Abstract
Objective
Animal data suggest that males, in particular, rely on PPAR-α activity to maintain normal muscle triglyceride metabolism. We sought to examine whether this was also true in men vs. women and its relationship to insulin sensitivity.
Materials/Methods
Normolipidemic obese men (n=9) and women (n=9) underwent an assessment of insulin sensitivity (IVGTT) and intramuscular triglyceride metabolism (GC/MS and GC/C/IRMS from plasma and muscle biopsies taken after infusion of [U-13C]palmitate) before and after 12 weeks of fenofibrate treatment.
Results
Women were more insulin sensitive (Si; 5.2(0.7 vs. 2.4(0.4 ×10−4/uU/ml, W vs. M, p<0.01) at baseline despite similar intramuscular triglyceride (IMTG) concentration (41.9(15.5 vs. 30.8(5.1 ug/mg dry weight, W vs. M, p=0.43), and IMTG fractional synthesis rate (FSR; 0.27(0.07 vs. 0.35(0.06/hr, W vs. M, p=0.41) as men. Fenofibrate enhanced FSR in men (0.35(0.06 to 0.54(0.06, p=0.05), with no such change seen in women (0.27(0.07 to 0.32(0.13, p=0.73), and no change in IMTG concentration in either group (23.0(3.9 in M, p=0.26 vs. baseline; 36.3(12.0 in W, p=0.79 vs. baseline). Insulin sensitivity was unaffected by fenofibrate (p>0.68). Lower percent saturation of IMTG in women vs. men before (29.1(2.3 vs. 35.2(1.7%, p=0.06) and after (27.3(2.8 vs. 35.1(1.9%, p=0.04) fenofibrate most closely related to their greater insulin sensitivity (R2=0.34, p=0.10), and was largely unchanged by the drug.
Conclusions
PPAR-α agonist therapy had little effect on IMTG metabolism in men or women. IMTG saturation, rather than IMTG concentration or FSR, most closely (but not significantly) related to insulin sensitivity and was unchanged by fenofibrate administration.
Keywords: Insulin resistance, isotopes, IMCL, muscle, sex
INTRODUCTION
Skeletal muscle is the major tissue responsible for insulin action on peripheral glucose uptake, and therefore has been implicated as a primary site for the development of insulin resistance and type 2 diabetes [1, 2]§. Considerable attention has been paid to examining the role of intramuscular triglyceride (IMTG), in particular, in this process. Repeated observations using different techniques have noted a positive linear relationship between IMTG concentration and insulin resistance [3–6]§ in both men and women. Nevertheless, when men and women are directly compared as first-degree relatives of people with diabetes [6]§, by age [7]§, or glucose tolerance status [8]§, women are generally more insulin sensitive than men despite as great or greater concentration of IMTG. Together these findings have led to speculation about the importance of composition and/or turnover of the IMTG pool, rather than its total size [9]§. Recently, Bergman et al. demonstrated the significance of low IMTG synthesis rate (a component of IMTG turnover) and high saturation of IMTG to insulin resistance [9, 10]§. However, these data were predominantly collected in men. Since women generally have a higher concentration of IMTG compared to men, it is possible that higher rates of IMTG synthesis and/or lower percent saturation in IMTG help explain sex differences in insulin sensitivity and in the progression from obesity to diabetes [8]§.
Of the vast array of possible regulators of IMTG metabolism, there is reason to believe that peroxisome proliferator activated receptor-α (PPAR-α) is particularly critical to maintain normal lipid trafficking and insulin action in male animal models. For example, etomoxir (a carnitine palmitoyltransferase -1 (CPT-1) inhibitor; CPT-1 being a known target gene of PPAR-α) is universally lethal when given to male, but not female, PPAR-α knockout mice [11]§. Further, the male mice can be rescued when pretreated with estradiol. In addition, the use of fenofibrate (PPAR-α agonist) has been shown to specifically reduce IMTG and improve insulin sensitivity proportionally in several male animal models [12–14]§. Whether fenofibrate administration could favorably alter IMTG dynamics and insulin action in obese men vs. women had not been previously explored, hence was the aim of the current study. We hypothesized that men would have lower baseline PPAR-α protein expression in muscle commensurate with lower IMTG fractional synthesis rate (FSR) – as a surrogate measure of IMTG turnover - and lower insulin sensitivity, and furthermore, that we could enhance IMTG FSR and insulin sensitivity with fenofibrate administration.
METHODS
Subjects
Overweight or obese non-smoking men (n=9) and post-menopausal women (n=9) between the ages of 45–70 were studied. All subjects were free of diabetes, but were at high risk for the disease by virtue of their age, body mass index and having a first-degree relative with type 2 diabetes. Volunteers were sedentary (<90 min/week planned activity) deemed healthy by history, physical examination and screening blood tests. Subjects were excluded for: fasting glucose > 6.9 mmol/l, glucose 2 hours post-75 g oral glucose load > 11.1 mmol/l, thyroid stimulating hormone <50 or >500 mU/l, fasting triglycerides >2.26 mmol/l, creatinine >130 mmol/l, elevated liver function tests (>2X normal), hematocrit < 38%, or WBC<3.0 × 103. Use of medications for lipid and/or glucose lowering also excluded enrollees. Approval for this study was obtained by the Colorado Multiple Institutional Review Board prior to its commencement. All volunteers provided their informed consent.
