Abstract
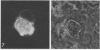
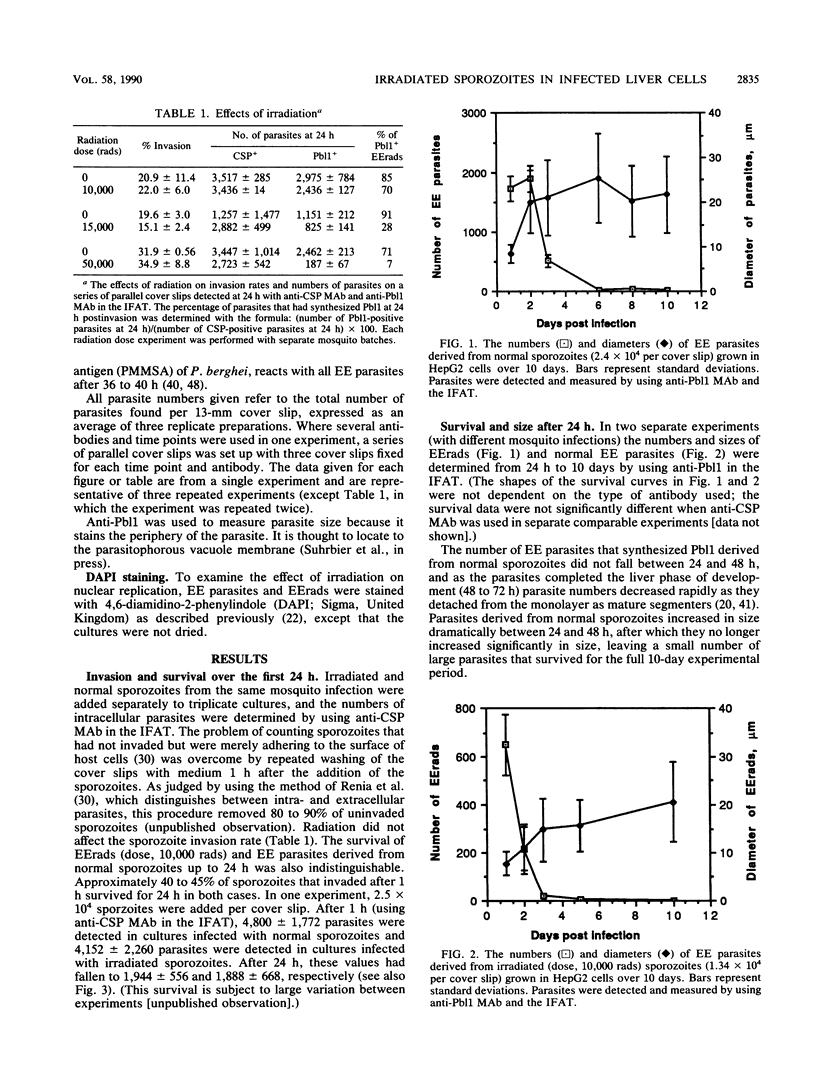
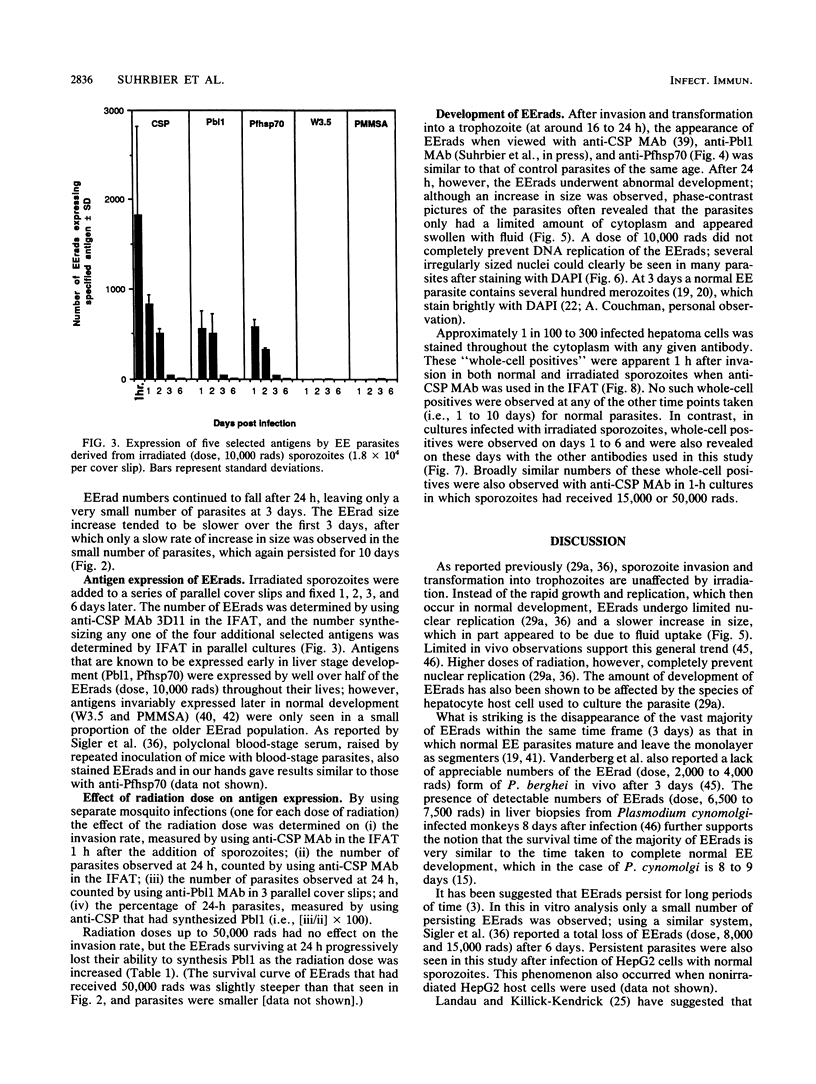
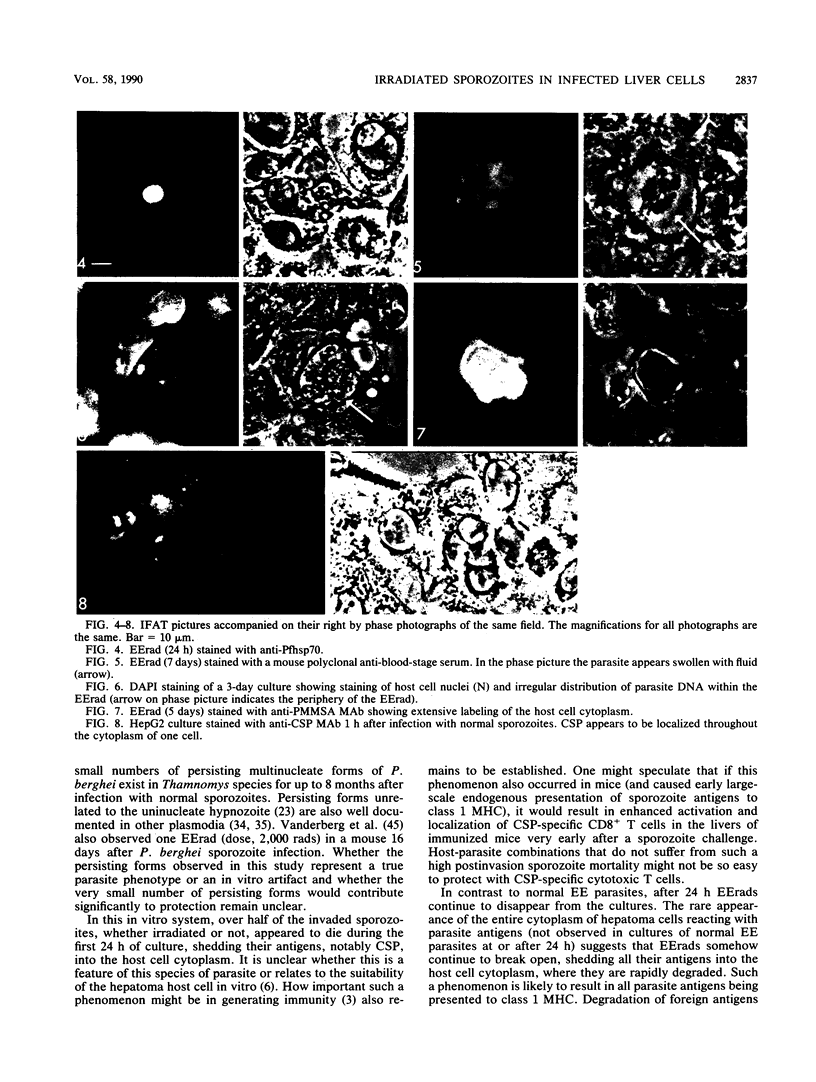
Exoerythrocytic (EE) stages of Plasmodium berghei derived from irradiated sporozoites were cultured in vitro in HepG2 cells. They synthesized several antigens, predominantly but not exclusively those expressed by normal early erythrocytic schizonts. After invasion, over half the intracellular sporozoites, both normal and irradiated, appeared to die. After 24 h, in marked contrast to the normal parasites, EE parasites derived from irradiated sporozoites continued to break open, shedding their antigens into the cytoplasm of the infected host cells. Increasing radiation dosage, which has previously been shown to reduce the ability of irradiated sporozoites to protect animals, correlated with reduced de novo antigen synthesis by EE parasites derived from irradiated sporozoites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Atkinson C. T., Aikawa M., Maloy W. L., Hollingdale M. R. Ultrastructural localization of Plasmodium falciparum circumsporozoite protein in newly invaded hepatoma cells. J Parasitol. 1987 Dec;73(6):1241–1245. [PubMed] [Google Scholar]
- Atkinson C. T., Millet P., Collins W. E., Aikawa M. Localization of circumsporozoite antigen in exoerythrocytic schizonts of Plasmodium cynomolgi. Am J Trop Med Hyg. 1989 Feb;40(2):131–140. doi: 10.4269/ajtmh.1989.40.131. [DOI] [PubMed] [Google Scholar]
- Beaudoin R. L., Strome C. P., Mitchell F., Tubergen T. A. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp Parasitol. 1977 Jun;42(1):1–5. doi: 10.1016/0014-4894(77)90054-6. [DOI] [PubMed] [Google Scholar]
- Bianco A. E., Crewther P. E., Coppel R. L., Stahl H. D., Kemp D. J., Anders R. F., Brown G. V. Patterns of antigen expression in asexual blood stages and gametocytes of Plasmodium falciparum. Am J Trop Med Hyg. 1988 Mar;38(2):258–267. doi: 10.4269/ajtmh.1988.38.258. [DOI] [PubMed] [Google Scholar]
- Coosemans M., Wery M., Van Marck E., Timperman G. Studies on the infectivity of Plasmodium berghei sporozoites in experimental hosts. Ann Soc Belg Med Trop. 1981 Sep;61(3):349–368. [PubMed] [Google Scholar]
