Abstract
Background
Hip fracture is associated with high morbidity and mortality. Pelvic external beam radiotherapy (EBRT) is known to increase the risk of hip fractures in women but the effect in men is unknown.
Methods
45,662 men aged ≥66 years, diagnosed with prostate cancer in 1992–2004 were identified from the SEER-Medicare database. Using Kaplan-Meier methods and Cox proportional hazards models, the primary outcome of hip fracture risk was compared among men who received radical prostatectomy (RP), EBRT, EBRT+androgen suppression therapy (AST) or AST alone, controlling for age, osteoporosis, race and other comorbidities. A secondary outcome was distal forearm fractures as an indicator of fragility fracture risk outside the radiation field.
Results
After controlling for covariates, EBRT increased the risk of hip fractures by 76% (HR 1.76, 95% CI 1.38–2.40) without increasing the risk of distal forearm fractures (HR 0.80, 95% CI 0.56–1.14). Combination therapy with EBRT+AST increased the risk of hip fracture 145% relative to RP (HR 2.45, 95% CI 1.88–3.19) and by 40% relative to EBRT (HR 1.40, 95% CI 1.17–1.68). EBRT+AST increased the risk of distal forearm fracture by 43% relative to RP (HR 1.43, 95% CI 0.97–2.10). The number needed to treat to result in 1 hip fracture through 10 years was 51 (95% CI 31–103).
Conclusion
In men with prostate cancer, pelvic 3-D conformal EBRT is associated with a 76% increased risk of hip fracture. This risk is slightly increased further by the addition of short-course AST to EBRT. This risk associated with EBRT is site-specific as there is no increase in the risk of fall-related fractures outside the radiation field.
INTRODUCTION
Hip fracture is associated with high morbidity and significant mortality.1, 2 Osteoporosis is the single greatest risk factor for fractures in general and hip fracture in particular. Factors associated with osteoporosis include age, body mass index, multiple comorbidities, glucocorticoid therapy, post-menopausal status in women and androgen suppression therapy (AST) for prostate cancer in men.3 Fragility fractures such as distal forearm and hip fracture are uniquely associated with falls.4 It is well accepted that pelvic external beam radiotherapy (EBRT) for gynecological malignancies and anorectal carcinoma in women increases the risk of hip fractures;5, 6 the mechanism is understood to be radiation-induced osteonecrosis that can occur as the beam path passes through the bone to reach the target site.7–9
The effect of pelvic EBRT on hip fracture risk in men is unknown. Prostate cancer is the most common solid organ malignancy in men, affecting nearly 200,000 men annually. In 2006, 28% of all men with newly diagnosed prostate cancer received EBRT within 6 months of diagnosis.10 Elderly men with prostate cancer are more commonly treated with EBRT, with or without AST, than younger men.11, 12 Elderly men are also the subpopulation at highest risk for hip fracture based on their age and comorbidities. Randomized clinical trials demonstrate improved survival with EBRT plus 6–36 months of AST (EBRT+AST) compared to either treatment alone in locally advanced prostate cancer13, 14 and 50% of high risk prostate cancer patients treated with EBRT also receive AST.15 Moreover, recent evidence shows that men with high risk prostate cancer treated with lifelong androgen deprivation have improved survival with the addition of EBRT.16
The prevalence of prostate cancer combined with high utilization of EBRT, particularly in the elderly, plus the frequent combination of EBRT+AST intensifies the need for understanding the effect of EBRT alone or in combination with AST on the risk of developing hip fractures in prostate cancer patients, especially among the elderly.
Our hypotheses were that compared to radical prostatectomy (RP), pelvic EBRT increases the risk of hip fractures but not fragility fractures outside the radiation field (distal forearm fractures) and that EBRT+AST would have a higher risk of hip fractures than EBRT alone. Our analysis was limited to 3-D conformal EBRT (henceforth termed EBRT) and did not include intensity modulated radiotherapy (IMRT).
METHODS
Data sources
After approval from the University of Minnesota Institutional Review Board, data were obtained from the Surveillance, Epidemiology, and End Results (SEER) cancer registry linked to Medicare enrollment and utilization data (SEER-Medicare). SEER contains patient and tumor characteristics as well as complete treatment information through 6 months after cancer diagnosis. The 17 geographic regions making up the SEER registry account for 26% of the U.S. population. An elderly subset of the SEER population may be followed beyond the initial year after diagnosis by linking their SEER information to claims data available through Medicare.
