Abstract
Background
Given the prevalence of alcohol use in adolescence, it is important to understand the consequences of chronic ethanol exposure during this critical period in development. The purpose of the present study was to assess possible age-related differences in susceptibility to tolerance development to ethanol-induced sedation and withdrawal-related anxiety, as well as voluntary ethanol intake after chronic exposure to relatively high doses of ethanol during adolescence or adulthood.
Methods
Adolescent and adult male Sprague-Dawley rats were assigned to one of five 10 day exposure conditions: chronic ethanol (4 g/kg every 48 hours), chronic saline (equivalent volume every 24 hours), chronic saline/acutely challenged with ethanol (4 g/kg on day 10), non-manipulated/acutely challenged with ethanol (4 g/kg on day 10) or non-manipulated. For assessment of tolerance development, loss of righting reflex was tested on the first and last ethanol exposure days in the chronic ethanol group, with both saline and non-manipulated animals likewise challenged on the last exposure day. Withdrawal-induced anxiety was indexed in a social interaction test 24 hrs after the last ethanol exposure, with ethanol-naïve chronic saline and non-manipulated animals serving as controls. Voluntary intake was assessed 48 hours after the chronic exposure period in chronic ethanol, chronic saline and non-manipulated animals using an 8 day 2 bottle choice, limited access ethanol intake procedure.
Results
Adolescents were less sensitive to the sedative effects of ethanol than adults. Adults, but not adolescents, developed chronic tolerance to the sedative effects of ethanol, tolerance that appeared to be metabolic in nature. Social deficits were observed after chronic ethanol in both adolescents and adults. Adolescents drank significantly more ethanol than adults on a g/kg basis, with intake uninfluenced by prior ethanol exposure at both ages.
Conclusion
Adolescents and adults may differ in their ability and/or propensity to adapt to chronic ethanol exposure, with adults, but not adolescents, developing chronic metabolic tolerance. However, this chronic exposure regimen was sufficient to disrupt baseline levels of social behavior at both ages. Taken together, these results suggest that, despite the age-related differences in tolerance development, adolescents are as susceptible as adults to consequences of chronic ethanol exposure, particularly in terms of disruptions in social behavior. Whether these effects would last into adulthood remains to be determined.
Keywords: Adolescence, Chronic Ethanol Exposure, Tolerance, Social Behavior, Ethanol Intake
Introduction
Alcohol use is prevalent in human adolescents, with binge-like patterns of drinking commonly reported among this age group. According to the 2008 Monitoring the Future survey, 11% of 8th graders, 22% of 10th graders and 25% of 12th graders reported consuming 5 or more drinks per occasion in the past two weeks (Johnson et al., 2009). Elevated alcohol consumption in adolescence has also been shown in animal models, with adolescent rats typically consuming 2–3 times more ethanol than their adult counterparts (Brunell & Spear, 2005; Doremus et al., 2005; Vetter & Spear, 2007; Vetter-O’Hagen et al., 2009). The extensive neural transformations that occur during adolescence (see Spear, 2000, 2010, for review) may make adolescence a time of particular vulnerability to lasting consequences of repeated alcohol use. Indeed, initial experiments suggest that chronic ethanol exposure may have a greater impact on adolescent rats than adults, in terms of ethanol-induced brain damage (Crews et al., 2000), learning deficits (Sircar & Sircar, 2005) and memory deficits (Markwiese et al., 1998; White & Swartzwelder, 2004). Thus, investigating why adolescents have a propensity to consume more alcohol than adults and the consequences of this adolescent ethanol exposure is of particular importance.
Decreased sensitivity to various adverse effects of ethanol, such as sedation, motor-impairment, and social impairment that serve as cues to limit or terminate intake may contribute to elevated ethanol consumption during adolescence (Doremus et al., 2003; Little et al., 1996; Moy et al., 1998; Silveri & Spear, 1998, 2001; Varlinskaya & Spear, 2002; White et al., 2002). This decreased sensitivity may be linked to age-related differences in the development of acute and chronic tolerance to ethanol-induced behavioral alterations. Adolescent rats typically express greater acute tolerance than their adult counterparts to ethanol-induced motor impairment (White et al., 2002), social impairment (Varlinskaya & Spear, 2006), and sedation (Draski et al., 2001; Silveri & Spear, 1998). However, research conducted in laboratory animals that focused on age-related differences in ethanol adaptations after repeated exposure to ethanol (chronic tolerance) has yielded mixed findings, with varying reports that adolescents, but not adults, developed chronic tolerance (Swartzwelder et al., 1998), that both adolescents and adults developed chronic tolerance (Varlinskaya & Spear, 2007), or even that, adolescents did not express chronic tolerance (Matthews et al., 2008). Further study is clearly needed to examine the ontogeny of chronic tolerance development and its effects on ethanol consumption.
In addition to tolerance, withdrawal severity may be a factor in promoting ethanol use and potentially abuse. Adolescent rats have been found to be less sensitive than adults to the anxiogenic effects to acute ethanol withdrawal (i.e., “hangover”) indexed via social inhibition (Varlinskaya & Spear, 2004) and elevated plus maze behavior (Doremus et al., 2003; Doremus-Fitzwater & Spear, 2007). The few studies to date that have examined the effects of chronic ethanol exposure in adults and adolescents on withdrawal-related behaviors have produced mixed results. Some studies have found adolescent rats to be more prone to withdrawal-related anxiety after chronic intermittent ethanol-exposure than adults (Wills et al., 2008, 2009). However, a recent study examining withdrawal after chronic ethanol administration found no age-related differences when adolescent and adult rats were maintained at similar BECs (Morris et al., 2010).