- Danforth H. D., Aikawa M., Cochrane A. H., Nussenzweig R. S. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J Protozool. 1980 May;27(2):193–202. doi: 10.1111/j.1550-7408.1980.tb04680.x. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Aikawa M., Cochrane A. H., Nussenzweig R. S. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J Protozool. 1980 May;27(2):193–202. doi: 10.1111/j.1550-7408.1980.tb04680.x. [DOI] [PubMed] [Google Scholar]
- De La Cruz V. F., Maloy W. L., Miller L. H., Good M. F., McCutchan T. F. The immunologic significance of variation within malaria circumsporozoite protein sequences. J Immunol. 1989 May 15;142(10):3568–3575. [PubMed] [Google Scholar]
- Druilhe P., Puebla R. M., Miltgen F., Perrin L., Gentilini M. Species- and stage-specific antigens in exoerythrocytic stages of Plasmodium falciparum. Am J Trop Med Hyg. 1984 May;33(3):336–341. doi: 10.4269/ajtmh.1984.33.336. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Enea V., Morimoto T., Nussenzweig V. Infectivity of Plasmodium berghei sporozoites measured with a DNA probe. Mol Biochem Parasitol. 1986 May;19(2):103–109. doi: 10.1016/0166-6851(86)90114-3. [DOI] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Miller L. H. The T cell response to the malaria circumsporozoite protein: an immunological approach to vaccine development. Annu Rev Immunol. 1988;6:663–688. doi: 10.1146/annurev.iy.06.040188.003311. [DOI] [PubMed] [Google Scholar]
- Guerin-Marchand C., Druilhe P., Galey B., Londono A., Patarapotikul J., Beaudoin R. L., Dubeaux C., Tartar A., Mercereau-Puijalon O., Langsley G. A liver-stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987 Sep 10;329(6135):164–167. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Suhrbier A., Nicholas J., Sinden R. E. Immunoelectron microscopic localization of circumsporozoite antigen in the differentiating exoerythrocytic trophozoite of Plasmodium berghei. Cell Biol Int Rep. 1988 Feb;12(2):123–129. doi: 10.1016/0309-1651(88)90126-9. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Isenbarger D., Long G. W., Sedegah M., Szarfman A., Waters L., Hollingdale M. R., van der Meide P. H., Finbloom D. S., Ballou W. R. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 1989 Jun 2;244(4908):1078–1081. doi: 10.1126/science.2524877. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Oster C. N., Mason C., Beier J. C., Sherwood J. A., Ballou W. R., Mugambi M., Chulay J. D. Human lymphocyte proliferative response to a sporozoite T cell epitope correlates with resistance to falciparum malaria. J Immunol. 1989 Feb 15;142(4):1299–1303. [PubMed] [Google Scholar]