Pre-Study Measures
Body composition was estimated from dual energy X-ray absorptiometry (DEXA). Intravenous glucose tolerance test (IVGTT): A modified frequently sampled IVGTT was performed as previously described by Bergman et al [15]§. Insulin sensitivity (Si) and secretion (acute insulin response (AIR) and the disposition index (Si × AIR = DI)) were calculated using the MINMOD computer program (Millenium version; MINMOD, Los Angeles, CA).
Pre-study diet control
Subjects were fed a control diet for 3 days prior to admission to the General Clinical Research Center (GCRC) for pre- and post-intervention study days. The control diet was isocaloric (calculated as 1.4 × [372 + (23.9 × fat-free mass)] calories per day), using the fat-free mass measured by DEXA. The diet composition was standardized as: 30% fat (saturated, polyunsaturated, and monounsaturated fats in a 1:1:1 ratio), 15% protein and 55% carbohydrate.
Study day
Subjects were fasted overnight (~12 hours) and were admitted to the GCRC at 07:30 on the morning of the IMTG turnover study. Upon admission, an intravenous catheter was placed in an antecubital vein for infusion, and sampling catheter was placed in a dorsal hand vein of the contralateral arm. For all blood samples, the heated hand technique was used to arterialize the blood. Background sampling began 30 minutes after sampling catheters had been placed. A baseline blood sample was drawn for determination of circulating hormone and substrate concentrations (catecholamines, insulin, glucose, c-peptide, glucagon, free fatty acids (FFA), glycerol, and lactate). Following the baseline blood draw, a continuous infusion of [U-13C]palmitate (Isotec, Miamisburg, OH) bound to human albumin was initiated at 0.0174 mmol/kg/min and continued throughout the study. Subjects remained semi-recumbent for 4 hours to allow for tracer incorporation into the intramuscular lipid pools. Blood samples were taken for hormone and substrate concentrations (as above) during the final 30 minutes of the 4-hour rest period. Indirect calorimetry was performed prior to blood sampling. Following the rest period, a vastus lateralis skeletal muscle biopsy was taken using the Bergstrom technique [16]§. Muscle was immediately flash frozen in liquid nitrogen and stored at −80°C until dissection and analysis.
Intervention
After completion of the study day, all subjects received open-label fenofibrate (TricorTM) 145 mg po qd × 12 weeks. Participants were counseled at length about possible side effects and allergic reactions. Information on tolerability was solicited weekly, and medication compliance assessed by final pill count. Subjects were asked to remain weight stable throughout the intervention period. The diet was no controlled except for the 3 days prior to the post-intervention study day.
Post-intervention study day
Twenty-four hours after ingestion of the final dose of fenofibrate, subjects returned to the GCRC at which time the study day (described above) was repeated.
Methods
Circulating hormone and substrate concentrations
All samples were stored at −80° C until analysis. Radioimmunoassay was used to determine insulin and glucagon (Linco Research Inc., St. Louis, MO), as well as c-peptide (Gamma counter, Diagnostic Products Corporation, Los Angeles, CA), concentrations. Standard enzymatic assays were used to measure glucose (COBA-Mira Plus, Roche Diagnostics, Mannheim, Germany), screening lipid panel (Beckman Coulter, Fort Collins, CO), lactate (Sigma Kit #826, St. Louis, MO), glycerol (Boehringer Mannheim Diagnostics, Mannheim, Germany), and FFA (NEFA Kit, Wako, TX). Epinephrine and norepinephrine concentrations were measured using high performance liquid chromatography (Dionex, Sunnyvale, CA).
Whole-body substrate oxidation
Whole-body substrate oxidation was measured using indirect calorimetry. Oxygen consumption and carbon dioxide production were used to calculate metabolic rate, as well as the oxidation of carbohydrate and fat using standard equations.
Muscle lipid analysis
Skeletal muscle samples were dissected free of extramuscular fat on ice as described by Guo et al. [17]§. Muscle (~70 mg) was lyophilized, added to 1 ml iced MeOH along with internal standards of tripentadecanoic acid, and dipentadencanoic acid, and homogenized (Omni TH, Omni International, Marietta, GA). Total lipids were extracted [18]§ and then added to solid phase extraction columns (Supelclean LC-NH2, 3 ml, Supelco Analytical, Bellefonte, PA) to isolate free fatty acids and IMTG as described by Kaluzny [19]§. The FFA fraction was methylated using 0.5 ml 2% sulfuric acid, and heated at 100°C for 1.5 hours. The IMTG fraction was converted to fatty acid methyl ester (FAME) by transmethylation using sodium methoxide. Stable isotope ratios of 13C in FAMEs were measured using a gas chromatography-combustion isotope ratio mass spectrometer (GC/C-IRMS) system (Thermo Electron Corp., Bremen, Germany). Enrichment was calculated based on a standard curve of known enrichments, and corrected for variations in abundance [20]§. Concentration and composition analysis was performed on an HP 6890 GC with a 30m DB-23 capillary column, connected to a HP 5973 MS. Peak identities were determined by retention time and mass spectra compared to standards of known composition.
Whole body palmitate oxidation
Two mL of breath CO2 was transferred into a 20 mL exetainer™ for the measurement of 13CO2/12CO2 with continuous flow isotope ratio mass spectrometry (IRMS) (Delta V, Thermo Electron, Bremen, Germany). Each sample was injected (1.2µL per injection) in duplicate for isotope ratio analyses, with an average standard deviation for all injections of 0.0001 atom percent.
