Abstract
Bioactive components from bitter melon (BM) have been reported to improve glucose metabolism in vivo, but definitive studies on efficacy and mechanism of action are lacking. We sought to investigate the effects of BM bioactives on body weight, muscle lipid content and insulin signaling in mice fed a high fat diet and on insulin signaling in L6 myotubes. Male C57BL/6J mice were randomly divided into low-fat diet control (LFD), high-fat diet (HFD) and HFD plus BM (BM) groups. Body weight, body composition, plasma glucose, leptin, insulin, and muscle lipid profile were determined over 12 weeks. Insulin signaling was determined in the mouse muscle taken at end of study and in L6 myotubes exposed to the extract. Body weight, plasma glucose, insulin, leptin levels, and HOMA-IR values were significantly lower in the BM fed HFD group when compared to the HFD group. BM supplementation significantly increased IRS-2, IR β, PI 3K and GLUT4 protein abundance in skeletal muscle as well as phosphorylation of IRS-1, Akt1 and Akt2 when compared with HFD (P<0.05 and P<0.01). BM also significantly reduced muscle lipid content in the HFD mice. BM extract greatly increased glucose uptake and enhanced insulin signaling in L6 myotubes. This study shows that bitter melon bioactives reduced body weight, improved glucose metabolism and enhanced skeletal muscle insulin signaling. A contributing mechanism to the enhanced insulin signaling may associate with the reduction in skeletal muscle lipid content. Nutritional supplementation with this extract, if validated for human studies, may offer an adjunctive therapy for diabetes.
Keywords: Bitter melon extract, acyl carnitine, IRS-1, PI 3Kinase, High fat diet
Introduction
Type 2 diabetes, a leading cause of death in the United States, has reached epidemic proportions in the US and worldwide (>18 million and 160 million individuals, respectively), and is projected to increase dramatically (1). Furthermore, the prevalence of insulin resistance, a major pathophysiologic parameter contributing to development of type 2 diabetes and an independent risk factor for cardiovascular disease and the metabolic syndrome, is even more widespread (2, 3). Thus, the prevention and treatment of diabetes and obesity present major challenges to the health care system over the coming decades.
As an alternative therapy for diabetes, bitter melon (BM), Momordica charantia, has been used historically in Asia, Africa, and Latin America, because of its reported hypoglycemic activity (4). BM has been proposed to contain bioactive components having anti-diabetic properties such as charantin, vicine, and polypeptide-p, as well as other unspecific bioactive components including antioxidants. Metabolic and hypoglycemic effects of bitter melon extracts have been demonstrated in cell culture, animal, and human studies (5, 6). Recently, a study observed that bitter melon extract was effective in ameliorating fructose diet-induced hyperglycemia, hyperleptinemia, hyperinsulinemia and hypertriglyceridemia as well as decreasing the levels of free fatty acid (FFA) in pre-clinical studies. The mechanism was proposed to be secondary to significantly increasing the expression of peroxisome proliferator-activated receptor gamma (PPAR γ) in white adipose tissue (WAT), decreasing the expression of leptin in WAT and increasing the mRNA expression and protein of glucose transporter 4 (GLUT4) in skeletal muscle (7). BM extract was able to act on a natural PPARγ signaling pathway in murine hepatoma cell line (8) and increased both glucose uptake and amino acid uptake in L6 cells (9). A recent study reported BM extract improved insulin sensitivity by increasing skeletal muscle insulin-stimulated IRS-1 tyrosine phosphorylation in rats fed a high fat diet (10) and increased hepatic IRS and phosphonositide-3 kinase interactions (11). However, precise mechanisms contributing to the in vivo actions of BM extract on carbohydrate metabolism, whether via regulation of insulin release or improvement in insulin signaling remains unknown. Clearly, in depth studies that combine the clinical effect with mechanistic studies of bitter melon for treating diabetes are needed.
Insulin is the major hormone controlling critical energy functions such as glucose and lipid metabolism. Insulin activates the insulin receptor tyrosine kinase IR, which phosphorylates and recruits different substrate adaptors including the IRS family of proteins. Tyrosine phosphorylated IRS then displays binding sites for numerous signaling partners. Among them, PI3K has a major role in insulin function mainly via the activation of Akt/PKB. Insulin stimulates glucose uptake in muscle and adipocytes via translocation of GLUT4 vesicles to the plasma membrane (12). In addition, lipotoxicity (increased tissue fat content) has been implicated in the development of muscle insulin resistance and type 2 diabetes mellitus (T2DM) (13). It was revealed that reduced adiposity in BM fed rats is associated with increased lipid oxidative enzyme activities and uncoupling protein expression (UCP) (14).
Thus, based on the proposed in vivo effects of bitter melon, we hypothesize that bitter melon mediates an anti-diabetic effect and improves glucose metabolism by enhancing insulin sensitivity and altering lipid content in muscle tissues. To test this hypothesis, we conducted a comprehensive study to evaluate mechanisms operative in vivo and in vitro.
2. Materials and Methods
2. 1. Materials
BM extract containing the proposed bioactives (aquatic extracted powder) was purchased from IdeaSphere Inc (American Fork, UT) and was standardized to contain > 39 % momordicosides A, F2, K, L. HPLC/LC-MS analysis was used to confirm the presence of molecular masses corresponding to each of the momordicosides as well as UV and MS chromatograms that were consistent with another commercially obtained extract of bitter melon. A high-fat diet containing 58% of energy from fat (D-12331) and a low-fat diet containing 10% Kcal from fat (D12250B) were purchased from Research Diets Inc (New Brunswick, NJ). [1,2-3H(N)] 2-Deoxy-D-glucose (2DG) and [r32P]ATP were purchased from PerkinElmer Life Science (Boston, MA). All other reagents were from Sigma-Aldrich (St. Louis, MO).