- Hollingdale M. R. Biology and immunology of sporozoite invasion of liver cells and exoerythrocytic development of malaria parasites. Prog Allergy. 1988;41:15–48. [PubMed] [Google Scholar]
- Hollingdale M. R., Leland P., Sigler C. I. In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei in irradiated hepatoma cells. Am J Trop Med Hyg. 1985 Jan;34(1):21–23. doi: 10.4269/ajtmh.1985.34.21. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R. Malaria and the liver. Hepatology. 1985 Mar-Apr;5(2):327–335. doi: 10.1002/hep.1840050230. [DOI] [PubMed] [Google Scholar]
- Hyman B. C., Macinnis A. J. Rapid detection of malaria and other bloodstream parasites by fluorescence microscopy with 4'6 diamidino-2-phenylindole (DAPI). J Parasitol. 1979 Jun;65(3):421–425. [PubMed] [Google Scholar]
- Krotoski W. A. Discovery of the hypnozoite and a new theory of malarial relapse. Trans R Soc Trop Med Hyg. 1985;79(1):1–11. [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Landau I., Killick-Kendrick R. Rodent plasmodia of the République Centrafricaine: the sporogony and tissue stages of Plasmodium chabaudi and P. berghei yoelii. Trans R Soc Trop Med Hyg. 1966;60(5):633–649. doi: 10.1016/0035-9203(66)90010-1. [DOI] [PubMed] [Google Scholar]
- Lockyer M. J., Marsh K., Newbold C. I. Wild isolates of Plasmodium falciparum show extensive polymorphism in T cell epitopes of the circumsporozoite protein. Mol Biochem Parasitol. 1989 Dec;37(2):275–280. doi: 10.1016/0166-6851(89)90159-x. [DOI] [PubMed] [Google Scholar]
- Mazier D., Miltgen F., Nudelman S., Nussler A., Renia L., Pied S., Goma J., Gentilini M. Pre-erythrocytic stages of plasmodia. Role of specific and nonspecific factors. Biol Cell. 1988;64(2):165–172. doi: 10.1016/0248-4900(88)90076-7. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967 Oct 14;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R., Vanderberg J., Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Mil Med. 1969 Sep;134(10):1176–1182. [PubMed] [Google Scholar]