The issue of adaptations to chronic ethanol exposure during adolescence is of particular importance, given that acquired tolerance to certain adverse and desired effects of ethanol could contribute to high ethanol intake during this developmental period. Additionally, a propensity to exhibit severe withdrawal symptoms upon cessation of repeated ethanol consumption may predispose certain individuals to continue to use and potentially abuse alcohol into adulthood. In line with the tolerance and withdrawal data, research assessing the influence of chronic ethanol exposure in adolescent animals on later ethanol consumption has provided mixed results, with some (Moore et al., 2010; Pascual et al., 2009; Siciliano & Smith, 2001; Strong et al., 2010), but not all (Tolliver & Samson, 1991; Vetter et al., 2007) studies reporting later increases in ethanol consumption in laboratory rodents.
The literature to date examining the effects of chronic ethanol exposure in adolescence on tolerance development, withdrawal and voluntary ethanol intake remains unclear. Procedural differences (e.g., different ethanol exposure regimens) across studies may be an important factor in the discrepant findings. The purpose of the present study is to examine all three aforementioned effects of chronic ethanol exposure using a chronic exposure regimen that is similar to reports from human adolescents of binge-like, intermittent consumption patterns. Thus, adolescents and adults were exposed to a chronic intermittent ethanol schedule (4 g/kg, i.p. every 48 hours), and tests of loss of righting reflex (LORR), social interaction and limited-access ethanol intake were used to index age-related differences in tolerance development, withdrawal-related anxiety and propensity to voluntarily consume alcohol, respectively.
Methods
Subjects
A total of 170 (108 experimental animals and 62 social partners) juvenile/adolescent and adult male Sprague-Dawley rats bred and reared in our colony at Binghamton University were used in this experiment. On the day after birth, postnatal day (P) 1, litters were culled to 8–10 pups, with 6 animals of one sex and 4 animals of the other retained whenever possible, with female offspring used in other projects. Pups were weaned on P21 and housed in same-sex littermate pairs unless otherwise noted. All animals were maintained in a temperature-controlled vivarium on a 14:10-h light: dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Animals used in this experiment were maintained and treated in accordance with guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Procedure
At the onset of the acclimation period, P21 for animals tested as juveniles and P66 for those tested as adults, each animal was re-housed with an age- and weight-matched, non-littermate partner and each pair was randomly assigned to one of the five experimental groups: chronic ethanol, chronic saline, chronic saline/acutely challenged with ethanol, non-manipulated/acutely challenged with ethanol and non-manipulated (n= 10–13 per group at each age). In order to reduce the impact of litter effects, no more than one animal from a given litter was placed into any of the 10 experimental conditions defined by the 2 (age) × 5 (group) factorial design (Holson & Pearce, 1992). Following three days of acclimation to their new housing situation, the chronic exposure period began.
Days 1–10: Chronic exposure (P24–33; P69–78)
On Day 1 of the 10-day exposure period, adolescents and adults in the chronic ethanol and both chronic saline groups were injected intraperitoneally (i.p) with saline (0.9% w/v) at an equivalent volume to that of a 20% v/v solution of 4 g/kg ethanol (2.52 % body weight). Beginning on Day 2, animals in the chronic ethanol condition were exposed to an intermittent ethanol schedule of 4 g/kg i.p. (20% solution in isotonic saline, v/v) every 48 hours, with an equivalent volume of saline administered on the days between ethanol exposures. Animals in the saline conditions continued to receive daily saline injections as on Day 1 for a total of either 10 days (chronic saline group) or 9 days (chronic saline/acutely challenged group). Animals in the chronic saline/acutely challenged and non-manipulated/acutely challenged conditions received a 4 g/kg i.p. injection of ethanol on Day 10. Injections were given between 10:00 AM and 12:00 PM, with solutions administered at room temperature.
Days 2 and/or 10: Test of ethanol-induced sedation (P25 and/or P33; P70 and/or P78)
For assessment of tolerance development to the sedative effects of ethanol, animals in the chronic ethanol condition were tested for their sensitivity to the sedative effects of ethanol on Days 2 and 10, the first and last ethanol exposure days; whereas animals in the two acutely challenged groups (chronic saline/acutely challenged and non-manipulated/acutely challenged) were examined following ethanol challenge on Day 10 only. After i.p. administration of the 4 g/kg ethanol dose on the day(s) of testing, animals were placed in a supine position in a V-shaped trough (v= 90° angle; sides of 12.5 × 19 cm for adolescents and 12.5 × 25.5 cm for adults) every 15 seconds until loss of righting reflex (LORR: inability to right on all four paws within 30 seconds) was observed. Animals were then maintained in a supine position until they were able to right themselves onto all four paws twice within 60 seconds [regain of righting reflex (RORR)]. Latency to LORR and the duration of LORR to RORR was recorded for each animal. Tail blood samples were collected for analysis of BECs immediately upon RORR.
Day 11: Social interaction test (P34; P79)
Animals chronically exposed to ethanol or saline were tested 24 hours after ethanol injection on Day 10 for withdrawal–related anxiety indexed via social inhibition during a social interaction test. Additionally, a non-manipulated group was included for assessments of effects of the chronic injection procedure per se on social behavior. The chronic saline and non-manipulated groups in the assessments from this point forward were not pre-exposed to ethanol, thus are not the same groups of animals tested for LORR.
For testing, each experimental animal was marked by a vertical line and placed individually into a social interaction chamber, a Plexiglas apparatus (30 × 20 × 20 cm for adolescents and 45 × 30 × 20 cm for adults) divided into two compartments of equal size by a clear Plexiglas partition with an aperture (7 × 5 cm for adolescents and 9 × 7 cm for adults) to allow movement between compartments. Each experimental animal remained in the social interaction chamber for 30 minutes to allow for apparatus acclimation and a sufficient amount of pre-test social deprivation to increase baseline levels of social behavior (see File, 1993). After the 30-minute pre-test period, a social partner was placed into the apparatus with the experimental animal for a 10-minute test period. Partners were weight-matched (within 10 grams) to the experimental animal and were always non-manipulated, non-socially deprived rats with no prior exposure to the apparatus or the experimental animal. A camera placed directly above the social interaction apparatus recorded each test period for later behavioral scoring by an experimenter blind to the animals’ pretest condition.
