Summary
Here we report that inactivation of the C. elegans dynamin-related protein DRP-1, a key component responsible for mitochondrial fission and conserved from yeast to humans, dramatically enhanced the effect of reduced insulin signaling (IIS) to extend lifespan. This represents the first report of a beneficial impact of manipulating mitochondrial dynamics on animal lifespan and suggests that mitochondrial morphology and IIS cooperate to modulate aging.
Keywords: mitochondria, fission protein DRP-1, insulin signaling, aging, C. elegans
Mitochondria are dynamic organelles able to undergo frequent morphological and numeral changes. A delicate balance between mitochondrial fusion and fission is critical for broad aspects of animal physiology, including apoptosis and control of mitochondrial inheritance and quality (Seo et al. 2010). From yeast to human, deregulations of mitochondrial network equilibrium as evident by disrupted mitochondrial morphology and accumulation of abnormally shaped mitochondria have been associated with senescence, aging and aging-related diseases (Sohal 1975; Yasuda et al. 2006; Lee et al. 2007). However, no direct evidence implicates mitochondrial dynamics in longevity determination in animals. The only evidence that mitochondrial plasticity positively impacts lifespan was shown in fungal models, in which reduced mitochondrial fission led to increased lifespan (Scheckhuber et al. 2007; Palermo et al. 2010). Mitochondrial dynamics are governed by molecular machineries that are highly conserved (Okamoto & Shaw 2005). The dynamin-related protein DRP-1 is the only profission protein identified in C. elegans and is demonstrated to control the scission of the mitochondrial outer membrane (Labrousse et al. 1999; Westermann 2010).
To explore how mitochondrial fission can impact animal lifespan, we monitored the lifespan of worms either treated with drp-1 RNAi or bearing a putative null-mutation in the drp-1 gene (Breckenridge et al. 2008). The mean lifespan of wild-type worms treated with drp-1 RNAi or drp-1 mutant worms was indistinguishable from that of control animals (Fig.1A-B, Table S1), even though their mitochondrial morphology are greatly disrupted (Labrousse et al. 1999, Fig.2B-C and S1), indicating that reduced mitochondrial fission does not affect C. elegans lifespan under normal culturing condition
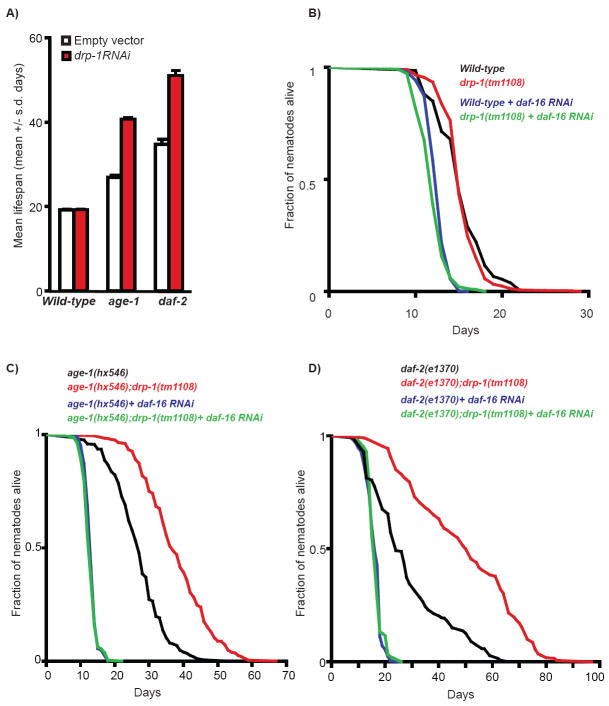
Figure 1. Loss of drp-1 enhances the longevity phenotype of IIS mutant worms in a daf-16-dependent manner.
(A) Mean adult lifespan (+/- s.d) of wild-type, age-1 and daf-2 mutant worms treated with empty vector (empty bars) or drp-1 RNAi (red bars). Note that quantitative PCR data showed that drp-1 mRNA expression was reduced by ~50% in all strains upon drp-1 RNAi treatment (data not shown). Adult lifespan of wild-type and drp-1 mutant worms (B); age-1 and age-1;drp-1 mutant worms (C); daf-2 and daf-2;drp-1 mutant worms (D) treated or not with daf-16 RNAi (as indicated). Quantitative data and statistical analysis are presented in Table S1.