- Nüssler A., Follezou J. Y., Miltgen F., Mazier D. Effect of irradiation on Plasmodium sporozoites depends on the species of hepatocyte infected. Trop Med Parasitol. 1989 Dec;40(4):468–469. [PubMed] [Google Scholar]
- Romero P., Maryanski J. L., Corradin G., Nussenzweig R. S., Nussenzweig V., Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989 Sep 28;341(6240):323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- Rénia L., Miltgen F., Charoenvit Y., Ponnudurai T., Verhave J. P., Collins W. E., Mazier D. Malaria sporozoite penetration. A new approach by double staining. J Immunol Methods. 1988 Sep 13;112(2):201–205. doi: 10.1016/0022-1759(88)90358-4. [DOI] [PubMed] [Google Scholar]
- SHORTT H. E., BRAY R. S., COOPER W. Further notes on the tissue stages of Plasmodium cynomolgi. Trans R Soc Trop Med Hyg. 1954 Mar;48(2):122–131. doi: 10.1016/0035-9203(54)90004-8. [DOI] [PubMed] [Google Scholar]
- SHORTT H. E., GARNHAM P. C. C. Demonstration of a persisting exo-erythrocytic cycle in Plasmodium cynomolgi and its bearing on the production of relapses. Br Med J. 1948 Jun 26;1(4564):1225–1228. doi: 10.1136/bmj.1.4564.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J. C., Ballou W. R., Baron L. S., Majarian W. R., Brey R. N., Hockmeyer W. T., Young J. F., Cryz S. J., Ou J., Lowell G. H. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988 Apr 15;240(4850):336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Sigler C. I., Leland P., Hollingdale M. R. In vitro infectivity of irradiated Plasmodium berghei sporozoites to cultured hepatoma cells. Am J Trop Med Hyg. 1984 Jul;33(4):544–547. doi: 10.4269/ajtmh.1984.33.544. [DOI] [PubMed] [Google Scholar]
- Stewart M. J., Vanderberg J. P. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool. 1988 Aug;35(3):389–393. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- Suhrbier A., Hamilton A. J., Nicholas J., Sinden R. E. The fate of the circumsporozoite antigens during the exoerythrocytic stage of Plasmodium berghei. Eur J Cell Biol. 1988 Apr;46(1):25–30. [PubMed] [Google Scholar]
- Suhrbier A., Holder A. A., Wiser M. F., Nicholas J., Sinden R. E. Expression of the precursor of the major merozoite surface antigens during the hepatic stage of malaria. Am J Trop Med Hyg. 1989 Apr;40(4):351–355. doi: 10.4269/ajtmh.1989.40.351. [DOI] [PubMed] [Google Scholar]