Frequencies of a number of social activities demonstrated by the test subject were scored and analyzed (Varlinskaya & Spear, 2002). Social investigation was defined as sniffing of any part of the body of the partner. Contact behavior included crawling over/under the partner and social grooming. Play fighting was scored as the sum of the frequencies of the following behaviors: pouncing or playful nape attack (experimental subject lunges at the partner with its forepaws extended outward), following and chasing (experimental animal rapidly pursues the partner), and pinning (the experimental subject stands over the exposed ventral area of the partner, pressing it against the floor). In the present study, subjects did not demonstrate serious fighting (i.e., aggressive behavior); therefore, this behavior was not scored. Social preference/avoidance was analyzed by scoring the number of crossovers (movement between compartments) the experimental animal demonstrated toward and away from the social partner. A preference/avoidance coefficient [Coefficient (%) = (crossovers to − crossovers from)/(crossovers to + crossovers from) × 100] was used to determine social motivation. Using this coefficient, positive scores reflect social preference, whereas negative numbers are an indication of social avoidance. Locomotor behavior in the social context was indexed by the total number of crossovers between the two compartments of the social interaction apparatus.
Days 12–20: Ethanol intake (P35–43; P80–88)
Beginning 24 hours after the social test, adolescents and adults in the chronic ethanol, chronic saline and non-manipulated groups were tested for limited access ethanol intake to assess the influence of chronic ethanol exposure, as well as the chronic injection procedure per se on subsequent voluntary consumption of ethanol. The intake procedure was modeled after the “supersac” paradigm developed by the Scripps Research Institute (Ji et al., 2008), but modified by the use of sucrose, rather than glucose, as a sweetener. On Day 12, animals were separated from their cage-mate by a mesh divider in their home cage and each animal of the pair was given access to a single bottle of supersac solution (3% sucrose and 0.125% saccharin w/v in tap water) for 2 hours. On Days 13–20, testing was conducted similarly, except the access period was reduced to one hour, and animals were given a two bottle choice between water and 10% EtOH in the supersac solution. Position of the two bottles was alternated daily to avoid position bias. On Days 16 and 20, tail blood samples were taken immediately following the intake test for analysis of BECs.
BEC Analysis
Tail blood samples were collected into heparinized tubes, rapidly frozen and maintained at −80 °C until analysis. Samples were assessed for BEC via headspace gas chromotography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials, which were then placed in a HP 7694E Auto Sampler that heated each vial for 8-minutes prior to extracting and injecting a 1.0 ml sample of the gas headspace into the gas chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
Data Analysis
Behavioral and BEC data were checked for outliers at each age, with scores > 2 standard deviations from the mean of each experimental condition excluded from analysis. Social interaction was the only measure that resulted in animal exclusion due to outliers, with a total of 2 adolescents and 3 adults (no more than two/experimental group) excluded prior to analysis. Each dependent measure was analyzed separately with factorial or repeated measures ANOVAs, and Tukey’s post-hoc tests were used to determine the locus of significant effects. Where appropriate, simple effects ANOVAs were used to analyze adolescent and adult data separately (e.g., body weight gain).
Results
Body Weight Gain
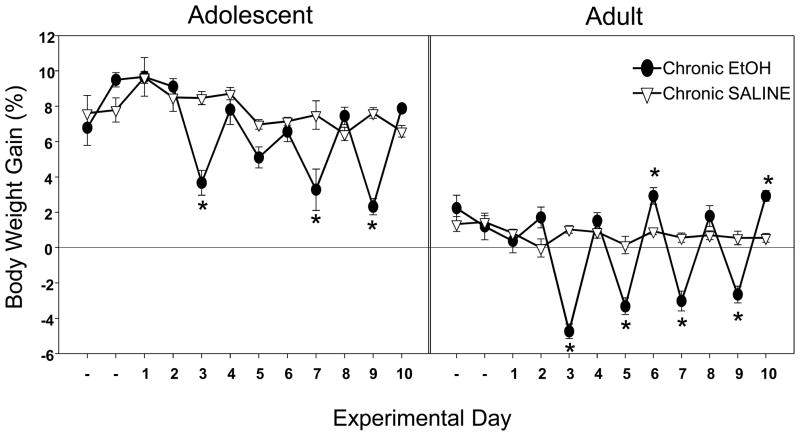
A 2 (condition: chronic ethanol, chronic saline) × 12 (day: acclimation + chronic exposure period) repeated measures ANOVA of percent body weight gain conducted separately at each age revealed main effects of condition [adolescent: F (1,18)=15.48, p< .001; adult: F (1,19)=22.72, p< .001] and day [F (11,198)=10.31, p< .001; F(11,209)=27.96, p< .001, respectively], as well as their interaction [F (11,198)=7.25, p<.001; F (11,209)=24.57, p<.001]. As shown in Figure 1, adolescents in the chronic ethanol condition gained significantly less weight than their saline-exposed counterparts during the 24-hour period following each ethanol injection on all days except Day 5. Adults in the chronic ethanol condition likewise showed significant weight loss during the 24-hour period after each ethanol injection, while gaining significantly more weight than their chronic saline-exposed counterparts on recovery Days 6 and 10 (see Figure 1).
Figure 1.
Percent body weight gain across the acclimation and chronic exposure period in adolescents (left) and adults (right) chronically exposed to saline or ethanol. Data are expressed as means ± SEM and asterisks (*) denote a significant difference (p ≤.05) between groups.