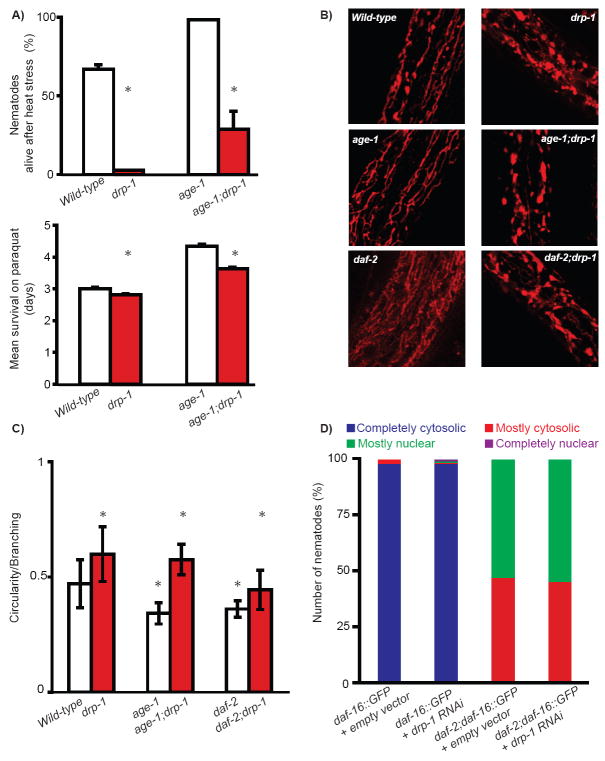
Figure 2. Loss of drp-1 affects mitochondrial morphology and stress resistance without affecting DAF-16 sub-cellular localization.
(A) Percentage of worms alive (+/- s.d) after 8 hours at 37°C (upper graph) and mean survival (+/- s.d) upon treatment with 25 mM paraquat (bottom graph) in the indicated strains. * indicates p<0.001 (Student’s t-test for heat stress and log-rank test for paraquat). TMRE staining (B) and circularity index (+/- s.d) (C) of the mitochondrial network in the indicated strains. In (C), * indicates p<0.005 when compared to single mutant counterpart and p<0.005 when compared to wild-type worms using a Student’s t-test. In agreement with previous studies (Labrousse et al. 1999), drp-1 mutant worms exhibited disrupted structure of the tubular mitochondrial network in which mitochondria tended to form large blebs as reflected by an increased circularity index. (D) DAF-16∷GFP was categorized by sub-cellular localization in daf-2 worms treated with empty vector or drp-1 RNAi. The data obtained at Day 3 of adulthood are presented.
The insulin/IGF-1 signaling (IIS) pathway is a key longevity pathway and C. elegans mutants with reduced IIS, such as the phosphatidyl-inositol 3-kinase age-1 mutant (Friedman & Johnson 1988; Morris et al. 1996) and the tyrosine kinase insulin/IGF receptor daf-2 mutant (Kenyon et al. 1993), are long-lived (Fig.1A,C-D). Given that mitochondrial fission plays a critical role in insulin secretion in mammals (Yoon et al. 2010), we next tested how inactivating drp-1 might affect the longevity of IIS mutants. Strikingly, both age-1 and daf-2 mutants treated with drp-1 RNAi showed substantial further increase in mean lifespan (>65% compared to age-1 and daf-2 single mutant on control RNAi; Fig.1A, Table S1). Similarly, age-1;drp-1 and daf-2;drp-1 mutants exhibited a >75% increase in mean lifespan and an extension by up to 30 days in maximum lifespan when compared to their single mutant counterparts (Fig.1C-D, Table S1). Importantly, the enhancement of both the mean and maximum lifespans of age-1 mutants by the drp-1 mutation could be rescued by a transgene expressing drp-1 (Fig.S2). We also tested whether drp-1 inactivation will impact the lifespan of other long-lived strains, including worms undergoing the bacteria deprivation paradigm of dietary restriction (Kaeberlein et al. 2006), and worms with mild impairment of the mitochondrial respiratory chain (Felkai et al. 1999; Feng et al. 2001). We detected no or marginal effects (Table S2). Altogether, we demonstrated that inactivating drp-1 specifically and robustly synergizes with reduced IIS to prolong longevity.
The longevity effect of reduced IIS is often associated with increased resistance to stress (Johnson et al. 2001). Surprisingly, wild-type and insulin mutant worms with drp-1 deletion were more sensitive to the oxidizing agent paraquat and to heat stress than their counterparts with wild-type drp-1 (Fig.2A, Table S3). The vulnerability of the age-1;drp-1 mutant to heat stress is due to drp-1 loss as it can be rescued by a transgene overexpressing drp-1 (Fig.S2). Therefore, inactivating drp-1 specifically prolongs longevity, but not stress resistance, of worms with reduced IIS, suggesting that manipulation of mitochondrial fission uncouples the function of IIS in aging and stress resistance. However, we cannot exclude that inactivating drp-1 causes an early stress in young worms but will confer stress resistance later in life.