2. 2. Animals
All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the Pennington Biomedical Research Center. Twenty male C57BL/6J mice at age 5-week were ordered from Charles River Laboratories, Inc. (Wilmington, MA) and were randomly divided into low-fat diet control (LFD as negative control, n=6), high-fat diet group (HFD as positive control, n=7), and a bitter melon extract treated HFD group (BM, n= 7). BM was administrated through food intake by incorporating BM extract into the high-fat diet at 1.2 % W/W, based on a previous study (14). Food intake (the difference of weight administered to the treatment groups and leftover plus spillage) and body weight were recorded weekly. Intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test (IIGTT) were performed at week 10 and week 11, respectively. Fasting (4 hr) plasma glucose, insulin and body composition were determined at week 0, 6 and 12 of the experimental period, respectively. Metabolic chamber experiments to assess energy expenditure were performed at week 8. At the end of the study, each group was divided into basal and insulin stimulated subgroups that were matched by body weight. The vastus lateralis muscle was collected at basal condition (n=3, fasting overnight, ~12 hr) and insulin stimulated condition (n=3 – 4, at 10 min post intraperitoneal injection insulin at dose of 2U/kg body weight). Plasma, skeletal muscle, and other tissues were also collected and quickly frozen in liquid nitrogen and stored at −80°C for later analysis.
3. Blood chemistry and hormone analysis
After 4 hours of fasting, blood samples were collected from the orbital sinus in unconscious mice after a few seconds of CO2 inhalation. Plasma glucose levels were measured by a colorimetric hexokinase glucose assay (Sigma Diagnostics, St Louis, MO). Plasma insulin level was determined by ultrasensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit from Crystal Chem (Downers Grove, IL). Homeostasis model assessment for determining insulin resistance (HOMA-IR) was calculated using the following formula: HOMA = [I0 (μU/ml) × G0 (mmol/liter)]/22.5 (15). Plasma leptin was measured as described in our previous study (16).
2. 4. Body composition measurement
Body composition for all animals was measured by nuclear magnetic resonance (NMR-Bruker, Newark, DE) (16). Total fat mass (FM) and free fat mass (FFM) were recorded.
2. 5. Metabolic chamber (Energy Expenditure) study
At week 8, the mice were placed into Oxymax System metabolic chambers (Columbus Instruments International Corporation, Columbus, OH) with ad libitum access to water and the indicated diet for seven days. Their locomotion, food and water intake, O2 consumption and CO2 emission were automatically monitored. The Respiratory Exchange Rate (RER, also known as RQ) was calculated as VCO2/VO2.
2. 6. Intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test (IPITT)
IPGTT and IPITT were performed at week 10 and week 11, respectively as described in a previous study (16). Briefly, after overnight fasting, IPGTT was performed by IP injection of 2 g glucose/kg body weight using 20% glucose in 0.9% NaCL or after 4 hrs fasting, IPITT was conducted by IP insulin injection of 0.75 U/kg body weight. Blood glucose concentrations were measured from nicked tail vein at time 0 (baseline), 15, 30, 60, and 120 minutes after glucose or insulin injections using the Freestyle blood glucose monitoring system (Thera Sense, Phoenix, AZ).
2. 7. Western blot analysis
Muscle tissue lysates were prepared by dissection and homogenized in buffer A (25 mM HEPES, pH 7.4, 1% Nonidet P-40 (NP-40), 137 mM NaCl, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml pepstatin, 5 μg/ml leupeptin) using a PRO 200 homogenizer (PRO scientific, Oxford, CT). The samples were centrifuged at 14,000 g for 20 minutes at 4°C and protein concentrations of the supernatant were determined by Bio-Rad protein assay kit (Bio-Rad laboratories, Inc. Hercules, CA). Supernatants (50 μg) were resolved by SDS-PAGE and subjected to immunoblotting. The protein abundance were detected with antibodies against IRS-1, IRS-2, anti-phospho-IRS-1(Tyr612), p85 of PI 3K, Akt1, Akt2, phosph-Akt1 (ser473) (Upstate, Lake Placid, NY) Phosph-Akt2(ser474) (GenScript Co, Piscataway, NJ), and β-actin using chemiluminescence reagent plus from PerkinElmer Life Science (Boston, MA), and quantified via densitometer. All the proteins were normalized by β-actin and specific protein phosphorylation was normalized by the corresponding protein shown in the legends.
2. 8. PI 3 kinase activity assay
IRS-1 associated PI 3 kinase activities of the muscle at baseline (0 min) and 10 min post insulin stimulation (2U/kg body weight via IP) were assessed as previously described (17). Briefly, 500 μg of muscle lysates was immunoprecipitated with 3 μg of IRS-1 antibody and protein A agarose. IRS immune complexes were incubated (10 min, 22 C) in 50 μl of 20 mM Tris/HCl (pH 7.0) buffer containing 50 μM [γ-32P]ATP (5 μCi, Perkin Elmer, Boston, MA), 10 mM MgCl2, 2 mM MnCl2, 100 mM NaCl, 2 mM EDTA, and 10 μg of phosphatidylinositol (PI). After thin layer chromatography (TLC), radiolabeled phosphatidylinositol 3-phosphate (PI-3P) was visualized by autoradiography and quantitated by a densitometer, BioRad Inc (Hercules, CA).
2. 9. Quantitation of muscle acyl carnitines
At 12 weeks, vastus lateralis muscle was collected from the mice, snap-frozen and muscle extracts prepared for analysis of fatty acyl carnitine by mass spectrometry at the School of Veterinary Medicine of Louisiana State University as previously described (18).
2. 10. Cell culture and BM extract treatment
L6 muscle cells were cultured to myotubes as described by Hajduch et al (19). L6 cells were grown in αMEM containing 2% (v/v) FBS and 1% (v/v) antibiotic/antimycotic solution (100 units/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B) at 37 °C with 5% CO2/95% oxygen. Fully differentiated myotubes were serum-starved for 18 h in Dulbecco’s modified Eagle’s medium containing 0.2% bovine serum albumin prior to experiments.
2. 11. 2-Deoxy-Glucose (2DG) uptake in muscle cells
L6 myotubes in 24 well-plates were treated with or without various doses of BM extract (1–10 μg/ml) overnight. After washing with PBS, cells were exposed to 0 or 100 nM insulin in Krebs-Ringer HEPES (KRH) buffer (50 mM HEPES, pH 7.4, 137 mM NaCl, 4.8 mM KCl, 1.85 mM CaCl2 and 1.3 mM MgSO4) for 15 min followed by an additional incubation period of 5 min with 2DG (100 μM, 0.5 μCi). The cells were washed four times with ice-cold KRH buffer, and lysed by adding 250 μL of 0.05 N NaOH, then transferred to vials with scintillation cocktail. The radioactivity in the cells was measured by liquid scintillation counter. Non-specific uptake was measured using the cells pretreated with 20 μM of cytochalasin B.