Plasma palmitate enrichment and TG composition
Methylation and extraction of plasma palmitate was performed as previously described [21]. Plasma triglyceride was isolated as described by Agren et al [22] and methylated using sodium methoxide as previously published [9]. Samples were run on an HP 6890 GC with a 30m DB-23 capillary column, connected to a HP 5973 MS. Enrichments were calculated based on a standard curve of known enrichments, and corrected for variations in abundance [20]. Peak identities were determined by retention time and mass spectra compared to standards of known composition.
Western Blotting
Frozen skeletal muscle samples were weighed, and homogenized on ice using a Kontes glass homogenizer (Kimble/Kontes, Vineland, NJ) in buffer [10]. Protein was extracted, concentration measured (Calbiochem, San Diego, CA), and 40 µg of sample protein and an internal standard were run on an SDS-PAGE 8% Bis-Tris gel (Invitrogen, Carlsbad, CA), using standard methods previously described [10]. Anti-human myosin A4.840 and A4.74 antibodies were purchased from the University of Iowa Hybridoma Bank (Iowa City, IA), anti-rabbit succinate dehydrogenase (SDH) and PPAR-α (Santa Cruz Biotechnology, Santa Cruz, CA), MAP4K4 (Abgent, San Diego, CA), IRS-1ser636 and IRS-1total (Cell Signaling Technology Inc., Danvers, MA), PKC-ε (Cell Signaling Technology, Beverly, MA) and CPT-1 (Alpha Diagnostics International, Inc., San Antonio, TX) antibodies were commercially available. The rabbit anti-4-HNE antibody was a generous gift from Dr. Dennis Peterson (University of Colorado Denver). Secondary antibodies were from Bio-Rad (Bio-Rad Hercules, CA).
Calculations
IMTG fractional synthesis rate was calculated as previously described by our laboratory [10].
% Saturation of IMTG = (laurate+myristate+palmitate+stearate)/(ΣFFA species) × 100
Where FFA represent concentration of individual FFA species in IMTG after transmethylation.
Palmitate rate of disappearance (Rd) and palmitate rate of oxidation were calculated using steady state kinetics and a whole body estimate of carbon label retention as previously described [23]. Calculation of palmitate oxidation rates was made using published values for the acetate recovery factor in obese humans at rest [24]. Non-plasma fatty acid oxidation was calculated as the difference between whole body fat oxidation and whole body palmitate oxidation.
Statistical Analysis
Pre-specified primary outcome data were insulin sensitivity and IMTG FSR, with secondary outcomes specified as IMTG concentration and saturation. Due to the small sample size, data were not corrected for multiple comparisons. Testing of the data revealed a non-normal distribution, therefore analyses were conducted on log-transformed values. Comparisons between groups were made using ANOVA for continuous variables and chi square (fisher exact where appropriate) for categorical variables (SPSS; Chicago, IL). All data are presented as mean +/− SEM. Overall significance was set at p≤0.05.
RESULTS
Subject Demographics
Men and women were of similar age (59±1.8 vs. 60±0.9 years, respectively, p=0.43). Body mass index was higher in men (32±1.4 vs. 28±0.7 kg/m2, p=0.03 vs. W), whereas body fat was higher in women (41±1.4 vs. 32±1.4% p<0.01 vs. M). Total cholesterol was higher in women (6.0±0.2 vs. 5.1±0.2 mmol/l, W vs. M, p=0.01), in part, due to higher high-density lipoprotein cholesterol (HDL-C; 1.4±0.1 vs. 1.0±0.1 mmol/l, W vs. M, p<0.01). Otherwise, baseline low-density lipoprotein cholesterol (LDL-C; 3.9±0.3 vs. 3.3±0.2 mmol/l, W vs. M, p=0.19) and plasma triglycerides (1.6±0.3 vs. 1.6±0.2 mmol/l, W vs. M, p=1.0) were similar between the sexes on the screening (no preceding diet control) lipid panel.
Hormone and Substrate Concentrations
Pre- and post-intervention hormone and substrate concentrations are summarized in Table 1. There were no sex differences in hormone and substrate concentrations at baseline except slightly higher norepinephrine in women vs. men (p=0.04). Fenofibrate lowered fasting glucose in both groups (p<0.01), and also insulin concentration in women (p=0.05) such that it was lower than in men post-intervention (p=0.03).
Table 1.