In order to assess potential recovery of weight loss following the exposure period, analyses of percent body weight gain and absolute weight gain (g) across the intake testing period were conducted separately at each age using separate 2 (condition: chronic ethanol, chronic saline) × 9 (day: intake training + 8 ethanol intake days) repeated measures ANOVAs. The analyses of percent body weight gain revealed a significant main effect of condition [adolescents: F (1,18)= 15.37, p= .001; adults: F (1,19)= 23.55, p<.001], with chronic ethanol pre-exposed animals at both ages gaining significantly more weight across the intake testing period than their saline pre-exposed age-mates (data not shown). However, despite evidence for compensatory weight gain following the ethanol exposure period in these percent body weight gain analyses, analyses of absolute body weight (g) revealed that this compensation was not sufficient to counter ethanol-induced weight deficits at either age [main effect of condition for adolescents: F (1,18)=9.39, p<.05; for adults: F (1,19)= 5.83, p<.05]. Even at the end of the intake period (i.e., 10 days after termination of the chronic injection regime), both adolescents (209.47 g ±17.77) and adults (411.59 g ± 40.92) pre-exposed to ethanol weighed significantly less than their saline pre-exposed counterparts (230.14 g ±17.75; 441.79 g ± 26.92, respectively).
Test of Ethanol-Induced Sedation
Latencies to LORR, LORR durations and BECs upon RORR were analyzed separately via both a: (a) within subjects analysis of the first and last ethanol exposures of animals in the chronic ethanol condition using a 2 (age) × 2 (day) repeated measures ANOVA and (b) between subjects analysis comparing acute and chronic ethanol exposure data on Day 10 using a 2 (age) × 3 (condition: chronic ethanol, chronic saline/acutely challenged and non-manipulated/acutely challenged) factorial ANOVA. No differences were found in latencies to LORR in either analysis.
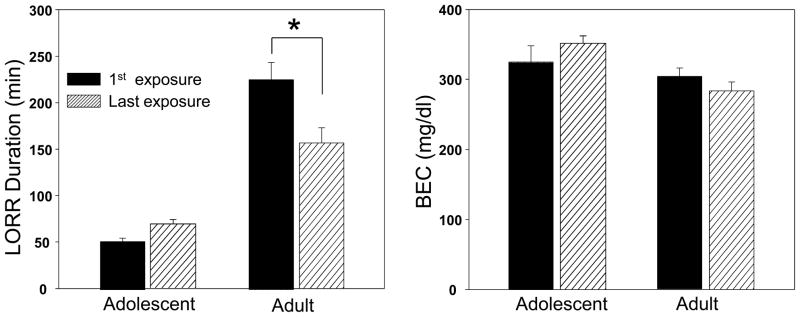
The within subjects analysis of LORR duration among the chronic ethanol-exposed animals revealed significant main effects of age [F (1,19)= 68.59, p< .001] and day [F (1,19)= 5.96, p<.05], and their interaction [F (1,19)= 18.96, p< .001]. Adolescents had significantly shorter LORR durations than adults on both assessment days. Adults displayed a significantly shorter LORR duration on the last exposure day (Day 10) than the first (Day 2), suggesting the emergence of chronic tolerance that was not seen in adolescents (see Fig. 2, left panel). Analysis of BECs at RORR (see Fig. 2, right panel) revealed only a significant main effect of age [F (1,19)= 9.33, p< .001], reflecting higher BECs in adolescents than adults upon RORR. The lack of significant differences in BECs at the time of awakening from the first to last exposure day, despite a significant reduction in duration of LORR across days among adults, likely reflects metabolic rather than functional tolerance.
Figure 2.
Loss of righting reflex (LORR) duration (left) and BECs (right) at regaining of righting reflex (RORR) on the first and last ethanol exposure days of adolescents and adults in the chronic ethanol group (within subjects). Data are expressed as means ± SEM and asterisks (*) denote a significant difference (p ≤.05) between the groups indicated.
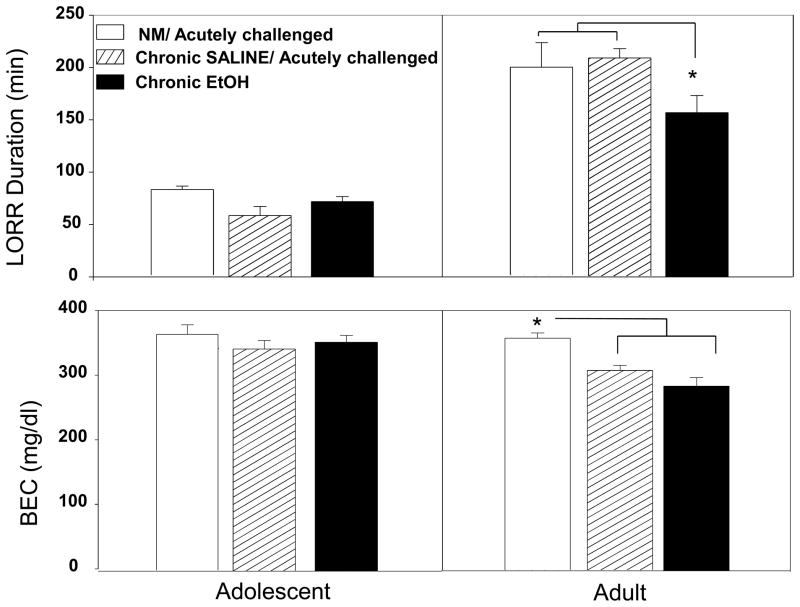
The between subjects analysis of the Day 10 LORR data revealed a significant main effect of age [F (1,61)= 137.67, p<.001] and an age × condition interaction [F (2,61)= 9.51, p<.05]. Adolescent LORR durations were significantly shorter than those of adults (Fig. 3, upper panel). Adults, but not adolescents, in the chronic ethanol group had significantly shorter LORR durations than adults exposed to ethanol for the first time on that day. Analysis of BECs at RORR revealed significant main effects of age [F (1,61)= 17.85, p<.001] and condition [F (2,61)= 7.30, p< .001] tempered by an interaction of these two factors [F (2,61)= 3.36, p< .05]. BECs at RORR were higher in non-manipulated/acutely challenged adults than in their age-mates chronically exposed to ethanol or saline, where BECs did not differ between the two chronic exposure groups (see Fig. 3, lower panel). Given the diminished duration of LORR, but similar BECs upon RORR when adults in the chronic ethanol group were compared with chronic saline controls, the results of the between-subjects analysis, like the within-subject analysis, provides further evidence that the chronic tolerance seen among adults was metabolic and not functional in nature.