Study subjects
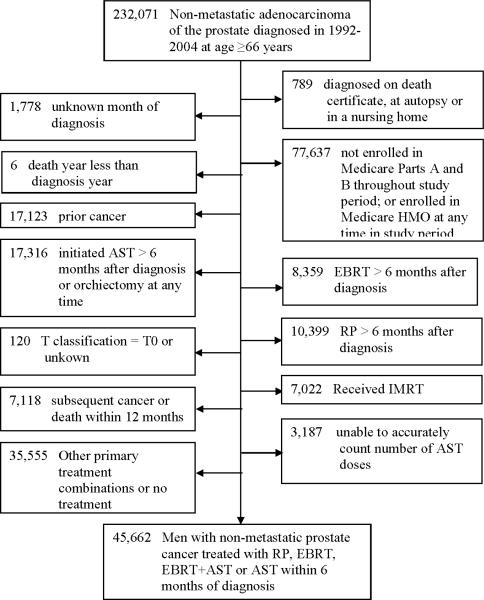
Men 66 years of age or older who received a first diagnosis of non-metastatic prostate cancer in the years 1992 through 2004 were identified in SEER (n=232,071; Figure 1). To ensure complete information, we limited our analysis to those most likely to have complete claims: we excluded patients who were not enrolled in both Part A and Part B Medicare for the 12 months before prostate cancer diagnosis or throughout the study period follow-up, whose prostate cancer had been diagnosed on autopsy or on a death certificate, and those for whom the month of diagnosis was unknown (n remaining = 151,867). 17,123 men with a prior cancer were excluded because they may have received treatment that could put them at risk for fracture. We limited our comparisons to those men managed with one of four non-overlapping treatment strategies within 6 months of prostate cancer diagnosis: (1) radical prostatectomy (RP) (without EBRT or AST), (2) EBRT (without AST), (3) EBRT+AST or (4) AST (without EBRT). EBRT was limited to three-dimensional conformal radiotherapy because IMRT was not used in significant numbers in the Medicare population until 2003 and the short follow-up available (through 2007) would be inadequate to draw conclusions about the long-term risk of hip fracture. We excluded men who received any of these therapies for the first time after 6 months in order to yield a uniform risk group who were all “exposed” to the etiology of interest soon after cancer diagnosis. Among those receiving EBRT+AST we included only those who received a range of AST doses supported by evidence: 6–36 months. These limitations excluded men who initiated AST later in their disease course (after 6 months for the RP and EBRT groups and after 36 months for the EBRT+AST group), thus minimizing the possibility of new metastatic disease after 6 months. Because AST as sole therapy in non-metastatic disease is not supported by evidence we did not place an upper limit on the length of therapy but did require that at least 3 months (commonly, 1 depot shot) be given. This yielded a final cohort of 45,662. Of these men, 7,854 also received brachytherapy, cryotherapy or thermal ablation. We alternately included, excluded and censored these 7,854 men and assessed for a change in outcomes.
Figure 1.
Cohort creation.
RP: radical prostatectomy; EBRT: external beam radiation therapy; AST: androgen suppression
Outcomes defined
Our primary outcome was the cumulative incidence of hospitalization for hip fracture through 2007, identified by Medicare inpatient claims. The cumulative incidence of distal forearm fracture was determined from Medicare Part A and B (i.e., hospital outpatient and Carrier file) claims for fracture of the radius/ulna. We excluded fractures that occurred in the year of diagnosis to allow for wash-out of differences in baseline risk and because such radiation effects are thought to be delayed in onset.
Demographic and cancer characteristics
Age was defined as the age at diagnosis of prostate cancer and was categorized in 5-year increments. A modification of the Charlson comorbidity index for use with Medicare claims data was utilized.17 Charlson score was categorized as 0, 1, ≥2. Disease grade and stage were obtained from the SEER database. Grade was categorized into World Health Organization strata. Stage was categorized using the 1997 modification (i.e., 2 substages within T2) of the TNM classification because more detailed stage information as reported in the 1992 modification (i.e., 3 substages within T2) is not available in SEER for the years 1998–2003. Race, year of diagnosis and SEER registry (i.e. geographic location) were determined from SEER. A history of osteoporosis was identified from diagnosis codes on Medicare Part A or B claims in the year prior to cancer diagnosis. Demographic and cancer characteristics were compared across primary cancer treatment groups and significant differences were inferred by chi square analysis.
Cumulative incidence of hip and distal forearm fracture
Kaplan-Meier time-to-event analysis was used to measure the unadjusted cumulative incidence of hip and distal forearm fracture, stratified by primary cancer treatment group. Fracture rates were compared across treatment groups using the log-rank test. Men were censored at diagnosis of a second cancer, death or end of study.
Mulitvariate model
Multivarate time-to-event analyses were performed with the use of Cox proportional-hazards regression. Separate models were constructed for the outcomes of hip and distal forearm fracture. The primary etiology of interest was the type of prostate cancer treatment. We were thus primarily interested in the hazard ratio of hip or distal forearm fracture in those managed with EBRT vs. RP, AST vs. RP and EBRT+AST vs. RP. We also report the risk of hip and distal forearm fracture after treatment with EBRT+AST vs. EBRT. Other covariates in the model included age, race, year of diagnosis, registry, comorbidities, osteoporosis, tumor stage and tumor grade. In order to provide an absolute measure of the impact of EBRT on hip fracture, the number of men who must be treated with EBRT to result in 1 hip fracture was calculated. This was done by calculating the inverse of the difference between the adjusted risk of hip fracture in the RP-treated and EBRT-treated men at 10 years. All analyses were 2-sided. Type 1 error rate was set at P<0.05. All analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
The final cohort of 45,662 consisted of 8,146 men managed with RP, 13,396 managed with EBRT, 6,974 managed with EBRT+AST and 17,146 managed with AST (Table 1). Median follow-up for the total cohort was 49 months and varied by treatment group (RP = 54 months, EBRT = 62 months, EBRT+AST = 46 months, AST = 38 months; Table 1). 10-year probability of overall survival likewise varied across treatment groups with RP-treated men having the highest likelihood of survival (77%) followed by EBRT (48%), EBRT+AST (40%) and AST (29%). Of the 45,662 men in the cohort, 1,636 (3.6%) developed hip fracture; distal forearm fracture occurred in 451 (1%; Table 1).