Hormone & Substrate Concentrations
| Pre- Fenofibrate |
Glucose | Insulin | Glucagon | FFA | Glycerol | Lactate | Epi | Norepi |
|---|---|---|---|---|---|---|---|---|
| (mmol/l) | (pmol/l) | (ng/l) | (umol/l) | (umol/l) | (mmol/l) | (pmol/l) | (nmol/l) | |
| Men | 5.7 ± 0.2 | 88 ± 15 | 78 ± 11 | 559 ± 41 | 87 ± 5.5 | 0.69 ± 0.07 | 153 ± 17 | 1.3 ± 0.1* |
| Women | 5.3 ± 0.2 | 57 ± 11 | 60 ± 5.3 | 663 ± 63 | 112 ± 12.2 | 0.54 ± 0.08 | 137 ± 17 | 1.9 ± 0.2 |
|
Post- Fenofibrate |
Glucose | Insulin | Glucagon | FFA | Glycerol | Lactate | Epi | Norepi |
| (mg/dl) | (uU/ml) | (pg/ml) | (umol/L) | (umol/L) | (mmol/L) | (pg/ml) | (pg/ml) | |
| Men | 4.8 ± 0.1§ | 73 ± 17 | 74 ± 10 | 639 ± 74 | 84 ± 9.2 | 0.56 ± 0.06 | 126 ± 12 | 1.3 ± 0.1 |
| Women | 4.6 ± 0.1§ | 31 ± 5§* | 57 ± 4.5 | 725 ± 62 | 104 ± 11.4 | 0.43 ± 0.10 | 153 ± 16 | 1.7 ± 0.2 |
p<0.05 men vs. women;
p<0.05 pre- vs. post-intervention
FFA = free fatty acids
Epi = epinephrine
Norepi = norepinephrine
Insulin Action & Secretion
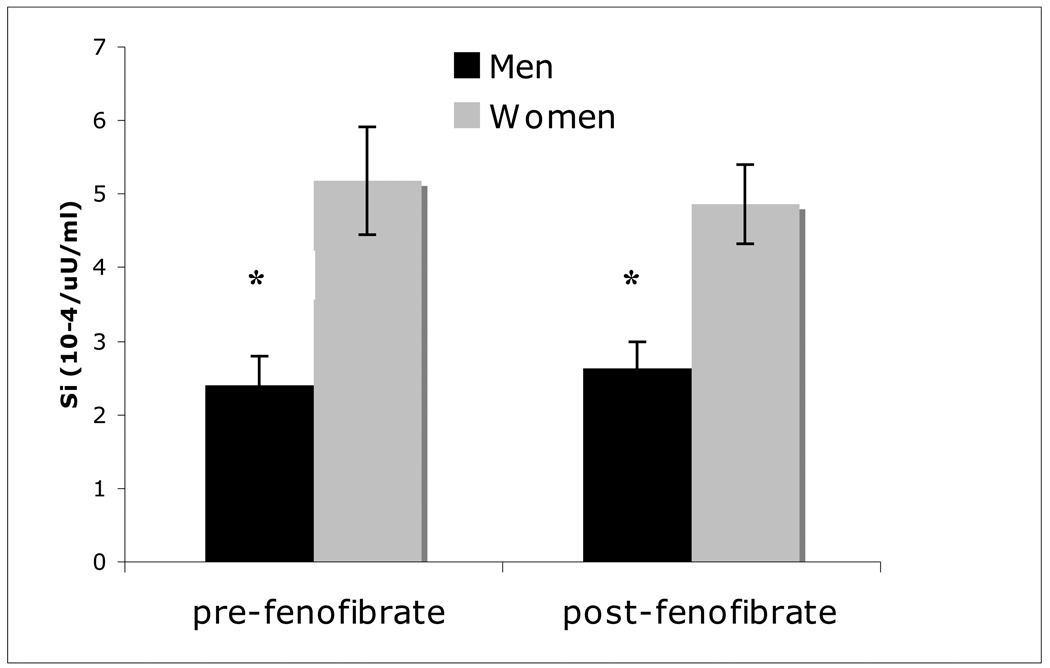
Insulin action (Si) was greater in women vs. men before (p=0.004) and after (p=0.004) fenofibrate, and was unchanged in either group (p≥0.68; Figure 1). Measures of insulin secretion (AIR and DI) were not different before (AIR 337±40 vs. 265±71 mU/ml/min, M vs. W, p=0.64; DI 649±241 vs. 1313±366 ×10−4/min, M vs. W, p=0.16) or after (p≥0.35) fenofibrate, and also were unchanged by the drug (p≥0.63).
Figure 1.
Insulin sensitivity (Si) pre- and post-fenofibrate in men and women. *p<0.05 men vs. women.
Whole Body Substrate Use
Respiratory exchange ratio (RER), a measure of whole body substrate use, was similar between groups at baseline (0.78±0.02 vs. 0.75±0.01, M vs. W, p=0.21). Following fenofibrate administration, there was a trend for an increase in RER in the men (p=0.09) such that the RER was higher than in women (0.83±0.02 vs. 0.77±0.01, M vs. W, p=0.004).