Figure 3.
LORR duration (top panel) and BECs at RORR (bottom panel) on day 10 of non-manipulated (NM)/acutely challenged with ethanol, saline/acutely challenged with ethanol and chronic ethanol exposed adolescents (left) and adults (right). Data are expressed as means ± SEM and asterisks (*) denote significance (p ≤.05) between the groups indicated.
Social Interaction Test: 24 hours post-exposure
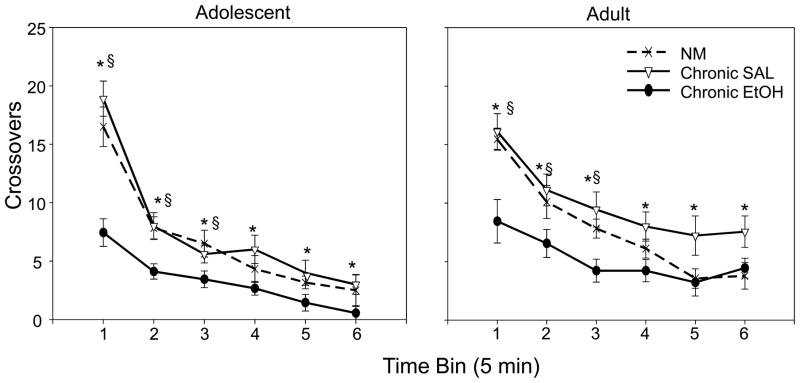
Number of crossovers between compartments in the social interaction apparatus during the 30-minute habituation period (used as an index of locomotor activity prior to the social interaction test) was scored in 5-minute bins and analyzed with a 2 (age) × 3 (condition: chronic ethanol, chronic saline and non-manipulated) × 6 (time bin) repeated measures ANOVA. Significant main effects of age [F(1,46)= 9.90; p<.01], condition [F(2,46)=16.84; p<.001] and time bin [F(5,230)=112.30; p<.001] emerged, tempered by significant interactions of time bin × condition [F(10,230)=6.12; p<.001] and time bin × age [F(5,230)=5.40; p<.001]. After the first 5-minute bin, adults displayed more locomotor activity in this context than adolescents. At both ages, animals in the chronic ethanol group showed decreased locomotor activity compared to the chronic saline group during the entire 30 minutes and compared to the non-manipulated group during the first three time bins (see Fig. 4).
Figure 4.
Locomotor activity (crosses) during the 30 minute habituation period prior to the social interaction test of non-manipulated (NM), chronic saline exposed and chronic ethanol exposed adolescents (left) and adults (right). Data are expressed as means ± SEM. Asterisks (*) denote a significant difference between chronic ethanol and chronic saline groups and (§) denote a significant difference between chronic ethanol and non-manipulated groups.
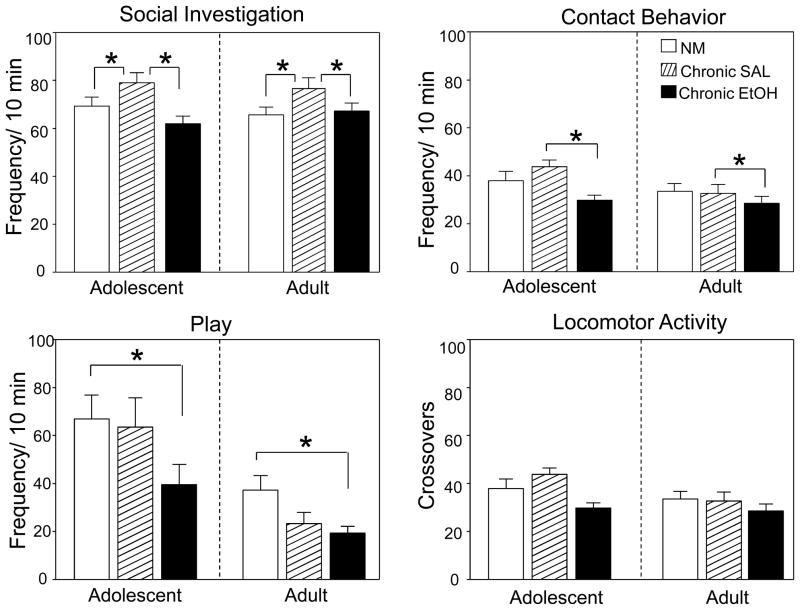
ANOVAs were conducted separately for each behavior measure (social investigation, contact, play fighting, preference/avoidance coefficient and overall locomotor activity). A 2 (age) × 3 (condition: chronic ethanol, chronic saline and non-manipulated) factorial ANOVA revealed significant main effects of age for contact and play behavior [F(1,51)= 4.60; p<.05 and F(1,51)=20.14; p<.01, respectively], with adolescents generally displaying more contact and play behaviors than adults, and significant main effects of condition for social investigation [F(2,51)=6.83, p<.01], contact [F(2,51)=4.34, p<.05], and play behavior [F(2,51)=3.87, p<.05], along with a trend for a condition effect for total crossovers [F (2,51)=2.83, p=.068], used as an index of general activity in this test. Tukey’s post-hoc tests conducted on data collapsed across age revealed that animals in the chronic ethanol condition displayed less play behavior when compared to non-manipulated animals, and less social investigation and contact behavior compared to animals in the chronic saline condition, with the latter effect seemingly driven by the adolescent animals (see Fig. 5, top panel). The only social measure in which non-manipulated and chronic saline animals differed was social investigation, with non-manipulated animals displaying significantly lower levels of this behavior compared to chronic saline exposed animals.
Figure 5.
Frequency of social investigating, social contact, play and locomotor activity (crosses) during the social interaction test of non-manipulated, chronic saline exposed and chronic ethanol exposed adolescents (left portion of each figure) and adults (right portion of each figure). Data expressed as means ± SEM and asterisks (*) denote a significant difference between groups indicated.