Table 1.
Description of Cohort. Demographic and clinical characteristics, stratified by prostate cancer treatment. Incidence of hip and forearm fracture, stratified by demographic and clinical characteristics.
| RP | EBRT | EBRT+AST | AST | Total | Hip fracture | Forearm fracture | |
|---|---|---|---|---|---|---|---|
| N (% of total) | 8146 (18%) | 13396 (29%) | 6974 (15%) | 17146 (38%) | 45665 | 1636 (3.6%) | 451 (1.0%) |
| Median Follow-up (months) | 54 | 62 | 46 | 38 | 49 | ||
| 10-year overall survival | 81% | 49% | 49% | 34% | 49% | ||
| 10-year incidence of hip fracture | 3% | 8% | 9% | 16% | 9% | ||
| Cohort Characteristics | Column percent | Overall percent | |||||
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 66–69 | 55.3% | 21.4% | 19.5% | 12.9% | 25.4% | 1.5% | 0.6% |
| 70–74 | 37.4% | 39.9% | 37.0% | 22.1% | 32.3% | 2.8% | 0.9% |
| 75–79 | 6.7% | 30.0% | 32.2% | 28.0% | 25.4% | 4.1% | 1.1% |
| 80–84 | 0.5% | 7.5% | 9.7% | 23.2% | 12.5% | 6.6% | 1.5% |
| 85+ | 0.1% | 1.1% | 1.6% | 13.8% | 5.8% | 7.9% | 1.2% |
| Charlson score | |||||||
| 0 | 85.0% | 74.8% | 72.0% | 69.0% | 74.0% | 3.3% | 0.9% |
| 1 | 12.1% | 18.1% | 19.2% | 20.1% | 18.0% | 4.4% | 1.2% |
| 2+ | 2.9% | 7.1% | 8.8% | 10.9% | 8.1% | 4.4% | 1.1% |
| Race | |||||||
| White | 82.2% | 82.4% | 80.1% | 74.5% | 79.1% | 3.8% | 1.1% |
| Black | 6.7% | 9.7% | 7.1% | 8.7% | 8.4% | 2.4% | 0.5% |
| Hispanic | 7.0% | 4.3% | 7.0% | 11.6% | 7.9% | 3.3% | 0.8% |
| Asian | 3.9% | 3.4% | 5.3% | 4.5% | 4.2% | 2.5% | 0.8% |
| Other | 0.3% | 0.2% | 0.5% | 0.7% | 0.4% | 2.0% | 2.4% |
| WHO grade | |||||||
| 1 | 3.0% | 9.3% | 2.3% | 4.0% | 5.1% | 5.8% | 1.5% |
| 2 | 82.0% | 73.8% | 57.9% | 64.8% | 69.4% | 3.2% | 0.9% |
| 3 | 14.4% | 13.7% | 37.6% | 28.2% | 22.9% | 4.1% | 1.1% |
| Undifferentiated | 0.6% | 3.2% | 2.2% | 3.0% | 2.5% | 5.1% | 0.9% |
| Tumor stage by T classification (all were N0M0) | |||||||
| 1 | 1.5% | 32.4% | 30.1% | 32.1% | 26.4% | 3.5% | 1.0% |
| 2 | 83.2% | 63.4% | 61.7% | 63.6% | 66.8% | 3.6% | 1.0% |
| 3 | 14.1% | 3.7% | 7.0% | 3.3% | 5.9% | 3.5% | 0.9% |
| 4 | 1.2% | 0.5% | 1.1% | 1.0% | 0.9% | 4.3% | 0.5% |
| Osteoporosis at baseline | |||||||
| 0 | 98.5% | 98.7% | 98.0% | 97.7% | 98.2% | 3.6% | 1.0% |
| 1 | 1.5% | 1.3% | 2.0% | 2.3% | 1.8% | 5.3% | 1.0% |
| Radical prostatectomy | |||||||
| 0 | 0.0% | 98.7% | 99.0% | 95.3% | 79.8% | 4.2% | 1.1% |
| 1 | 100.0% | 1.3% | 1.0% | 4.7% | 20.2% | 1.1% | 0.6% |
| Subsequent cancer | |||||||
| 0 | 94.9% | 87.0% | 91.4% | 94.2% | 91.8% | 3.7% | 1.0% |
| 1 | 5.1% | 13.0% | 8.6% | 5.8% | 8.2% | 2.8% | 0.3% |
| Brachytherapy | |||||||
| 0 | 100.0% | 84.5% | 77.8% | 79.0% | 84.2% | 4.0% | 1.1% |
| 1 | 0.0% | 15.5% | 22.2% | 21.0% | 15.8% | 1.2% | 0.5% |
| High Dose Rate Brachytherapy | |||||||