2. 12. Statistical analysis
All data were expressed as mean ± SEM. Comparisons of groups were done by t test (2-sided) or ANOVA for experiments with more than two subgroups. P value <0.05 was considered significant.
3. Results
3. 1. Effect of BM extract on body weight, food intake, energy expenditure and body composition in mice
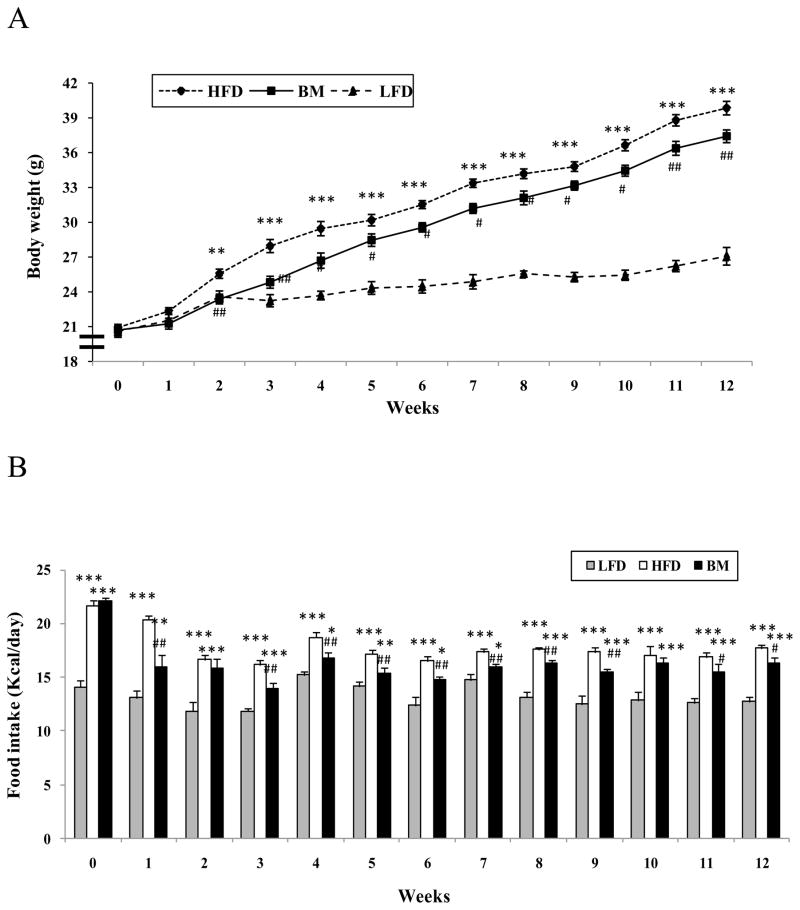
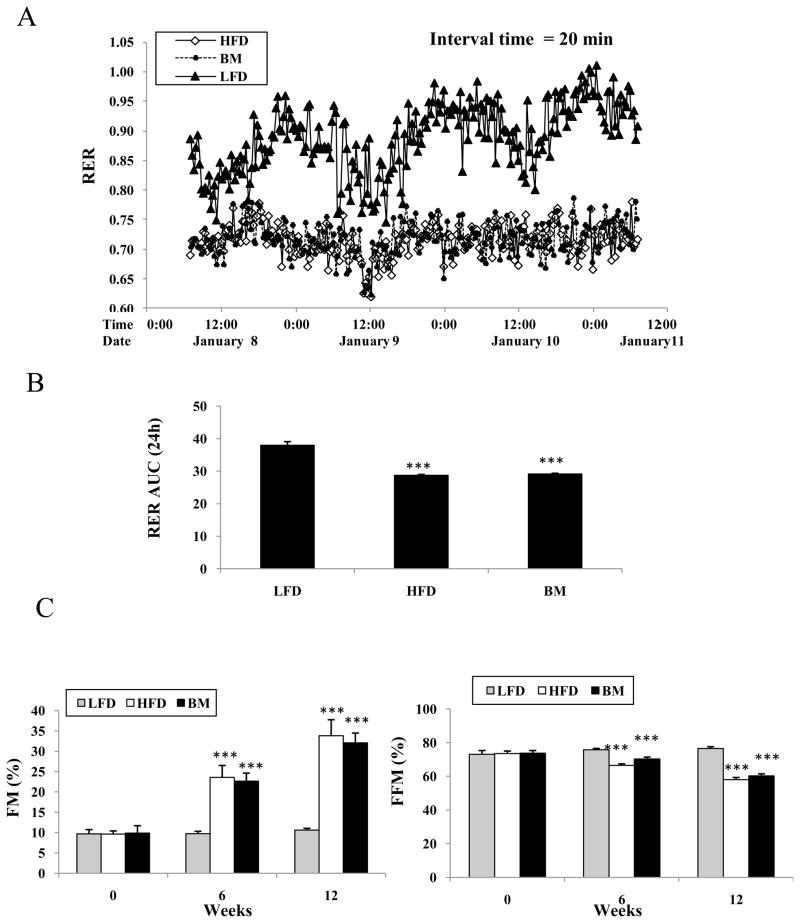
At baseline (week 0), the body weights of the three groups of animals were 20.8 ± 0.98 g, 21.3 ± 1.02 g and 21.2 + 0.87 g, then increased at week 12 to 27.1 ± 1.1 g, 39.9 ±0.7 g, and 37.4 ± 0.4 g in LFD, HFD, and BM groups, respectively (LFD vs HFD, P<0.01 and BM vs HFD, P<0.05, Fig. 1A). The food intake (normalized by body weight) was significantly higher in both HFD and BM groups than in LFD control mice in the first three weeks, but was significantly lower in the BM group than in the HFD group beginning at the first week on diets (Fig. 1B, P<0.01 and P<0.05). After 4 week feeding, there was no significant difference among all three groups. Analysis of metabolic parameters demonstrated that the RER rate was significantly lower in the HFD and the BM groups than in the LFD control group, but there was no difference between the BM and the HFD groups (Fig. 2A and 2B). The body composition results demonstrated that the HFD feeding resulted in a significantly increased fat mass (FM) accumulation (increase about 2-fold at week 6 and 3-fold at week 12 in comparison with their baseline). The FM was slightly increased with time in the LFD mice. However, free fat mass (FFM) was significantly lower in both the HFD and BM groups than LFD group (P<0.001, Fig. 2C). FFM was slightly higher in the HFD group than in the BM group, but was not felt to be significantly different.