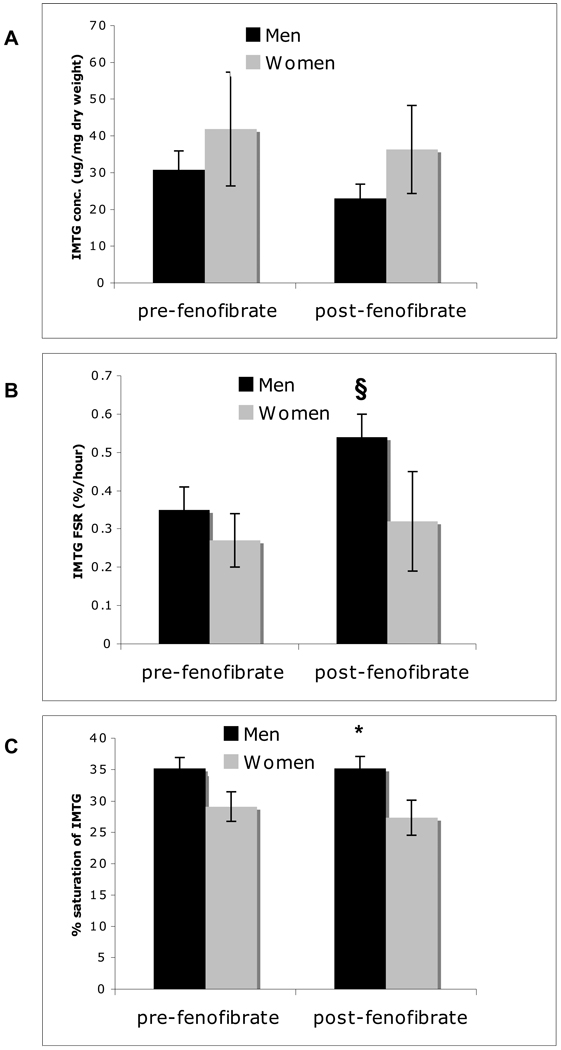
IMTG Concentration, Composition and Synthesis Rate
IMTG concentration was similar at baseline (p=0.43) and after fenofibrate (p=0.22; Figure 2A). IMTG FSR (a measure of IMTG turnover) increased in men (pre- vs. post-treatment, p=0.05) with no change observed in the women (p=0.73; Figure 2B). A trend for lower IMTG saturation seen in the women pre-treatment (p=0.06) became significant post-treatment (p=0.04; Figure 2C) despite little change in either group (p≥0.65). A non-significant trend relating IMTG saturation to insulin sensitivity (Si; R2=0.34, p=0.10) was observed in the cohort as a whole. In examining the individual fatty acid species in IMTG, women had more oleic acid before (p=0.01) and after (p=0.02) fenofibrate, whereas the men had more palmitic (p=0.04) and stearic (p=0.03) acid, as well as lower palmitoleic acid (p=0.02) vs. women post-fenofibrate (Table 2). Ratios of 16:1/16:0 (0.25±0.06 vs. 0.13±0.01, W vs. M post-treatment, p=0.02) and 18:1/18:0 (11.4±2.6 vs. 5.1±0.9, W vs. M post-treatment, p=0.02), a surrogate for stearyl CoA desaturase-1 (SCD-1) activity [25], were both significantly higher in women post-treatment but not different between groups pre-treatment. Palmitate Rd was similar between men and women pre-drug (3.44±0.78 vs. 3.53±0.46 umol/kg/min, M vs. W, p=0.92), yet a marked decline in Rd in men was seen post-drug, such that men had a significantly lower palmitate disposal post-treatment (1.85±0.30 vs. 4.22±0.51 umol/kg/min, M vs. W, p=0.003). No differences were noted between the sexes with respect to palmitate incorporation or oxidation before or after fenofibrate (data not shown).
Figure 2.
Intramuscular triglyceride (IMTG) concentration (A), fractional synthesis rate (FSR) (B), and saturation (C) pre- and post- fenofibrate in men and women. *p<0.05 men vs. women; §p<0.05 pre- vs. post-intervention.
Table 2.
Composition of Plasma and Intramuscular Triglyceride Pre- and Post-Fenofibrate
| Plasma | myristic (mmol/l) |
palmitic (mmol/l) |
palmitoleic (mmol/l) |
stearic (mmol/l) |
oleic (mmol/l) |
linoeate (mmol/l) |
γ-linolenic (mmol/l) |
α-linolenic (mmol/l) |
arachidonic (mmol/l) |
|---|---|---|---|---|---|---|---|---|---|
| Pre-fenofibrate | |||||||||
| Men | 0.12±0.02* | 1.04±0.08 | 0.12±0.01 | 0.40±0.04* | 1.19±0.07 | 1.01±0.10 | 0.03±0.00 | 0.07±0.01 | 0.11±0.01* |
| Women | 0.06±0.01 | 0.83±0.12 | 0.13±0.03 | 0.27±0.03 | 1.11±0.19 | 0.91±0.11 | 0.02±0.00 | 0.07±0.01 | 0.08±0.01 |
| Post-fenofibrate | |||||||||
| Men | 0.07±0.01 | 0.79±0.09 | 0.11±0.02 | 0.27±0.05 | 0.90±0.07 | 0.66±0.05 | 0.03±0.00 | 0.04±0.01 | 0.11±0.01 |
| Women | 0.04±0.01 | 0.63±0.10 | 0.11±0.02 | 0.20±0.03 | 0.86±0.15 | 0.62±0.12 | 0.02±0.00 | 0.04±0.01 | 0.09±0.01 |
| Muscle |
myristic (ug/mg dry wt) |
palmitic (ug/mg dry wt) |
palmitoleic (ug/mg dry wt) |
stearic (ug/mg dry wt) |
oleic (ug/mg dry wt) |
linoeate (ug/mg dry wt) |
linolenic (ug/mg dry wt) |
arachidonic (ug/mg dry wt) |
|
| Pre-fenofibrate | |||||||||
| Men | 1.84±0.21 | 24.19±0.62 | 4.51±0.57 | 9.14±1.52 | 33.56±3.29* | 16.13±0.33 | 0.84±0.05 | 0.85±0.11 | |
| Women | 2.24±0.16 | 23.88±0.86 | 5.00±0.35 | 6.23±0.59 | 45.89±1.68 | 15.21±0.40 | 0.86±0.05 | 0.68±0.10 | |
| Post-fenofibrate | |||||||||
| Men | 2.23±0.20 | 25.46±0.69* | 3.40±0.31* | 9.35±1.03* | 42.19±1.36* | 15.02±0.06 | 0.91±0.04 | 0.89±0.08 | |
| Women | 1.78±0.31 | 21.62±1.62 | 5.25±0.74 | 5.26±1.24 | 49.40±2.39 | 14.89±0.05 | 0.85±0.04 | 0.73±0.09 | |
p<0.05 men vs. women
p<0.05 pre- vs. post-fenofbrate
wt = weight
Plasma Triglyceride Concentration and Composition
Plasma triglyceride (TG) concentration (assessed by GC/MS post-diet control) trended to be higher in men (1.37±0.08 mmol/l) vs. women (1.04±0.15 mmol/l, p=0.09) at baseline. However, fenofibrate significantly decreased total plasma TG in men (p=0.03; not women, p=0.28) by preferentially decreasing unsaturated fatty acids such as oleic acid (p=0.01), linoleate (p=0.01) and linolenic acid (p=0.02) (Table 2). Men tended to have more overall saturated plasma TG at baseline and following fenofibrate (p=0.06 for both). Women had less myristic acid (p=0.02) and stearic acid (p=0.02), as well as more arachadonic acid (p=0.03), at baseline, whereas no such differences were noted post-treatment (Table 2).