Ethanol Intake
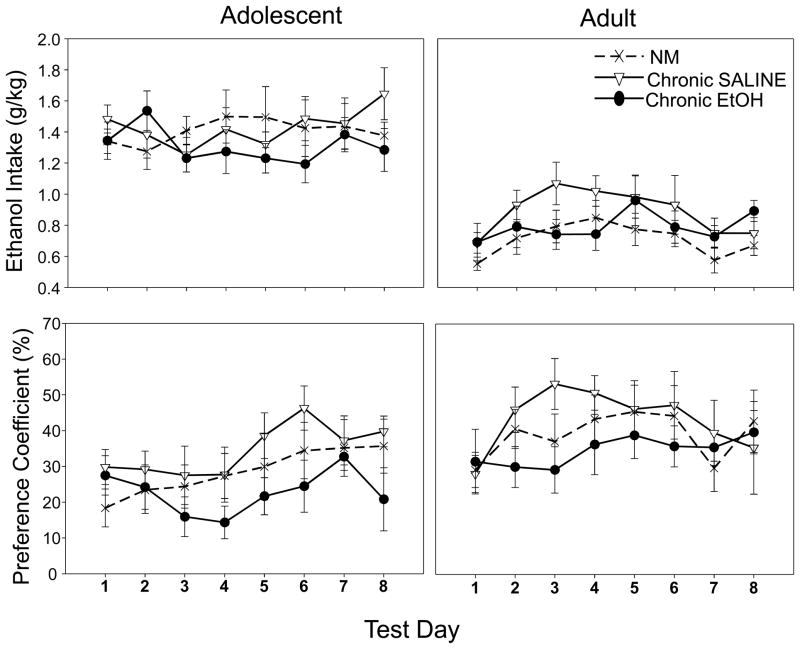
A 2 (age) × 3 (condition: chronic ethanol, chronic saline and non-manipulated) × 8 (ethanol intake days) repeated measures ANOVA of the voluntary ethanol consumption data revealed only a significant effect of age [F(1,56)=112.88, p<.001], with adolescents drinking significantly more ethanol (g/kg) than adults (see Fig. 6, top panel).
Figure 6.
Ethanol intake g/kg (top panel) and percent preference (bottom panel) across the 8 day limited access intake procedure in adolescents (left) and adults (right). Data expressed as means ± SEM.
A 2 (age) × 3 (condition: chronic ethanol, chronic saline and non-manipulated) × 8 (ethanol intake days) repeated measures ANOVA was conducted to assess percent preference for the ethanol supersac solution [calculated as: (ethanol sol (g) − water (g))/(ethanol sol (g) + water (g)) × 100, with positive values reflecting ethanol preference]. Both ages showed a preference for the ethanol supersac solution, although a main effect of age emerged [F(1,56)=10.25, p<.05], with adults showing a higher preference for the ethanol supersac solution than adolescents (see Fig. 6, bottom panel). No other significant main effects or interactions emerged in these analyses.
A 2 (age) × 3 (condition: chronic ethanol, chronic saline and non-manipulated) × 2 (day: 4, 8) repeated measures ANOVA of BECs after the 1-hour intake sessions revealed a significant day × age interaction [F(1,56)=4.88, p<.05], with adult Day 4 BECs (20.55 ± 4.52) higher than adolescent BECs on Day 4 (8.16 ± 3.01) and higher than BECs at both ages on Day 8 (adolescent: 10.30 ±2.93; adult: 9.65 ± 2.20). No other significant effects emerged.
Discussion
Even at ethanol doses sufficient to induce hypnosis throughout the exposure period and disrupt normal body weight gain, sensitivity to the sedative effects of ethanol was unaltered across the 10 day exposure period in adolescent animals. In contrast to the lack of tolerance expression among adolescents, chronically exposed adults exhibited metabolic tolerance—an attenuated response to ethanol challenge associated with more rapid ethanol metabolism/elimination as indexed by shorter LORR durations, but with equivalent BECs at recovery. Though age-related differences emerged in the ability to counteract the sedative effects of ethanol, both adolescents and adults displayed social deficits when tested 24 hours after the chronic exposure period. Despite evidence of metabolic tolerance in adults and deficits in social behavior in both adolescents and adults, this chronic ethanol regimen did not subsequently influence voluntary ethanol intake.
Adolescent animals showed shorter durations of LORR and higher BECs upon RORR than adults on the first and last ethanol exposure days, regardless of chronic exposure condition. These results are consistent with previous findings that adolescents are less sensitive to the sedative effects of ethanol than adults (Draski et al., 2001; Linsenbardt et al., 2009; Little et al., 1996; Silveri & Spear, 1998; Swartzwelder et al., 1998), and extend them to demonstrate that these age differences are retained across repeated ethanol exposures.
The lack of tolerance expression after chronic ethanol exposure among adolescents in the present study is reminiscent of that reported previously, with adolescent rats not showing chronic tolerance either to the sedative effects of ethanol (indexed via LORR) during 20 days of exposure to 4 g/kg ethanol every 48 hours administered i.p. (Matthews et al., 2008), or to ethanol-induced hypothermia after 7 days of moderate chronic ethanol vapor exposure (Ristuccia & Spear, 2005). Similarly, Linsenbardt et al. (2009) reported chronic tolerance development in adult, but not adolescent mice following 4 days of i.p. exposure to 4 g/kg ethanol, with this tolerance being metabolic in nature. Reports of metabolic tolerance development are a fairly consistent finding of studies examining chronic tolerance in adult rodents (Cao et al., 1995; Linsenbardt et al., 2009; Tampier et al., 1991; Varlinskaya & Spear, 2007; York & Chan, 1994). In other studies, however, adolescents were found to develop tolerance to ethanol-induced sedation after chronic exposure to 4 g/kg administered intragastrically (i.g.) twice daily for 7 days (Swartzwelder et al., 1998) or 5 g/kg i.p. every 48 hours for 20 days (Silvers et al., 2003). Taken together, the results of these studies suggest that expression of chronic tolerance to the sedative effects of ethanol in adolescent animals may require repeated exposure to high doses of ethanol for a relatively long period of time-- an exposure pattern more pronounced than that necessary to induce chronic tolerance expression in adults (at least via induction of metabolic tolerance). Given the relative insensitivity of adolescents to the sedative effects of ethanol (Silveri & Spear, 1998 Silveri & Spear, 2004; Swatrzwelder et al., 1998), it is perhaps not surprising that adolescent animals may require higher doses (and possibly longer ethanol exposures) than adults to induce sufficient perturbations to precipitate adaptations such as the development of tolerance (see Silveri & Spear, 2001). However, it is not simply the case that tolerance development in adolescents requires relatively high ethanol exposure levels, given that both adolescents and adults were found to develop chronic tolerance to ethanol-induced social impairment after 7 days of 1 g/kg i.p. ethanol exposure (Varlinskaya & Spear, 2007).