Figure 1.
Effects of BM extract on body weight and food intake in mice. Body weight and food intake were recorded weekly. Panel A shows body weight and panel B illustrates food intake in
All three groups of mice. Data are presented as Mean ± SEM (n=6–7/group), * P<0.05, ** P<0.01 and ***P<0.001, HFD or BM group vs LFD control. #P<0.05, ##P<0.01 BM vs HFD.
Figure 2.
Energy expenditure and body composition in mice groups. At week 8, mice were placed into metabolic chambers with water and food for seven days. Animals’ locomotion, O2 consumption and CO2 emission were automatically monitored. RER (Respiratory Exchange Rate) was calculated by VCO2/VO2 values and showed in panel A and B. Penal C shows body composition measured at week 0, 6 and 12. Fat mass (FM) and free fat Mass (FFM) results were presented as percent of body weight in the three group animals. Mean ± SEM (n=6–7/group), * P<0.05, ** P<0.01 and ***P<0.001, HFD or BM vs LFD control. #P<0.05, ##P<0.01 BM vs HFD.
3. 2. BM extract improves glucose metabolism and decreases leptin concentration in high-fat diet fed mice
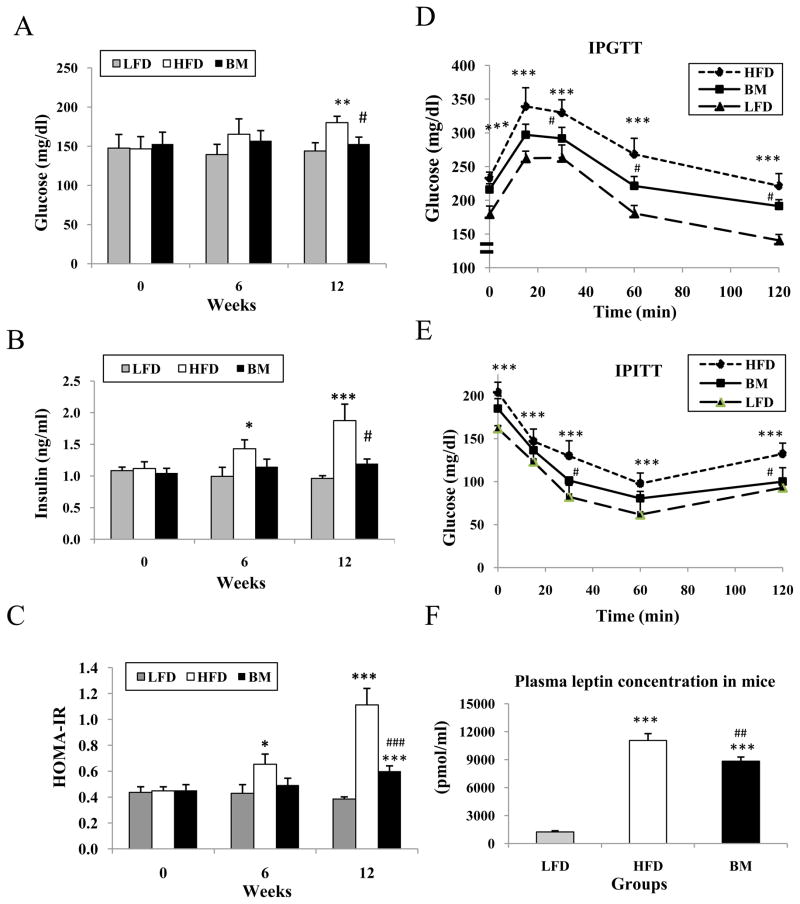
Fasting plasma glucose and insulin levels as well as HOMA-IR values were significantly increased in the HFD group (Fig. 3A–C). Animals randomized to the HFD and supplemented with BM (BM group) were observed to have significantly lower glucose, insulin and HOMA-IR compared to HFD. Furthermore, BM improved glucose tolerance measured by IPGTT (Fig. 3D). Specifically, total areas under the glucose curve (GluAUC) were calculated by the trapezoidal rule with IPGTT data and showed GluAUC was 866 ± 32, 1181± 56 and 998 ± 49 mg/dl in LFD, HFD and BM groups, respectively. The GluAUC was significantly higher in HFD and BM groups than in the LFD group (P<0.001 and P<0.01), but was significantly lower in the BM group when compared to the HFD group (P<0.01). IPITT data revealed that glucose disposal was the highest in the LFD group among all groups after injection of insulin, and BM extract treatment significantly improved insulin sensitivity when compared with the HFD group (Fig. 3E). At 12 weeks, the plasma leptin concentrations in the HFD and BM groups were significantly higher than in the LFD group (Mean ± SEM was 8.93 ± 0.6 fold and 7.12 ± 0.36 fold of control, respectively, P<0.001), but leptin concentration was approximately 20% lower in the BM group than in the HFD group(P<0.01, Fig. 3F).
Figure 3.
Glucose, insulin and leptin concentration, and IPGTT and IPITT in mice. Four hour fasting serum was collected at week 0, 6 and 12 respectively for measuring glucose and insulin concentration. Panel A is glucose levels, panel B shows insulin concentration and Panel C is HOMA results in the mice. IPGTT was carried out at week 10 after overnight fasting and IPITT was performed at week 11 after 4 hour fasting respectively. These data were showed in penal D and penal E. Panel F illustrated plasma leptin concentration of week 12 in the mice. Mean ± SEM (n=6–7/group), * P<0.05 and *** P<0.001 respectively LFD vs HFD. # P<0.05 and ## P<0.001, BM vs HFD.