| 0 | 99.9% | 96.7% | 95.3% | 99.1% | 98.0% | 3.6% | 1.0% |
| 1 | 0.1% | 3.3% | 4.7% | 0.9% | 2.0% | 1.4% | 0.4% |
| Thermal ablation | |||||||
| 0 | 100.0% | 99.7% | 99.9% | 100.0% | 99.9% | 3.6% | 1.0% |
| 1 | 0.0% | 0.3% | 0.1% | 0.0% | 0.1% | 4.7% | 2.3% |
| Cryotherapy | |||||||
| 0 | 100.0% | 99.9% | 99.9% | 98.2% | 99.3% | 3.6% | 1.0% |
| 1 | 0.0% | 0.1% | 0.1% | 1.8% | 0.7% | 0.3% | 0.3% |
RP: radical prostatectomy; EBRT: external beam radiation therapy; AST: androgen suppression therapy; WHO: World Health Organization
Demographic and cancer characteristics stratified by cancer treatment
Men treated with RP were the youngest and healthiest group and had the best 10-year overall survival, whereas those managed with AST were the oldest and were more likely to have multiple comorbidities and the lowest 10-yr overall survival (Table 1). Men treated with EBRT or EBRT+AST were of intermediate age, had intermediate levels of comorbidities and had intermediate survival. Known osteoporosis at baseline was rare in all groups (1.3–2.3%). 14% of both RP-treated and EBRT-treated men had high grade disease whereas the rate of high grade disease in those receiving EBRT+AST was 38% and in those receiving AST was 28%. <5% of men in the EBRT, EBRT+AST and AST groups also underwent RP. By definition, no man in the RP group received brachytherapy. Brachytherapy was utilized in 15–22% of the other groups. High dose rate brachytherapy was used in 3 and 5% of men in the EBRT and EBRT+AST groups, respectively, and was rare in the other groups. Thermal ablation and cryotherapy were rare in all groups (<1% in most cases)
Unadjusted cumulative incidence of hip or distal forearm fracture
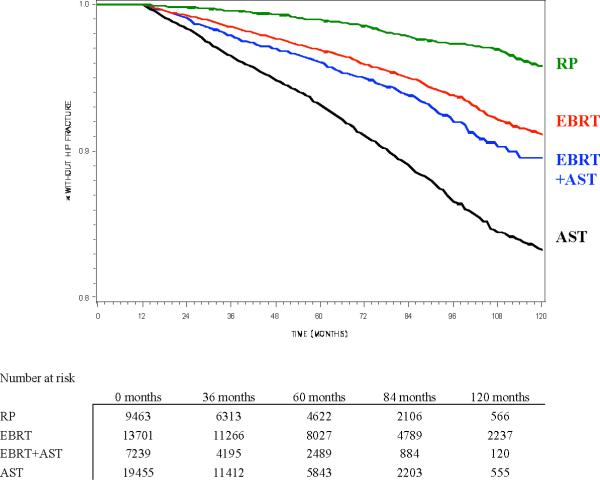
The cumulative incidence of hip fracture at 10 years was 2.6% in men undergoing RP and 8.4% in men undergoing EBRT (p<0.001; Figure 2a and Table 1). The addition of AST (mean = 11 monthly AST doses) to EBRT slightly increase the risk of hip fracture above that with EBRT alone (8.7% vs. 8.4%, p=0.014). Hip fracture risk was highest in those managed with AST alone (16.2%, mean = 19 monthly AST doses). Except for EBRT vs. EBRT+AST, all other pair-wise comparisons by log-rank test (i.e. EBRT vs. RP, EBRT vs. AST, AST vs. RP, AST vs. EBRT+AST and RP vs. EBRT+AST) were statistically significant at p<0.0001.
Figure 2.
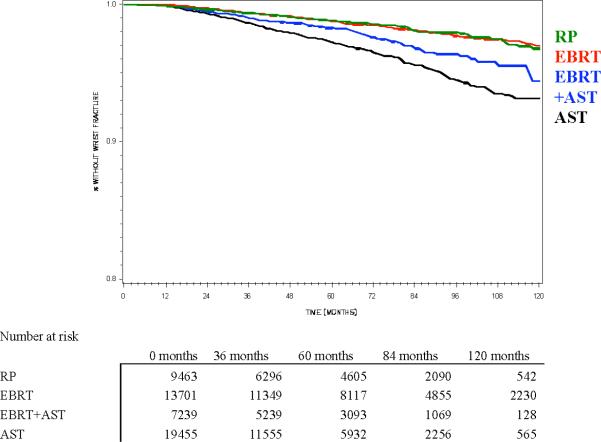
Kaplan-Meier estimate of probability of freedom from (a) hip fracture or (b) distal forearm fracture, stratified by primary prostate cancer treatment (RP = radical prostatectomy, EBRT = external beam radiotherapy, AST = androgen suppression therapy). All pair-wise comparisons are significant by log-rank test except freedom from distal forearm fracture with RP vs. EBRT (Figure 2b).