We next examined how inactivation of drp-1 affects mitochondrial morphology by comparing the indices of circularity (a mathematical estimation of circular shapes) of individual mitochondrion (see Supplementary Materials and Methods). We found that mitochondrial morphology was similarly disrupted in single drp-1 mutant and in IIS;drp-1 mutants (Fig.2C), suggesting DRP-1 does not cooperate with IIS to regulate mitochondrial fission. Interestingly, our data also revealed that mitochondria of daf-2 and age-1 single mutants presented lower indices of circularity than those of wild-type animals (Fig.2B-C).
Signaling from DAF-2 and AGE-1 results in cytoplasmic retention of the FOXO transcription factor DAF-16, and DAF-16 translocation into the nucleus in age-1 and daf-2 mutants enables DAF-16-mediated regulation of target genes that contribute to diverse functional outcomes (Hekimi et al. 2001; Lee et al. 2001). Because the prolonged lifespan of age-1 and daf-2 mutants are fully dependent on daf-16 (Dorman et al. 1995), we next tested whether the extraordinary longevity of the IIS;drp-1 mutants also requires daf-16. We found that daf-16 RNAi completely abolished the synergistic effect of drp-1 and daf-2/age-1 mutations (Fig.1C-D, Table S1). Interestingly, we did not detect any changes in DAF-16∷GFP sub-cellular localization upon drp-1 inactivation (Fig.2D). Thus, the mechanism allowing DRP-1 to cooperate with IIS in modulating lifespan fully depends on DAF-16 but is unlikely to be mediated via DAF-16’s nuclear translocation.
In considering a mechanism that enables drp-1 inactivation to specifically synergize with IIS mutants to extend lifespan, several possibilities come to mind. In mammals, mitochondrial fission is critical for insulin secretion (Zorzano et al. 2009; Yoon et al. 2010). Thus, drp-1 inactivation in worms may similarly interfere with insulin secretion and further reduce IIS in age-1/daf-2 mutants. This possibility is unlikely however as DAF-16 nuclear translocation was not enhanced in IIS;drp-1 double mutants. Although we found that age-1 and daf-2 single mutants show slight alterations in mitochondrial morphology, our data do not support the possibility that mitochondrial dynamics was further perturbed in IIS;drp-1 double mutants, as mitochondrial morphology in those mutants were similar to drp-1 single mutant. On the other hand, because the drp-1 single mutant has a brood size defect (Breckenridge et al. 2008), it is possible that germline proliferation is inhibited when drp-1 is inactivated, and defective germline proliferation is known to synergize with IIS mutant to increase lifespan (Hsin & Kenyon 1999; Spanier et al.). Alternatively, similar to what was shown with mitochondrial prohibitins (Artal-Sanz & Tavernarakis 2009), inactivation of drp-1 may perturb lipid metabolism, a process highly dependent on mitochondrial activity, to synergize with IIS mutants to extend lifespan. Lastly, C. elegans DRP-1 is shown to regulate apoptosis under sensitized conditions (Breckenridge et al. 2008). Intriguingly, daf-2 mutants exhibit enhanced germline apoptosis (Pinkston et al. 2006). It is possible that the apoptotic function of DRP-1 is induced in IIS mutants, and loss of drp-1 may block apoptosis in IIS mutants and further promote longevity. Interestingly, in fungal models, mitochondrial fission modulates wild-type lifespan by interfering with apoptosis (Scheckhuber et al. 2007; Palermo et al. 2010). It is possible that fungal aging is more closely connected to apoptosis (Rockenfeller & Madeo 2008) than C. elegans aging, providing an explanation for why reduced mitochondrial fission affects wild-type lifespan in fungi but not in worms.
This is the first report of a manipulation of mitochondrial dynamics that positively impacts lifespan in an animal. As components of the mitochondrial fission/fusion machinery and IIS are highly conserved, our observations are likely relevant to mitochondrial biology and longevity in mammals.
Materials and Methods
All strains were obtained from the Caenorhabditis Genetic Center with the exception of the drp-1 (tm1108) strain provided by the National Bioresource Project for the Nematode. Lifespan assays were performed as in (Li et al. 2008) with slight modifications (see Supplementary Materials and Methods).
DAF-1 6 s u b-cellular localization was evaluated using daf-2(e1370);daf-16(mgDf47);xrls87 transgenic worms as in (Li et al. 2008) and (Padmanabhan et al. 2009) with slight modifications (see Supplementary Materials and Methods). The data shown in Fig.2D were obtained at Day 3 of adulthood.
Mitochondrial networks were visualized using tetramethylrhodamine (TMRE) staining or a Pmyo-3:mito∷GFP construct. See Supplementary Materials and Methods for more detailed procedures.