3. 3. Effect of HFD and BM extract on fatty acyl carnitine content in mice muscle tissues
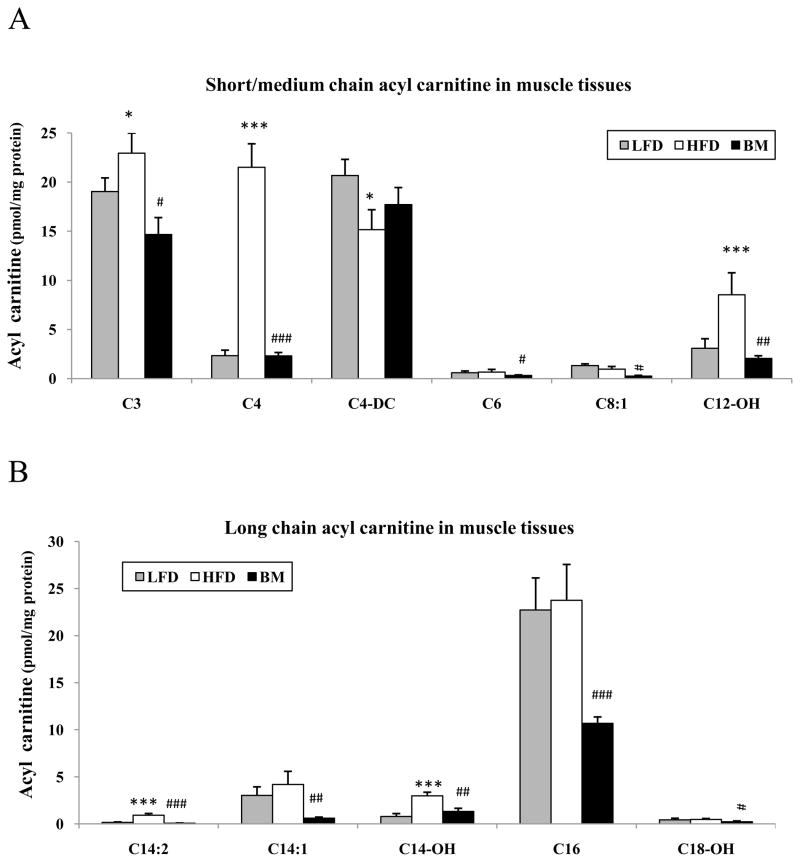
Fatty acyl carnitine profiles were assessed by tandem mass spectrometry in muscle extracts from mice randomized to the LFD, HFD and BM groups. HFD significantly increased both short/medium chain acyl carnitine contents (C3, C4, and C12-OH) and long-chain acyl carnitine contents (C14:2, C14-OH), but decreased C4-DC contents in the muscle when compared with the LFD control. However, HFD supplementation with BM modulated the HFD induced lipid changes in muscle by significantly reducing fatty acyl carnitine content (Fig. 4A and B).
Figure 4.
Fatty acyl carnitine profiles were obtained by tandem mass spectrometry in muscle extracts from mice fed with low-fat (n=6, grey bars), high-fat diet (n=7, white bars) and BM extract supplementation (n=7, black bars). The effect of HFD and BM on short/medium chain (penal A) and long chain fatty acyl carnitine (penal B) was assessed by ANOVA as described in the methods. For each acyl carnitine subtype, C3, acrylylcarnitine; C4, butyrylcarnitine/isobutyrylcarnitine; C4DC, succinyl carnitine; C6, 3-hydroxy-cis-5-0ctenoyl carnitine; C8:1, octanoyl-L-carnitine; C12-DC, dodecenoyl-L-carnitine; C14-2, tetradecadienyl-L-carnitine; C14-OH, tetradecenoyl-L-carnitine; C16, hexadecanoyl-L-carnitine; C18-OH, 3-hydroxysteraroylcarnitine. Mean ± SEM, * P<0.05, ** P<0.01 and *** P<0.001 respectively HFD or BM vs LFD. # P<0.05 and ## P<0.01, BM vs HFD.
3. 4. BM extract enhances insulin signaling in muscle tissues in the high fat diet fed mice
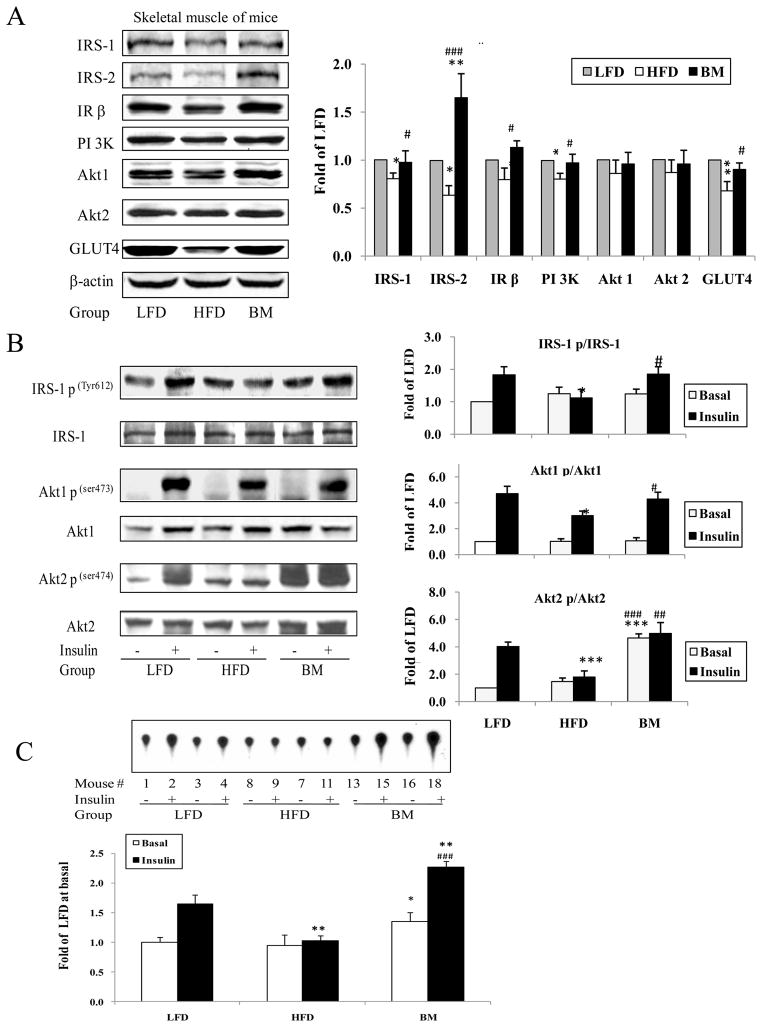
The data of insulin signaling pathway protein analysis demonstrated that IRS-1, IRS-2, PI 3K, and GLUT4 protein abundance were significantly lower in HFD than in LFD (P<0.05 and P <0.001). BM extract supplementation significantly increased IRS-1, IRS-2, PI 3K and GLUT4 plus IR β protein abundance in comparison with the HFD group (Fig. 5A). Insulin stimulated phosphorylation of IRS1, Akt1 and Akt2 in muscle was significantly lower in the HFD group than in the LFD, but basal phosphorylation of these proteins was not different among the three groups. However, BM supplementation significantly increased basal Akt2 phosphorylation as well as insulin-stimulated phosphorylation of IRS-1, Akt1 and Akt2 when compared with HFD animals (P<0.001, P<0.05 and P<0.01 respectively, Fig. 5B). There were no differences in basal IRS-1 p and Akt 1 p as well as insulin-stimulated IRS-1 p, Akt 1 p and Akt 2 p between LFD and BM groups except basal Akt 2 p content was significantly higher in BM than in LFD mice. The results of IRS-1 associated PI-3 kinase (PI 3K) activity in muscle revealed that the HFD did not significantly alter basal PI 3kinase activity, but significantly reduced insulin-stimulated PI3K activation. Moreover, BM supplementation significantly increased basal and insulin-stimulated PI 3K activities by 1.4- and 2.2-fold in comparison with HFD (P<0.05 and P<0.001, respectively, Fig. 5C).