The 10-year incidence of distal forearm fracture was 1.6% for men treated with either RP or EBRT (p=.6313) but 2.5% in those undergoing EBRT+AST and 4.4% in those on AST (Figure 2b and Table 1). Except for EBRT vs. RP, all other pair-wise comparisons by log-rank test (i.e. EBRT vs. AST, EBRT vs. EBRT+AST, AST vs. RP, AST vs. EBRT+AST and RP vs. EBRT+AST) were statistically significant at p<0.002.
Multivariate-Adjusted Cox Models
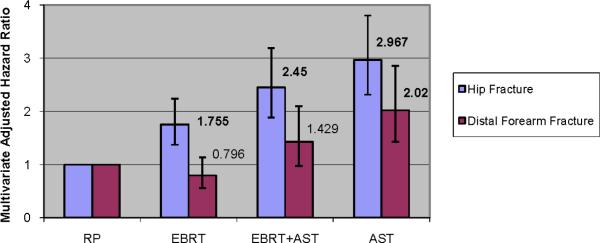
After controlling for measurable confounders, men managed with EBRT were at a 76% increased risk of hip fracture compared to men managed with RP (HR1.76, 95% CI 1.38–2.40) but at no increased risk of distal forearm fracture (HR 0.80, 95% CI 0.36–1.78) (Figure 3 and Table 2). EBRT+AST increased the risk of hip fracture by 145% relative to RP (HR 2.45, 95% CI 1.88–3.19) and increased the risk of distal forearm fracture by 43% relative to RP (HR 1.43, 95% CI 0.97–2.10). Finally, AST tripled the risk of hip fracture (HR 2.97, 95% CI 2.32–3.80) and doubled the risk of distal forearm fracture (HR 2.02, 95% CI 1.43–2.85). EBRT+AST increased the risk of hip fracture by 40% compared to EBRT alone (HR 1.40, 95% CI 1.17–1.68). Other factors associated with hip and distal forearm fracture included age, comorbidities, race and a baseline diagnosis of osteoporosis (see Table 2 for details). Year of diagnosis was associated with hip fracture but not distal forearm fracture. There was no change in the HR of hip or distal forearm fracture associated with the various treatment groups when patients who received brachytherapy, cryotherapy or thermal ablation were excluded or censored at the time of therapy rather than included. The number needed to treat to result in 1 hip fracture through 10 years was 51 (95% CI 31–103).
Figure 3.
Multivariate-adjusted hazard ratio of hip or wrist fracture, stratified by primary prostate cancer treatment (RP = radical prostatectomy, EBRT = external beam radiotherapy, AST = androgen suppression therapy). 95% confidence interval represented by whiskers.
Table 2.
Complete results of Cox proportional hazards models predicting the time to hip or distal forearm fracture.
| Hip fracture | Distal forearm fracture | |||||
|---|---|---|---|---|---|---|
| Covariates* | OR | 95% CI | P-value | OR | 95% CI | P-value |
| EBRT vs. RP | 1.755 | (1.375,2.239) | <.0001 | 0.796 | (0.559,1.135) | 0.2084 |
| EBRT + AST vs. RP | 2.45 | (1.881,3.192) | <.0001 | 1.429 | (0.974,2.095) | 0.0676 |
| AST vs. RP | 2.967 | (2.315,3.802) | <.0001 | 2.02 | (1.43,2.852) | <.0001 |
| 1993 vs. 1992 | 0.931 | (0.747,1.161) | 0.5261 | 0.764 | (0.429,1.36) | 0.3593 |
| 1994 vs. 1992 | 0.859 | (0.682,1.082) | 0.198 | 1.355 | (0.857,2.142) | 0.1942 |
| 1995 vs. 1992 | 0.772 | (0.608,0.982) | 0.0347 | 0.924 | (0.559,1.525) | 0.7559 |
| 1996 vs. 1992 | 0.742 | (0.581,0.948) | 0.0169 | 1.005 | (0.614,1.646) | 0.983 |
| 1997 vs. 1992 | 0.644 | (0.5,0.829) | 0.0006 | 0.796 | (0.47,1.347) | 0.3946 |
| 1998 vs. 1992 | 0.613 | (0.476,0.789) | 0.0002 | 0.923 | (0.56,1.524) | 0.7551 |
| 1999 vs. 1992 | 0.63 | (0.491,0.809) | 0.0003 | 0.781 | (0.472,1.292) | 0.3357 |
| 2000 vs. 1992 | 0.685 | (0.543,0.865) | 0.0015 | 0.607 | (0.358,1.029) | 0.0637 |
| 2001 vs. 1992 | 0.515 | (0.401,0.663) | <.0001 | 0.944 | (0.593,1.501) | 0.8065 |
| 2002 vs. 1992 | 0.506 | (0.387,0.662) | <.0001 | 0.832 | (0.514,1.347) | 0.454 |
| 2003 vs. 1992 | 0.632 | (0.467,0.856) | 0.0031 | 0.797 | (0.479,1.328) | 0.384 |
| 2004 vs. 1992 | 0.574 | (0.357,0.925) | 0.0224 | 0.572 | (0.298,1.099) | 0.0935 |
| Age (continuous variable) | 1.097 | (1.087,1.107) | <.0001 | 0.792 | (0.357,1.757) | 0.5669 |
| Charlson 1 vs. 0 | 1.404 | (1.246,1.583) | <.0001 | 1.394 | (1.116,1.741) | 0.0034 |
| Charlson 2+ vs. 0 | 1.618 | (1.369,1.913) | <.0001 | 1.438 | (1.038,1.992) | 0.0288 |
| Grade 2 vs. 1 | 1 | (0.831,1.202) | 0.9974 | 0.848 | (0.602,1.194) | 0.3443 |
| Grade 3 vs. 1 | 1.116 | (0.911,1.367) | 0.2886 | 1.01 | (0.693,1.472) | 0.9579 |
| Grade Unknown vs. 1 | 1.038 | (0.762,1.414) | 0.8127 | 0.698 | (0.364,1.338) | 0.2788 |
| T2 vs. T1 | 1.029 | (0.917,1.155) | 0.6274 | 0.989 | (0.797,1.227) | 0.9184 |
| T3 vs. T1 | 1.165 | (0.923,1.469) | 0.1984 | 0.88 | (0.562,1.378) | 0.5769 |