In addition to age-related differences in tolerance development, the results of this experiment also suggest that adolescents and adults may differ in consequences of the chronic injection/handling process, an effect that has been previously observed in our laboratory (see Ristuccia et al., 2007). In comparison to previously non-manipulated adults, chronic saline exposed adults regained the righting reflex at lower BECs despite similar LORR durations. These data suggest that the chronic injection/handling process itself may increase ethanol metabolism and possibly, sensitivity to ethanol-induced sedation in adults. Adolescents, however, were not influenced in this manner by chronic injection/handling. Alterations in ethanol sensitivity in adults, but not adolescents, due to handling/injection have been previously reported in our laboratory, although the observed changes in ethanol sensitivity were opposite in nature, with attenuated ethanol-induced hypothermia (Ristuccia et al., 2007).
Both ages showed evidence of decreased locomotor activity as a result of the repeated ethanol exposure. This effect emerged during the habituation period in the social interaction apparatus, where ethanol exposed animals of both ages were less active than their chronic saline-exposed and non-manipulated counterparts. These findings are consistent with prior reports of withdrawal-related hypoactivity, which is commonly observed (e.g., Ristuccia and Spear, 2005; Slawecki and Roth, 2004; Zhao et al., 2007). Interestingly, this apparent withdrawal-related hypoactivity was diminished upon introduction of a social partner (despite the presence of social deficits in these animals).
Whereas the suppression in locomotor activity after chronic ethanol exposure was less evident in a social setting, such exposure did attenuate social behaviors (investigation, contact, and play) at both ages. Previous research from our laboratory assessing acute withdrawal (i.e., hangover) found decreased play and contact behavior in adult, but not adolescent males, with adolescents actually showing an increase in play behavior during the withdrawal (“hangover”) phase following acute ethanol challenge with 4 g/kg i.p. (Varlinskaya & Spear, 2004), whereas the current study found that both adolescents and adults showed an attenuation of contact and play behaviors after chronic ethanol exposure. Whether these effects would recover with time remains to be determined.
The observed decreases in social activity at both ages could potentially be related to withdrawal-induced increases in anxiety, given that social behavior is extremely sensitive to different anxiogenic manipulations (Doremus-Fitzwater et al., 2009; File & Seth, 2003). This suggestion is weakened, however, by prior research findings that only two social measures--social preference and social investigation-- are consistently influenced by anxiogenic and/or anxiolytic manipulations, whereas play, and locomotor activity have not been found to be as sensitive to these manipulations (Doremus-Fitzwater et al., 2009; Varlinskaya & Spear, 2002, 2006). Given that chronic exposure to ethanol in the present study resulted in widespread decrease in several measures of social behavior, while social preference (indexed by the preference/avoidance coefficient) was unaltered, these effects appear more likely to reflect ethanol-induced social deficits rather than withdrawal-induced anxiety per se. Moreover, although social investigation was decreased in animals chronically exposed to ethanol when compared with those in the chronic saline condition, non-manipulated animals also showed less social investigation than chronic saline-exposed animals. Decreased social investigation in non-manipulated animals could possibly be driven by anxiogenic effects of experimenter handling prior to testing in these previously non-manipulated animals, whereas chronic saline exposed animals were handled daily and perhaps had habituated to some extent to this type of manipulation. This explanation, however, again raises the possibility that the decreased investigation observed in chronic ethanol exposed animals might be driven by withdrawal-related anxiety, but without a likewise decrease in social preference this argument is substantially weakened. Further investigation of stress/handling effects on social behavior would help in interpreting these effects.
As in our previous studies (Brunell & Spear, 2005; Doremus et al, 2005; Vetter & Spear, 2007), ethanol intake differed as a function of age, with adolescent animals drinking more ethanol (g/kg) than their adult counterparts. Yet, intake was not influenced at either age by prior chronic ethanol exposure. These results suggest that under the circumstances of the present study, chronic ethanol exposure does not increase subsequent ethanol consumption in either adolescents or adults. Taken together with previous studies (Gurkovskaya et al., 2009; Tolliver & Samson, 1991; Vetter et al., 2007), these data are consistent with the conclusion that mere exposure to ethanol during adolescence may not be sufficient to increase subsequent ethanol intake. However, a number of other studies have reported increases in voluntary ethanol consumption in adulthood after chronic ethanol exposure during adolescence (Pascual et al., 2009; Siciliano & Smith, 2001; Walker & Ehlers, 2009; Yoshimoto, 2002; Yoshimoto, 1988). Critical variables may include age of ethanol initiation, as well as duration, amount and type of exposure (forced vs. voluntary), as well as ethanol intake paradigms utilized in adulthood. Age of subsequent intake testing may also be important, with few studies assessing adolescent exposure effects with intake tests beginning during the adolescent period as in the present study. Further studies are needed to explain consequences of various types of adolescent exposures on subsequent ethanol intakes later in adolescence and in adulthood.