Protein Expression
Enzymes influencing IMTG metabolism were examined by western blot [10]. Skeletal muscle oxidative capacity, assessed by succinate dehydrogenase (SDH), was lower in men (0.75±0.06 AU) compared to women (1.30±0.24 AU, p=0.02) post-treatment, but not pre-treatment (p=0.17). Fenofibrate also lowered CPT-1 in men (1.19±0.10 vs. 0.81±0.11 AU, p=0.03). Otherwise protein abundance for myosin A4.840 (type 1 muscle fibers), myosin A4.74 (type 2 muscle fibers), mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4), 4-hydroxynonenal (4-HNE), insulin receptor substrate-1 serine-phosphorylated at position 636 (IRS-1ser636), ratio of IRS-1ser636/IRS-1total, PPAR–α, protein kinase C isoform epsilon (PKC-ε) were not different between groups (data not shown).
Medication Tolerability and Compliance
Fenofibrate at 145 mg orally per day × 12 weeks was well tolerated in all subjects. There were no drop-outs, no serious adverse events, and compliance was >95% (no difference between men and women). There was no significant change in weight in either group (0.58±0.36 vs. 0.63±0.25 kg weight change, M vs. W, p=0.92).
DISCUSSION
Increasing evidence suggests that obese men and women may develop diabetes differently. Specifically, we have previously shown altered intramuscular triglyceride metabolism relates to diminished insulin action in men, but not women, in the progression to diabetes [8]. Specifically, lower insulin sensitivity, higher IMTG concentration and lower IMTG FSR cluster in men with pre-diabetes (vs. simple obesity), with no such differences observed in women at the same stages of glucose intolerance. PPAR-α is a known mediator of intramuscular lipid trafficking, particularly requisite in male animal models [11]. The significance of PPAR-α in skeletal muscle of male humans at risk for diabetes had not been previously explored and was the aim of the current study. Major findings from this study demonstrated that fenofibrate (a PPAR-α agonist) administration 1) increased IMTG FSR in men, but 2) did not change IMTG concentration or saturation in either sex, and 3) ultimately did not impact insulin sensitivity in men or women. Notably, IMTG saturation, rather than concentration or FSR, most closely (but not significantly) related to insulin resistance in the cohort as a whole. Fenofibrate lowered total plasma TG concentration in the normolipidemic men in this study, but did so at the expense of unsaturated fatty acids. In contrast, presumed enhancement of SCD-1 activity further desaturated IMTG in women and may have contributed to their greater insulin sensitivity.
Reports examining the effect of fenofibrate on insulin sensitivity are mixed, largely due to differences in PPAR-α distribution in insulin sensitive tissues that vary widely between species. For example, improved insulin sensitivity post-fibrate treatment has been universally observed in rodent models [13, 26, 27], but virtually never in human studies [28–30], including ours. We did observe a decrease in both circulating glucose and insulin concentration in women, as well as a lower circulating glucose concentration in men, post-fenofibrate, but these subtle changes did not significantly impact the index of whole body insulin sensitivity derived by the IVGTT. Further, markers of insulin signaling in muscle from the western blots, although non-insulin stimulated, were unchanged post-treatment in either sex. Together, these data serve as the first to demonstrate that PPAR-α agonists have no major effect on insulin action in human skeletal muscle in vivo.
Increasing evidence suggests that IMTG concentration and insulin sensitivity can be dissociated [31–33]. Our results suggest the same may be true for IMTG FSR and insulin sensitivity. Collectively, these observations underscore the complexity and continued interest in the link between IMTG dynamics and insulin action [34, 35]. Recent studies in both animals and humans have concluded that high IMTG concentration may not be deleterious to insulin sensitivity as long as IMTG flux is preserved [31–33]. Nevertheless, the current study increased IMTG FSR in men a priori and did not observe the expected improvement in insulin sensitivity. Of note, in the basal state, IMTG FSR approximates IMTG degradation, thus serves as a surrogate marker of turnover in the IMTG pool. It is possible that fenofibrate had mixed actions on IMTG metabolism in men that obscured an improvement in insulin sensitivity from increased FSR. For example, markers of lipid uptake (palmitate Rd and CPT-1) and oxidation (SDH) were suppressed by fenofibrate. Unchanged IMTG concentration amidst an increase in IMTG FSR would suggest that the degradation rate of IMTG also increased to match the synthesis rate. Taken together, the FFAs liberated during IMTG degradation likely were utilized for IMTG re-synthesis, rather than oxidized. These findings support the notion that PPAR-α activity has distinct effects on tissue lipid partitioning in men, and these are independent of insulin action.