Figure 5.
Effect of BM extract on insulin signaling pathway proteins in mice muscle tissues. IRS-1, IRS-2, IR β, PI3K, Akt1, Akt2 and GLUT4 in the muscle lysates were determined by western blotting. Panel A shows interested protein abundance in the muscle lysates. Results were normalized by β-actin. Panel B is the phosphorylation of IRS-1, Akt1 and Akt2 by normalized with corresponding protein levels. Panel C showed muscle PI 3 kinase activity in the mice. A 500 μg of muscle lysates was immunoprecipitated with anti-IRS-1 antibody and protein A agarose. IRS-1 associated PI 3K activity was measured by adding reaction buffer containing [r32P]ATP, PI and MgCl for 20 min, detail was described in the methods. Data are presented as mean ± SEM (n=6–7/group), * P<0.05, **P<0.01 and ***P<0.001, HFD or BM vs LFD. #P<0.05, ## P<0.01 and ### P<0.001 BM vs HFD respectively.
3. 5. Effect of BM extract on glucose uptake and insulin signaling in cultured muscle cells
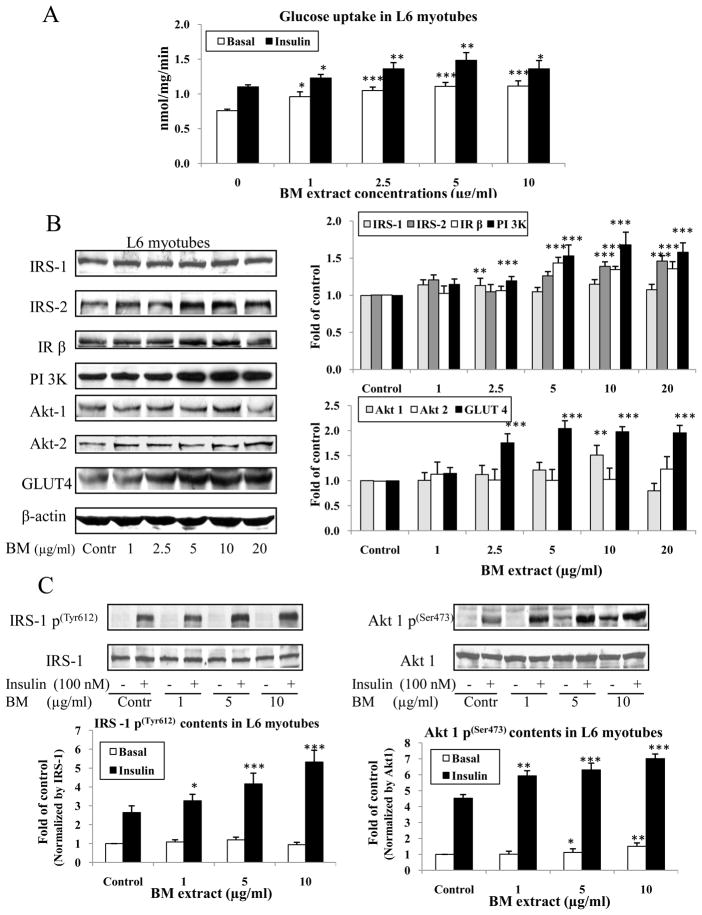
To test if BM extract has a direct beneficial effect on improving glucose metabolism and insulin signaling independent of possible confounders, i.e. calorie intake, as would be present in the animal study, we conducted in vitro experiments to measure 2-Deoxy--glucose uptake and insulin signaling in L6 myotubes treated with various doses of BM extract (indicated in the legends of figures). We demonstrated that BM extract treatment dramatically increased both basal and insulin-stimulated glucose uptake (P<0.05 and P<0.01, respectively, Fig. 6A). BM also substantially increased IR β, IRS-2, PI 3K, and GLUT 4 protein abundance in L6 cells at BM dose over 2.5 μg/ml (P <0.01 and P<0.001, Fig. 6B). Furthermore, BM extract not only significantly increased basal Akt 1 p abundance at dose over 5 μg/ml, but also enhanced insulin-stimulated phosphorylation of IRS-1 and Akt1 in a dose dependent manner (P<0.05, P<0.01 and P<0.001 respectively, Fig. 6C).
Figure 6.
Glucose uptake and insulin signaling measurements in BM extract treated L6 myotubes. At day 6 post differentiation, cells were treated with various doses of BM extract showed in legends overnight (16 h). 2-D-glucose uptake was measured with [3H]-2-D-glucose assay and insulin signal transduction pathway protein contents were analyzed by western blotting. Panel A showed glucose uptake results. Panel B reveals insulin signaling protein abundance normalized by β-actin in BM treated L6 myotubes. Panel C illustrates that phosphorylation of IRS-1 and Akt1 normalized by IRS-1 and Akt1 protein levels respectively. Data were presented as mean ± SEM from three independent experiments. * P<0.05, ** P<0.01 and *** P<0.001, BM vs control respectively.