| T4 vs. T1 | 1.279 | (0.796,2.057) | 0.3094 | 0.454 | (0.112,1.842) | 0.2689 |
| Black vs. White | 0.663 | (0.534,0.823) | 0.0002 | 0.482 | (0.308,0.755) | 0.0015 |
| Hispanic vs. White | 0.902 | (0.74,1.101) | 0.3113 | 0.721 | (0.485,1.071) | 0.1056 |
| Asian vs. White | 0.545 | (0.386,0.77) | 0.0006 | 0.679 | (0.377,1.222) | 0.1967 |
| Other vs. White | 0.531 | (0.199,1.42) | 0.2071 | 2.254 | (0.926,5.488) | 0.0735 |
| Osteoporosis vs. None at cancer diagnosis | 1.657 | (1.225,2.242) | 0.0011 | 1.001 | (0.495,2.021) | <0.0001 |
Significant findings are marked in bold. The outcomes of interest are the three treatment group variables (EBRT vs. RP, EBRT+AST vs. RP, and AST vs. RP) and also appear in the main body of the paper. The remainder of the variables were controlled for in the model but are not the focus of this paper and are found only here in the table.
RP: radical prostatectomy; EBRT: external beam radiation therapy; AST: androgen suppression therapy; WHO: World Health Organization
DISCUSSION
We demonstrate that 3-D conformal pelvic EBRT increases the risk of hip fracture by 76% in men over 65 years of age being treated for prostate cancer. To analyze the site-specific risk modification of the EBRT we also test the effect of EBRT on the risk of fall-related fractures outside the radiation field, specifically distal forearm fracture. Indeed, we show that whereas EBRT increased the risk of hip fracture it did not increase the risk of distal forearm fracture. As reference points, the added hip fracture risk due to EBRT treatment is similar in scale to the added risk from a 7 year increase in age (HR=1.097 per year) or having a Charlson comorbidity score of ≥2 vs. a score of 0 in our model, the risk imparted by a baseline diagnosis of osteoporosis, or the increased risk others have shown due to being a current smoker vs. never smoker.6 In absolute risk terms, 51 men need to be treated with EBRT to induce 1 hip fracture through 10 years of follow-up. Given that over 28% of the nearly 200,000 men diagnosed with prostate cancer each year receive EBRT, 3-D conformal EBRT may be linked to approximately 1,000 hip fractures each year.
We have previously shown that pelvic EBRT is a significant risk factor for hip fractures but not other fractures in women with gynecologic and colorectal malignancies.5 Similarly, the Stockholm trial of short-course radiotherapy for rectal cancer resulted in a doubling of fracture incidence in the radiated group for both men and women.18 The bony pelvis lies in close proximity to genitourinary pelvic organs and their lymphatics. Therefore, when radiation is used to treat the prostate and/or the pelvic lymph nodes, nearby bony structures are also irradiated.
Radiation damage occurs in the bone matrix, at the cellular level and at the vascular level.7 Radiation can lead to death of osteoblasts, osteocytes, and osteoclasts, resulting in a reduction in bone matrix production. In addition, radiation damage to the vascular supply to the bone may lead to further bone loss.9 Radiotherapy has been associated with fractures of the femur, pubic rami, and pubic symphysis; acetabular failure; and avascular necrosis of the hip.8, 9, 19 Adding to the complexity of the problem, fractures after radiotherapy are more difficult to treat; hip replacement after radiotherapy is associated with an increased risk of infection and malfunction.8, 20
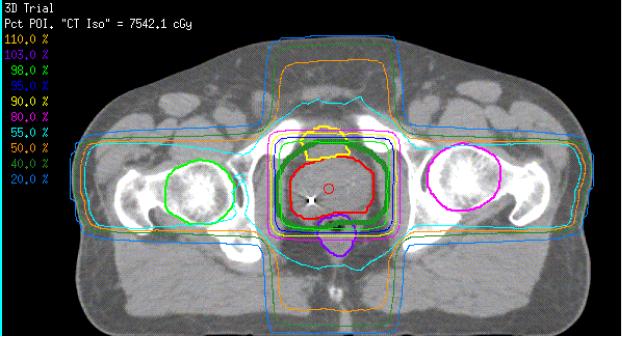
This study did not analyze the association between IMRT and hip fractures. Prostate IMRT utilization has increased greatly over the last decade since it was first approved for payment by Medicare in 2002. Whereas some of the 3-D conformal EBRT beams pass through the hip en route to the prostate (Figure 4), IMRT can allow planners decrease the dose to the hip or completely avoid the hip when necessary without compromising dose to the prostate or increasing scatter to the rectum and bladder.21 This may translate into a lower risk for hip fracture with IMRT. However, we did not include IMRT in this analysis because it was introduced late in our study period and our Medicare claims follow-up would have been too short (3–4 years) to measure the long-term risk of hip fracture in this group.