- Suhrbier A., Janse C., Mons B., Fleck S. L., Nicholas J., Davies C. S., Sinden R. E. The complete development in vitro of the vertebrate phase of the mammalian malarial parasite Plasmodium berghei. Trans R Soc Trop Med Hyg. 1987;81(6):907–909. doi: 10.1016/0035-9203(87)90346-4. [DOI] [PubMed] [Google Scholar]
- Suhrbier A., Wiser M. F., Winger L., Harte P., Newton M. F., Hodivala K. J., Nicholas J., Sinden R. E. Contrasts in antigen expression in the erythrocytic and exoerythrocytic stages of rodent malaria. Parasitology. 1989 Oct;99(Pt 2):165–170. doi: 10.1017/s0031182000058595. [DOI] [PubMed] [Google Scholar]
- Szarfman A., Lyon J. A., Walliker D., Quakyi I., Howard R. J., Sun S., Ballou W. R., Esser K., London W. T., Wirtz R. A. Mature liver stages of cloned Plasmodium falciparum share epitopes with proteins from sporozoites and asexual blood stages. Parasite Immunol. 1988 May;10(3):339–351. doi: 10.1111/j.1365-3024.1988.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Vanderberg J. P., Nussenzweig R. S., Most H., Orton C. G. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. II. Effects of radiation on sporozoites. J Parasitol. 1968 Dec;54(6):1175–1180. [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser M. F. Characterization of monoclonal antibodies directed against erythrocytic stage antigens of Plasmodium berghei. Eur J Cell Biol. 1986 Oct;42(1):45–51. [PubMed] [Google Scholar]
- Wiser M. F. Plasmodium antigens external to the parasite but with the infected erythrocyte. Parasitol Res. 1989;75(3):206–211. doi: 10.1007/BF00931277. [DOI] [PubMed] [Google Scholar]
- Zavala F., Hollingdale M. R., Schwartz A. L., Nussenzweig R. S., Nussenzweig V. Immunoradiometric assay to measure the in vitro penetration of sporozoites of malaria parasites into hepatoma cells. J Immunol. 1985 Feb;134(2):1202–1205. [PubMed] [Google Scholar]
- de Groot A. S., Johnson A. H., Maloy W. L., Quakyi I. A., Riley E. M., Menon A., Banks S. M., Berzofsky J. A., Good M. F. Human T cell recognition of polymorphic epitopes from malaria circumsporozoite protein. J Immunol. 1989 Jun 1;142(11):4000–4005. [PubMed] [Google Scholar]