The finding that BECs on Day 4 of the intake were higher in adults than in adolescents is peculiar, given that adolescents drank significantly more ethanol on a g/kg basis than did adults. Given that the Day 8 data did not replicate this finding, it is possible that the age effect seen on Day 4 may have been spurious. Alternatively, there may be age-related differences in pharmacokinetics and/or drinking patterns across the 1-hour drinking period that could contribute to these differences. In voluntary, limited access intake tests, animals often consume much of their intake during the first 15–20 min of the session (see Bell et al., 2006), raising the possibility that age-related differences in the patterning of intake may make it difficult to directly relate intake levels to BECs at the end of the 1-hour drinking period. Careful delineation of drinking patterns and blood ethanol levels across the intake period may help clarify potential age-related differences in the relationship between these variables.
Taken together, these results suggest that, despite the age-related differences in tolerance development, adolescents are as susceptible as adults to consequences of chronic ethanol exposure, particularly in regards to social deficits evident during the immediate withdrawal period. Given that binge level ethanol consumption is pervasive during adolescence in humans, it will be important to assess potential lasting impacts of repeated adolescent perturbations with ethanol on behavior and neurobiological function in adulthood.
Acknowledgments
The research presented in this paper was supported by NIAAA grants R01AA018026, R37AA012525 (to LPS) and R01AA012453 (to EIV).
References
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biology. 2006;11(3–4):270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 2005;29(9):1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Cao W, Wehner JM, Collins AC. Chronic intragastric infusion produces tolerance to ethanol in LS and SS mice. Alcohol (Fayetteville, NY) 1995;12(3):241–246. doi: 10.1016/0741-8329(94)00101-i. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, RCS, Knapp DJ. Binge Ethanol Consumption Causes Differential Brain Damage in Young Adolescent Rats Compared With Adult Rats. Alcoholism: Clinical and Experimental Research. 2000;24(11):1712–1723. [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism, Clinical And Experimental Research. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2003;75(2):411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Age-related differences in anxiolytic actions of ethanol and diazepam in adolescent and adult male rats 2006 [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcoholism, Clinical And Experimental Research. 2007;31(9):1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & Behavior. 2009;97(3–4):484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacology, Biochemistry, And Behavior. 2001;70(2–3):387–396. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. European Journal Of Pharmacology. 2003;463(1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Gurkovskaya OV, Leonard ST, Lewis PB, Winsauer PJ. Effects of pregnanolone and dehydroepiandrosterone on ethanol intake in rats administered ethanol or saline during adolescence. Alcoholism, Clinical And Experimental Research. 2009;33(7):1252–1264. doi: 10.1111/j.1530-0277.2009.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural Pharmacology. 2008;19(1):1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcoholism, Clinical And Experimental Research. 2009;33(3):464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcoholism, Clinical And Experimental Research. 1996;20(8):1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JA. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol. 2008;42(8):617–621. doi: 10.1016/j.alcohol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL. Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcoholism, Clinical And Experimental Research. 2010;34(4):734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2010;44(1):89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcoholism, Clinical And Experimental Research. 1998;22(7):1485–1492. [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal Of Neurochemistry. 2009;108(4):920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Hernandez M, Wilmouth CE, Spear LP. Differential expression of ethanol-induced hypothermia in adolescent and adult rats induced by pretest familiarization to the handling/injection procedure. Alcoholism, Clinical And Experimental Research. 2007;31(4):575–581. doi: 10.1111/j.1530-0277.2007.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcoholism, Clinical And Experimental Research. 2005;29(10):1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiology & Behavior. 2001;74(4–5):637–643. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism-Clinical and Experimental Research. 1998;22(3):670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: Observations when equating ethanol perturbation across age. Alcoholism-Clinical and Experimental Research. 2001;25(9):1301–1308. [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcoholism, Clinical And Experimental Research. 2003;27(10):1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent Rats Exposed to Repeated Ethanol Treatment Show Lingering Behavioral Impairments. Alcoholism: Clinical and Experimental Research. 2005;29(8):1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcoholism, Clinical And Experimental Research. 2004;28(4):598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Spear L. The Behavioral Neuroscience of Adolescence. 1. W.W. Norton & Company Inc; New York, NY: 2010. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Hormones And Behavior. 2010;58(1):82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol (Fayetteville, NY) 1998;15(4):311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Tampier L, Quintanilla ME. Effect of 3-amino-1,2,4-triazole on the hypothermic effect of ethanol and on ethanol tolerance development. Alcohol (Fayetteville, NY) 1991;8(4):279–282. doi: 10.1016/0741-8329(91)90369-8. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacology, Biochemistry, And Behavior. 1991;38(3):575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism-Clinical and Experimental Research. 2004;28(1):40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicology and Teratology. 2007;29(1):23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time Course of Elevated Ethanol Intake in Adolescent Relative to Adult Rats Under Continuous, Voluntary-Access Conditions. Alcoholism: Clinical and Experimental Research. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol And Alcoholism (Oxford, Oxfordshire) 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behavioral Neuroscience. 2009;123(4):926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcoholism, Clinical And Experimental Research. 2002;26(7):960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2002;73(3):673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Differential dietary ethanol intake and blood ethanol levels in adolescent and adult rats: effects on anxiety-like behavior and seizure thresholds. Alcoholism, Clinical And Experimental Research. 2008;32(8):1350–1360. doi: 10.1111/j.1530-0277.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcoholism, Clinical And Experimental Research. 2009;33(3):455–463. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL, Chan AW. Age effects on chronic tolerance to ethanol hypnosis and hypothermia. Pharmacology, Biochemistry, And Behavior. 1994;49(2):371–376. doi: 10.1016/0091-3057(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Hori M, Sorimachi Y, Watanabe T, Yano T, Yasuhara M. Increase of Rat Alcohol Drinking Behavior Depends on the Age of Drinking Onset. Alcoholism: Clinical and Experimental Research. 2002;26(s1):63s–65s. doi: 10.1097/01.ALC.0000026977.19902.61. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism, Clinical And Experimental Research. 2007;31(9):1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]