Figure 4.
EBRT Treatment Plan for Prostate Cancer. Note how lateral beams pass through the hip bones en route to the prostate. (From Kathryn Dusenbery, M.D., Department of Therapeutic Radiology, University of Minnesota)
AST is known to increase the risk of fractures.22 Because EBRT+AST combination therapy in high risk prostate cancer is known to improve survival compared to either treatment alone,13, 14, 16 we were interested in the combined effect of these modalities on the risk of hip fracture. As all cancer therapy should be aimed at balancing benefits and risks, an examination of the risk of combination therapy is important. As expected, hip and distal forearm fracture risk was highest in the group treated with AST alone (mean = 19 monthly doses). Although the addition of short course AST to EBRT (mean =11 monthly doses) did increase the risk of hip fracture by 40% compared to EBRT alone, the added effect of AST was not as dramatic as that seen at the higher number of doses used with AST monotherapy. Given that current trial evidence favors 36 months over 6 months of AST when combined with EBRT,14 it would be interesting to know how more AST doses affects hip fracture risk in the EBRT+AST group. However, a low number of events (hip fractures) in each group after stratification by the number of AST doses prevented us from investigating for such a dose-response relationship.
Certain limitations deserve mention. As our findings are based on a population of men > 65 years of age, conclusions may not be applicable in younger patients. Claims-based research can be an inexact measure of minor events; however, hip fracture nearly always results in acute hospitalization and claims-based methods have been shown to be valid in such disease models.23 It may be argued that the older age and higher number of comorbidities of our EBRT-treated cohort are evidence that men who select EBRT are generally more ill and at higher baseline risk for hip fracture. We have controlled for such a selection bias in two ways. First we demonstrate that EBRT-treated patients were not at higher risk for distal forearm fractures. In a way, distal forearm fracture serves as an internal control – if the increase in hip fracture risk seen in the EBRT-treated men was due to selection bias then one would expect their risk of distal forearm fracture to have been higher too. Second, our multivariate model demonstrates that after controlling for these other risk factors (e.g. age and comorbidities), EBRT still increases the risk of hip but not distal forearm fracture. Of note, there may be residual confounding due to differences in the prevalence of risk factors that we are not able to measure such as patient weight, smoking, glucocorticoid therapy, alcohol use and radiation dose to the hip -- some of these risk factors may be correlated with the receipt of EBRT. Finally, we were only able to exclude metastatic disease through 6 months after diagnosis. Should new bony metastases occur after 6 months these may result in fracture. This may confound measurement of our outcome if the likelihood of delayed metastatic disease differs between RP and EBRT-treated men. We controlled for the risk of confounding due to metastases by excluding men who initiated AST or underwent orchiectomy more than 6 months after diagnosis. Furthermore, previous studies have shown that even among men with advanced prostate cancer managed with AST, only 7–16% of fractures are due to metastases.24, 25 Randomized studies would be needed to confirm these findings. Such studies should have extended follow-up (10 years) and be of sufficient size to be powered to detect a difference in events that occur in <10% of men. These studies should control for the risk factors we were unable to directly measure.
CONCLUSION
In men with prostate cancer, pelvic 3-D conformal EBRT is associated with a 76% increase in the risk of hip fracture. This risk is slightly increased further by the addition of short-course AST to EBRT. This risk associated with EBRT is site-specific as no increase in the risk of fall-related fractures outside the radiation field is seen. An assessment of baseline bone health may prove useful in men considering EBRT.
Acknowledgments
Source of Funding: This study was funded in part by a grant from the National Institutes of Health: Grant No. 5K12-RR023247-03.