In contrast to the changes observed in men, fenofibrate administration appeared to have virtually no effect in women. Women were more insulin sensitive than men both before and after the intervention, as has been previously reported in carefully matched men and women [8, 36, 37]. Interestingly, degree of saturated IMTG, rather than IMTG concentration or FSR, most closely (although not significantly) related to insulin sensitivity in this cohort, as in others [9, 10]. Our women trended toward having less saturated IMTG before treatment that became significantly lower after treatment. This may have been due to an increase in skeletal muscle SCD-1 activity, as suggested by an increase in 16:1/16:0 and 18:1/18:0 ratios [25]. Similar to skeletal muscle, plasma TG also tended to be less saturated in women before and after treatment. Understanding how women maintain a state of less saturated TG, and how fenofibrate may have influenced this, may prove a novel pathway for insulin sensitization.
The observed changes in IMTG composition and FSR were presumed due to PPAR-α activation in skeletal muscle by fenofibrate. Most surprising was the lack of change in skeletal muscle PPAR-α protein expression following treatment. Interestingly, PPAR-α is expressed most prominently in human skeletal muscle [38], but its activity appears to be far greater in liver [39, 40]. Specifically, fenofibrate accelerates the clearance of triglyceride-rich lipoproteins [30], likely affecting the delivery of FFA to muscle for uptake into IMTG. Thus, we speculate that changes in IMTG metabolism may have been indirect, through the enhanced action of PPAR-α in the liver, affecting downstream delivery of substrate to muscle, not altered PPAR-α activity in muscle itself. This speculation cannot be confirmed, as we did not measure PPAR-α protein expression in liver. However, the decline in plasma TG in the men is consistent with this contention. In short, much work is still needed to elucidate the sexual dimorphism in response to PPAR-α agonists noted here and in recent clinic trials [41].
There are several limitations of the current study worth noting. First, a published, not individually measured, acetate recovery factor was used in the calculation of palmitate oxidation. This was done because IMTG dynamics, not palmitate oxidation, was the primary outcome of interest. Second, all subjects received open label fenofibrate and none received placebo. Thus, day-to-day variation in our outcome measures was not captured and additional studies will be needed to confirm our findings. It is also possible that use of the hyperinsulinemic/euglycemic clamp may have been a more precise measure of insulin sensitivity, but numerous validation studies have demonstrated close correlation between Si (from the IVGTT) and glucose infusion rate (from the clamp). Lastly, our small sample size likely reduced the ability to detect subtle differences in some parameters of interest. It should also be noted that the data are not corrected for multiple comparisons, thus should be interpreted with caution, generating hypotheses rather than fully conclusive.
In summary, the current study examined the role of PPAR-α as a possible mediator for the sex differences observed in intramuscular lipid metabolism in obese people at risk for diabetes. We observed an increase in IMTG FSR in men; a change that has been previously shown to relate to enhanced insulin sensitivity in men [8], however no change in insulin sensitivity was observed in the current study. Insulin sensitivity appears more closely related to the degree of IMTG saturation, than to its concentration or synthesis rate [9, 10]. Lower TG saturation in plasma and/or muscle of women may be vital in maintaining their higher insulin sensitivity in this study and others. These data suggest changing plasma and/or IMTG saturation may be an avenue for future therapies to enhance insulin sensitivity and prevent the development of diabetes.
Acknowledgements
We owe the success of this work to the research subjects who volunteered their time to participate, the committed staff of the General Clinical Research Center, as well as to the National Institutes of Health, who funded this work (grant # NIH DK-064811, DK-059739, and RR-0036).
Abbreviations
- IMTG
intramuscular triglyceride
- PPAR-α
peroxisome proliferator activated receptor-α
- FSR
fractional synthesis rate
- DEXA
dual energy X-ray absorptiometry
- IVGTT
Intravenous glucose tolerance test
- Si
Insulin sensitivity
- AIR
acute insulin response
- DI
disposition index
- GCRC
General Clinical Research Center
- FFA
free fatty acids
- FAME
fatty acid methyl ester
- GC/C-IRMS
gas chromatography-combustion isotope ratio mass spectrometer
- IRMS
isotope ratio mass spectrometry
- SDH
succinate dehydrogenase
- IRS
insulin receptor substrate
- CPT-1
carnitine palmitoyltransferase -1
- MAP4K4
mitogen-activated protein kinase kinase kinase kinase 4
- PKC
protein kinase C
- Rd
rate of disappearance
- ANOVA
analysis of variance
- SEM
standard error of the mean
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- RER
Respiratory exchange ratio
- SCD-1
stearyl CoA desaturase-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no conflicts of interest related to this work.