4. Discussion
In this study, we report on the effects of bioactives from BM extract to favorably enhance glucose metabolism and insulin signaling in skeletal muscle tissues from mice fed a high-fat diet. Our results demonstrate that bioactives in BM, when supplemented in a high fat diet, significantly increased insulin sensitivity in vivo as assessed by HOMA index and IPITT. In addition, cellular insulin signaling in skeletal muscle such as increased IRS-1, IRS-2, IR β, PI 3K, and GLUT4 protein abundance and elevated PI 3K activity, was enhanced by BM as assessed in both in vivo and in vitro studies. Moreover, BM supplementation significantly modulated muscle fatty acyl carnitine content when compared with the HFD group. We also noted no differences in energy expenditure and body composition between the BM and HFD animals. Consistent with prior reports in rats (14), we observed that the significantly reduced body weight gain of BM mice is not caused by lower energy intake.
The main finding from this study lies in the effects of BM extract on reducing plasma leptin and insulin concentrations as well as enhancing muscle insulin signaling in the high fat diet fed mice. We observed that mice developed insulin and leptin resistance during diet-induced obesity. Leptin behaves as a potent anorexic and energy-enhancing hormone in most young or lean animals, but its effects are diminished or lacking in the obese state associated with a normal genetic background (20). In diet-induced obesity, hypothalamic and systemic inflammatory factors trigger intracellular mechanisms that lead to resistance to the main adipostatic hormones, leptin and insulin (21). In vitro studies have demonstrated inhibitory effect of leptin on insulin signaling, Morrison et al recently reported that there is a crosstalk between leptin and insulin signaling during the development of DIO (20–22). Further evidence has shown that leptin impairs insulin signaling in rat adipocytes (23). Interestingly, metformin, a hypoglycemic drug, can restore leptin sensitivity in high-fat-fed obese rats with leptin resistance (24). It is established that leptin increases PI 3K activity in liver, leading to lowered hepatic triglyceride levels in lean rats, but not in obese animals on a high-fat diet (25). Since PI 3K couples leptin and insulin signaling pathways via IRS-1 and IRS-2, it could be argued it could be argued that a defective activation of PI 3K could be a novel mechanism of peripheral leptin or insulin resistance (26). Recently, Nerurkar et al. reported that BM extract significantly reduced plasma apolipoprotein B-100 and increased hepatic IRS-1 and PI 3K activity in mice fed high fat diet (13). Our findings support the concept that defective PI 3K activity may also contribute to insulin resistance in skeletal muscle in the HFD induced obese animals. We demonstrated that HFD results in insulin and leptin resistance in mice with significantly reduced insulin stimulated PI 3K activity in the muscle. Consistent with Sridhar’s study (10), we observed BM significantly increased muscle IRS-1 phosphorylation in the mice fed a HFD. Thus, our study suggests that bioactives in the BM extract was able to attenuate the effect of a HFD on the insulin signaling pathway with significant reduction of plasma insulin and leptin levels. BM-mediated reductions in insulin and leptin may partially result from decreasing body weight in mice fed a HFD. Emerging evidence suggests that leptin resistance predisposes the animal to exacerbated diet-induced obesity (DIO) and the elevated plasma concentration of leptin in obesity is correlated with adipose tissue mass (20). Furthermore, we demonstrated that BM directly increases IRS, PI 3K and GLUT4 protein expression in cultured muscle cells, an in vitro model and condition for which clinical factors, i.e. calorie intake and body weight, would not be confounding variables (Fig. 6B–C).
Akt1 and Akt2 are the key downstream intermediates within the PI 3K pathway linked to insulin action on GLUT4 in adipocytes and skeletal muscle (24). Moreover, an Akt2 gene mutation leads to a dominantly inherited syndrome of insulin resistant diabetes and partial lipodystrophy (27). Interestingly, the isoforms of Akt, i.e. Akt1 and Akt2 are not functionally redundant. Mouse gene knockout studies, as well as knockdown studies in 3T3L1 adipocytes using small-interfering RNA (siRNA), have shown that reducing only Akt1 does not alter insulin sensitivity, whereas reducing Akt2 levels decreases insulin sensitivity and reduces glucose disposal (27). In addition, the metabolic phenotype was more profound when both Akt1 and Akt2 protein levels were reduced (28, 29). Therefore, both Akt1 and Akt2 are required for insulin signaling. Direct interaction between PI 3K and membrane vesicles leads to mobilization of GLUT4 glucose transporters in response to insulin stimulation in adipose and muscle cells (30).
Another major finding of our study is that BM may modulate lipid content in skeletal muscle in HFD fed mice. Evidence has been accumulating that insulin resistance is accompanied by mitochondrial dysfunction in skeletal muscle and liver, and that this could be the proximal cause of impaired lipid oxidation and accumulation of intramyocellular lipid (IMCL) (31–33). It was believed that incomplete muscle long-chain fatty acid (LCFA) β-oxidation, increases tissue accumulation of acetyl-CoA and generates chain-shortened acyl carnitine molecules implicated in insulin resistance, but molecular links between mitochondrial fat catabolism and insulin action remain controversial (34, 35). To study if LCFA combustion is associated with insulin resistance, Adams and coworkers analyzed plasma acyl carnitine profile in BMI- and age-matched overweight to obese type 2 diabetic and non-diabetic African-American women, and demonstrated that inefficient tissue LCFA β-oxidation, due in part to a relatively low tricarboxylic acid cycle capacity, increases tissue accumulation of acetyl-CoA and generates chain-shortened acyl carnitine molecules that activate proinflammatory pathways implicated in insulin resistance (36). Thus, HFD compromised acylcarnitine metabolism with increased skeletal muscle accumulation of acyl carnitine esters and insulin resistance (36, 37). It was reported that reduced acyl carnitine reverses marked perturbation in mitochondrial fuel metabolism including low rates of complete fatty acid oxidation, elevated incomplete β-oxidation and impaired substrate switching from fatty acid to pyruvate in muscle of obese rats (38). A recent study showed that pioglitazone improved insulin resistance in T2DM in association with mobilization of fat and toxic lipid metabolites out of muscle (13). Our study demonstrated that HFD significantly increased fatty acyl carnitine content in skeletal muscle when compared with the LFD mice; while BM supplementation substantially attenuated high-fat diet induced fatty acyl carnitine accumulation in muscle. Thus, the modulation of lipid metabolism by BM in muscle may be associated with the improved insulin action, but clearly our data does not suggest cause and effect. Our data support a model in which mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance (39). Although there was no difference in energy expenditure between HFD and BM groups, we confirmed that BM extract treatment significantly increased fatty acid oxidation and decreased triglyceride content in cultured muscle cells (data not shown) and the similar finding of BM increased lipid oxidative enzyme activities and UCP expression was observed in a rat study (14). Therefore, the reduction of muscle lipids by BM may play a role in enhancing insulin signaling.