Stress assays were performed on synchronized populations at the young adult stage or Day 3 of adulthood. For heat stress, synchronized worm populations were placed at 37°C for 8 hours and scored immediately after for survival. For paraquat-induced stress, synchronized worm populations were transferred onto plates seeded with a final concentration of 25mM paraquat. Similar results were obtained using 15 mM paraquat. The population was kept at 20°C and scored every day for survival. Each assay was repeated at least 3 times.
Supplementary Material
Acknowledgments
We are grateful to the members of the Lee Laboratory and the Cornell C. elegans groups for discussions during the course of this work. We thank Carol Bayles of the Microscopy and Imaging Facility at Cornell University and Rada Omanovic for technical support as well as the members of the Aguilaniu lab and Cécile Bedet from the LBMC, ENS-Lyon. LW is supported by a grant from the United Mitochondrial Disease Foundation (UMDF) and from the Marie Curie IRG. SSL is supported by the Senior Scholar Award in Aging from the Ellison Medical Foundation, the Glenn Award for Research in Biological Mechanisms of Aging and R01 grant AG024425 from the NIA.
References
- Artal-Sanz M, Tavernarakis N. Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans. Nature. 2009;461:793–797. doi: 10.1038/nature08466. [DOI] [PubMed] [Google Scholar]
- Breckenridge DG, Kang BH, Kokel D, Mitani S, Staehelin LA, Xue D. Caenorhabditis elegans drp-1 and fis-2 regulate distinct cell-death execution pathways downstream of ced-3 and independent of ced-9. Mol Cell. 2008;31:586–597. doi: 10.1016/j.molcel.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman JB, Albinder B, Shroyer T, Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141:1399–1406. doi: 10.1093/genetics/141.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felkai S, Ewbank JJ, Lemieux J, Labbe JC, Brown GG, Hekimi S. CLK-1 controls respiration, behavior and aging in the nematode Caenorhabditis elegans. Embo J. 1999;18:1783–1792. doi: 10.1093/emboj/18.7.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi S, Burgess J, Bussiere F, Meng Y, Benard C. Genetics of lifespan in C. elegans: molecular diversity, physiological complexity, mechanistic simplicity. Trends Genet. 2001;17:712–718. doi: 10.1016/s0168-9525(01)02523-9. [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Johnson TE, de Castro E, Hegi de Castro S, Cypser J, Henderson S, Tedesco P. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol. 2001;36:1609–1617. doi: 10.1016/s0531-5565(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- Li J, Ebata A, Dong Y, Rizki G, Iwata T, Lee SS. Caenorhabditis elegans HCF-1 functions in longevity maintenance as a DAF-16 regulator. PLoS Biol. 2008;6:e233. doi: 10.1371/journal.pbio.0060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S, Mukhopadhyay A, Narasimhan SD, Tesz G, Czech MP, Tissenbaum HA. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo V, Falcone C, Calvani M, Mazzoni C. Acetyl-L-carnitine protects yeast cells from apoptosis and aging and inhibits mitochondrial fission. Aging Cell. 2010;9:570–579. doi: 10.1111/j.1474-9726.2010.00587.x. [DOI] [PubMed] [Google Scholar]
- Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science. 2006;313:971–975. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- Rockenfeller P, Madeo F. Apoptotic death of ageing yeast. Exp Gerontol. 2008;43:876–881. doi: 10.1016/j.exger.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheckhuber CQ, Erjavec N, Tinazli A, Hamann A, Nystrom T, Osiewacz HD. Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nat Cell Biol. 2007;9:99–105. doi: 10.1038/ncb1524. [DOI] [PubMed] [Google Scholar]
- Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RD. Mitochondrial changes in flight muscles of normal and flightless Drosophila melanogaster with age. J Morphol. 1975;145:337–353. doi: 10.1002/jmor.1051450307. [DOI] [PubMed] [Google Scholar]
- Spanier B, Rubio-Aliaga I, Hu H, Daniel H. Altered signalling from germline to intestine pushes daf-2;pept-1 Caenorhabditis elegans into extreme longevity. Aging Cell. 2010;9:636–646. doi: 10.1111/j.1474-9726.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- Westermann B. Mitochondrial dynamics in model organisms: what yeasts, worms and flies have taught us about fusion and fission of mitochondria. Semin Cell Dev Biol. 2010;21:542–549. doi: 10.1016/j.semcdb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Ishii T, Suda H, Akatsuka A, Hartman PS, Goto S, Miyazawa M, Ishii N. Age-related changes of mitochondrial structure and function in Caenorhabditis elegans. Mech Ageing Dev. 2006;127:763–770. doi: 10.1016/j.mad.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial Dynamics in Diabetes. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Liesa M, Palacin M. Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch Physiol Biochem. 2009;115:1–12. doi: 10.1080/13813450802676335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.