Author contributions:
| Design | data collection | analysis | interpret | writing | |
|---|---|---|---|---|---|
| Leigh Perreault | X | X | X | X | X |
| Bryan Bergman | X | X | X | ||
| Devon Hunerdosse | X | X | |||
| David Howard | X | X | |||
| Robert Eckel | X | X |
REFERENCES
- 1.DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983;32:35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 4.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 5.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 6.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 7.Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59:89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perreault L, Bergman BC, Hunerdosse DM, Eckel RH. Altered Intramuscular Lipid Metabolism Relates to Diminished Insulin Action in Men, but Not Women, in Progression to Diabetes. Obesity(Silver Spring) 2010;18(11):2093–2100. doi: 10.1038/oby.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol. 2010;108:1134–1141. doi: 10.1152/japplphysiol.00684.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes. 2009;58:2220–2227. doi: 10.2337/db09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djouadi F, Weinheimer CJ, Saffitz JE, et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha- deficient mice. J Clin Invest. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou CJ, Haluzik M, Gregory C, et al. WY14,643, a peroxisome proliferator-activated receptor alpha (PPARalpha) agonist, improves hepatic and muscle steatosis and reverses insulin resistance in lipoatrophic A-ZIP/F-1 mice. J Biol Chem. 2002;277:24484–24489. doi: 10.1074/jbc.M202449200. [DOI] [PubMed] [Google Scholar]
- 13.Furuhashi M, Ura N, Murakami H, et al. Fenofibrate improves insulin sensitivity in connection with intramuscular lipid content, muscle fatty acid-binding protein, and beta-oxidation in skeletal muscle. J Endocrinol. 2002;174:321–329. doi: 10.1677/joe.0.1740321. [DOI] [PubMed] [Google Scholar]
- 14.Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Watanabe RM, Rebrin K, Ader M, Steil GM. Toward an integrated phenotype in pre-NIDDM. Diabet Med. 1996;13:S67–S77. [PubMed] [Google Scholar]
- 16.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 17.Guo Z, Mishra P, Macura S. Sampling the intramyocellular triglycerides from skeletal muscle. J Lipid Res. 2001;42:1041–1048. [PubMed] [Google Scholar]
- 18.Rosendal J, Knudsen J. A fast and versatile method for extraction and quantitation of long-chain acyl-CoA esters from tissue: content of individual long-chain acyl-CoA esters in various tissues from fed rat. Anal Biochem. 1992;207:63–67. doi: 10.1016/0003-2697(92)90500-7. [DOI] [PubMed] [Google Scholar]
- 19.Kaluzny MA, Duncan LA, Merritt MV, Epps DE. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985;26:135–140. [PubMed] [Google Scholar]
- 20.Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism. 1998;47:706–712. doi: 10.1016/s0026-0495(98)90035-x. [DOI] [PubMed] [Google Scholar]
- 21.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 22.Agren JJ, Julkunen A, Penttila I. Rapid separation of serum lipids for fatty acid analysis by a single aminopropyl column. J Lipid Res. 1992;33:1871–1876. [PubMed] [Google Scholar]
- 23.Wolfe R. Radioative and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- 24.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 2000;49:2102–2107. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- 25.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–E37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HJ, Choi SS, Park MK, et al. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats. Biochem Biophys Res Commun. 2002;296:293–299. doi: 10.1016/s0006-291x(02)00822-7. [DOI] [PubMed] [Google Scholar]
- 27.Nadeau KJ, Ehlers LB, Aguirre LE, Reusch JE, Draznin B. Discordance between intramuscular triglyceride and insulin sensitivity in skeletal muscle of Zucker diabetic rats after treatment with fenofibrate and rosiglitazone. Diabetes Obes Metab. 2007;9:714–723. doi: 10.1111/j.1463-1326.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 28.Abbasi F, Chen YD, Farin HM, Lamendola C, Reaven GM. Comparison of three treatment approaches to decreasing cardiovascular disease risk in nondiabetic insulin-resistant dyslipidemic subjects. Am J Cardiol. 2008;102:64–69. doi: 10.1016/j.amjcard.2008.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belfort R, Berria R, Cornell J, Cusi K. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J Clin Endocrinol Metab. 95:829–836. doi: 10.1210/jc.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabbrini E, Mohammed BS, Korenblat KM, et al. Effect of Fenofibrate and Niacin on Intrahepatic Triglyceride Content, Very Low-Density Lipoproteins Kinetics, and Insulin Action in Obese Subjects with Nonalcoholic Fatty Liver Disease. J Clin Endocrinol Metab. 2010;95(6):2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- 34.AbouRjaili G, Shtaynberg N, Wetz R, Costantino T, Abela GS. Current concepts in triglyceride metabolism, pathophysiology, and treatment. Metabolism. 59:1210–1220. doi: 10.1016/j.metabol.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Newsom SA, Schenk S, Li M, Everett AC, Horowitz JF. High fatty acid availability after exercise alters the regulation of muscle lipid metabolism. Metabolism. 2010 Sep 24; doi: 10.1016/j.metabol.2010.08.004. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuutila P, Knuuti MJ, Maki M, et al. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes. 1995;44:31–36. doi: 10.2337/diab.44.1.31. [DOI] [PubMed] [Google Scholar]
- 37.Yki-Jarvinen H. Sex and insulin sensitivity. Metabolism. 1984;33:1011–1015. doi: 10.1016/0026-0495(84)90229-4. [DOI] [PubMed] [Google Scholar]
- 38.Loviscach M, Rehman N, Carter L, et al. Distribution of peroxisome proliferator-activated receptors (PPARs) in human skeletal muscle and adipose tissue: relation to insulin action. Diabetologia. 2000;43:304–311. doi: 10.1007/s001250050048. [DOI] [PubMed] [Google Scholar]
- 39.Catapano AL. Mode of action of fibrates. Pharmacol Res. 1992;26:331–340. doi: 10.1016/1043-6618(92)90232-z. [DOI] [PubMed] [Google Scholar]
- 40.Shepherd J. Mechanism of action of fibrates. Postgrad Med J. 1993;69 Suppl 1:S34–S41. [PubMed] [Google Scholar]
- 41.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]