32.Gagos S, Chiourea M, Christodoulidou A, Apostolou E, Raftopoulou C, Deustch S, et al. Pericentromeric instability and spontaneous emergence of human neoacrocentric and minute chromosomes in the alternative pathway of telomere lengthening. Cancer Res. 2008;68:8146–55. doi: 10.1158/0008-5472.CAN-08-0945. [DOI] [PubMed] [Google Scholar]
- 33.Perrem K, Bryan TM, Englezou A, Hackl T, Moy EL, Reddel RR. Repression of an alternative mechanism for lengthening of telomeres in somatic cell hybrids. Oncogene. 1999;18:3383–90. doi: 10.1038/sj.onc.1202752. [DOI] [PubMed] [Google Scholar]