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database
Footnotes
Financial disclosures: no author has any financial conflict of interest with any product discussed in this manuscript.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
REFERENCES
- 1.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–82. doi: 10.1016/S0140-6736(98)09075-8. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10093980. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103(2A):12S–17S. doi: 10.1016/s0002-9343(97)90022-x. discussion 17S–19S. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9302893. [DOI] [PubMed] [Google Scholar]
- 3.Sweet MG, Sweet JM, Jeremiah MP, Galazka SS. Diagnosis and treatment of osteoporosis. Am Fam Physician. 2009;79(3):193–200. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19202966. [PubMed] [Google Scholar]
- 4.Chen JS, Simpson JM, March LM, Cameron ID, Cumming RG, Lord SR, et al. Risk factors for fracture following a fall among older people in residential care facilities in Australia. J Am Geriatr Soc. 2008;56(11):2020–6. doi: 10.1111/j.1532-5415.2008.01954.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18811606. [DOI] [PubMed] [Google Scholar]
- 5.Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294(20):2587–93. doi: 10.1001/jama.294.20.2587. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16304072. [DOI] [PubMed] [Google Scholar]
- 6.Tosteson AN, Gottlieb DJ, Radley DC, Fisher ES, Melton LJ., 3rd Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18(11):1463–72. doi: 10.1007/s00198-007-0429-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17726622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41(3):208–11. doi: 10.1002/mpo.10338. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12868120. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs JJ, Kull LR, Frey GA, Gitelis S, Sheinkop MB, Kramer TS, et al. Early failure of acetabular components inserted without cement after previous pelvic irradiation. J Bone Joint Surg Am. 1995;77(12):1829–35. doi: 10.2106/00004623-199512000-00006. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8550650. [DOI] [PubMed] [Google Scholar]
- 9.Mumber MP, Greven KM, Haygood TM. Pelvic insufficiency fractures associated with radiation atrophy: clinical recognition and diagnostic evaluation. Skeletal Radiol. 1997;26(2):94–9. doi: 10.1007/s002560050200. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9060100. [DOI] [PubMed] [Google Scholar]
- 10.Our unpublished analysis of the SEER public use file.
- 11.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 28(7):1117–23. doi: 10.1200/JCO.2009.26.0133. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schymura MJ, Kahn AR, German RR, Hsieh MC, Cress RD, Finch JL, et al. Factors associated with initial treatment and survival for clinically localized prostate cancer: results from the CDC-NPCR Patterns of Care Study (PoC1) BMC Cancer. 10:152. doi: 10.1186/1471-2407-10-152. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20403178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–6. doi: 10.1016/s0140-6736(02)09408-4. Available from >http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12126818. [DOI] [PubMed] [Google Scholar]
- 14.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–27. doi: 10.1056/NEJMoa0810095. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19516032. [DOI] [PubMed] [Google Scholar]
- 15.Alanee SL, Jarosek SA, Virnig BA, Elliott SP. How does level I evidence affect treatement trends of EBRT+AST combination therapy for prostate cancer?. American Urological Association; San Francisco, CA. 2010. [Google Scholar]
- 16.Warde PR MM, Sydes MR, Gospodarowicz MK, Swanson GP, Kirkbride P, Kostashuk E, Hetherington J, Ding K, Parulekar W. NCIC CTG PR.3/ MRC PRO7/ SWOG JPR3 investigators Intergroup randomized phase III study of androgen deprivation therapy (ADT) plus radiation therapy (RT) in locally advanced prostate cancer ASCO. 2010 [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11146273. [DOI] [PubMed] [Google Scholar]
- 18.Randomized study on preoperative radiotherapy in rectal carcinoma. Stockholm Colorectal Cancer Study Group. Ann Surg Oncol. 1996;3(5):423–30. doi: 10.1007/BF02305759. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8876883. [DOI] [PubMed] [Google Scholar]
- 19.Fu AL, Greven KM, Maruyama Y. Radiation osteitis and insufficiency fractures after pelvic irradiation for gynecologic malignancies. Am J Clin Oncol. 1994;17(3):248–54. doi: 10.1097/00000421-199406000-00015. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8192113. [DOI] [PubMed] [Google Scholar]
- 20.Massin P, Duparc J. Total hip replacement in irradiated hips. A retrospective study of 71 cases. J Bone Joint Surg Br. 1995;77(6):847–52. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7593093. [PubMed] [Google Scholar]
- 21.Kung JH, Reft H, Jackson W, Abdalla I. Intensity-modulated radiotherapy for a prostate patient with a metal prosthesis. Med Dosim. 2001;26(4):305–8. doi: 10.1016/s0958-3947(01)00079-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11747995. [DOI] [PubMed] [Google Scholar]
- 22.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–64. doi: 10.1056/NEJMoa041943. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15647578. [DOI] [PubMed] [Google Scholar]
- 23.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(8 Suppl):IV, 62–8. doi: 10.1097/00005650-200208001-00009. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12187170. [DOI] [PubMed] [Google Scholar]
- 24.Melton LJ, 3rd, Alothman KI, Khosla S, Achenbach SJ, Oberg AL, Zincke H. Fracture risk following bilateral orchiectomy. J Urol. 2003;169(5):1747–50. doi: 10.1097/01.ju.0000059281.67667.97. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12686824. [DOI] [PubMed] [Google Scholar]
- 25.Townsend MF, Sanders WH, Northway RO, Graham SD., Jr. Bone fractures associated with luteinizing hormone-releasing hormone agonists used in the treatment of prostate carcinoma. Cancer. 1997;79(3):545–50. doi: 10.1002/(sici)1097-0142(19970201)79:3<545::aid-cncr17>3.0.co;2-3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9028366. [DOI] [PubMed] [Google Scholar]