It was previously reported that incubation of L6 myotubes with different concentrations of BM extract resulted in time-dependent increases in 2-Deoxy-glucose with maximal uptakes occurring at a concentration of 5 μg/ml (40). Consistent with this report, we observed similar optimal concentration of BM in increasing glucose uptake and insulin signaling proteins in L6 myotubes. It was reported that BM extract mimics insulin in its ability to exert a hypoglycemic effect, and stimulate amino acid uptake into skeletal muscle cells (9). We also demonstrated the effect of BM on insulin signaling with significantly increasing basal phosphorylation of Akt1 and Akt2, and PI 3K activity in mice muscle. In addition, BM not only significantly increased basal glucose uptake, PI 3K, and GLUT4 protein expression of L6 myotubes in a dose-dependent manner, but also significantly increased basal Akt 1 phosphorylation, beyond its insulin synergy. Therefore, our study suggests that the effect of BM on improving glucose metabolism and enhancing insulin signaling may mimic the action of insulin. Nevertheless, the effect of BM on insulin signaling in cultured cells may not precisely mirror the mechanism or response seen in vivo. Clearly, the difference between the in vivo and in vitro studies may be due to the variance of BM dosages or its absorptive bioactive components in the animals.
The limitation of the current study is that we are unable to determine whether BM extract affects processes via actions in the central nervous system or whether BM extract alters secretion of gut hormones. Another limitation is that at this time, we are not able to specifically measure the proposed bioactives in plasma and there is limited data currently on bioavailability and plasma appearance of bioactives after ingestion.
CONCLUSION
Bitter melon extract significantly reduced body weight, improved glucose metabolism and enhanced insulin signaling in mice fed with HFD. In addition, BM modulated acylcarnitine patterns in muscle observed with HFD feeding. As such, nutritional supplementation with this extract may provide an adjunctive therapy for the treatment of obesity and diet-induced insulin resistance.
Acknowledgments
Z. Q. Wang and W.T. Cefalu designed research; X.H Zhang and Y.M Yu conducted research experiments; D Ribnicky and A Poulev analyzed BM extract by using HPLC/LC-MS; Z.Q Wang performed statistical analysis, and wrote the manuscript and E.Z Floyd reviewed it. Z.Q Wang had primary responsibility for final content. All authors read and approved the final manuscript. We thank Drs. Thomas Gettys and Donald K Ingram for the valuable comments and suggestion. We also thank Connie David (Analytical Systems Laboratory, School of Veterinary Medicine, LSU) for her excellent technical support.
Supported by P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) which funds the Botanical Research Center of Pennington Biomedical Research Center and The Biotech Center of Rutgers University
Abbreviations
- IPGTT
intraperitoneal glucose tolerance test
- IRS-1
insulin receptor substrate 1
- GLUT4
glucose transporter 4
Footnotes
Author disclosures: Z.Q. Wang, X. H Zhang, YM. Yu, D Ribnicky and W.T. Cefalu have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1998;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): A review of efficacy and safety. Am J Health-Syst Pharm. 2003;60:356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- 5.McCarty MF. Does bitter melon contain an activator of AMP-activated kinase? Med Hypotheses. 2004;63:340–343. doi: 10.1016/j.mehy.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Krawinkel MB, Keding GB. Bitter gourd (Momordica Charantia): A dietary approach to hyperglycemia. Nutr Rev. 2006;64(7 Pt 1):331–337. doi: 10.1301/nr.2006.jul.331-337. [DOI] [PubMed] [Google Scholar]
- 7.Shih CC, Lin CH, Lin WL, Wu JB. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009;123(1):82–90. doi: 10.1016/j.jep.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Chao CY, Huang CJ. Bitter gourd (Momordica charantia) extract activates Peroxisome proliferator-activated receptors and upregulates the expression of the acyl CoA oxidase gene in H4IIEC3 hepatoma cells. J Biomed Sci. 2003;10(6 Pt 2):782–791. doi: 10.1159/000073966. [DOI] [PubMed] [Google Scholar]
- 9.Cummings E, Hundal HS, Wackerhage H, Hope M, Belle M, Adeghate E, Singh J. Momordica charantia fruit juice stimulates glucose and amino acid uptakes in L6 myotubes. Mol Cell Biochem. 2004;261(1–2):99–104. doi: 10.1023/b:mcbi.0000028743.75669.ab. [DOI] [PubMed] [Google Scholar]
- 10.Sridhar MG, Vinayagamoorthi R, Arul Suyambunathan V, Bobby Z, Selvaraj N. Bitter gourd (Momordica charantia) improves insulin sensitivity by increasing skeletal muscle insulin-stimulated IRS-1 tyrosine phosphorylation in high-fat-fed rats. Br J Nutr. 2008;99(4):806–12. doi: 10.1017/S000711450783176X. [DOI] [PubMed] [Google Scholar]
- 11.Nerurkar PV, Lee YK, Motosue M, Adeli K, Nerukar VR. Momordica Charantia (bitter melon) reduces plasma apolipoprotein B-100 and increases hepatic insulin receptor substrate and phosphonositide-3 kinase interactions. British J nutrition. 2008;100:751–759. doi: 10.1017/S0007114508937430. [DOI] [PubMed] [Google Scholar]
- 12.Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413(2):201–15. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW, Monroy A, Koul S, Sriwijitkamol A, Musi N, Shulman GI, Defronzo RA. Effects of Pioglitazone on Intramyocellular Fat Metabolism in Patients with Type 2 Diabetes Mellitus. Clin Endocrinol Metab. 2010 Feb 15; doi: 10.1210/jc.2009-0911. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan LLY, Chen Q, Go AGG, Lam EKY, Li ETS. Reduced adiposity in bitter Melon (Momordica charantia)-fed rats is associated with increased lipid oxidative enzyme activities and uncoupling protein expression. J Nutr. 2005;135:2517–2523. doi: 10.1093/jn/135.11.2517. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZQ, Zuberi AR, Zhang XH, Macgowan J, Qin J, Ye X, Son L, Wu Q, Lian K, Cefalu WT. Effects of dietary fibers on weight gain, carbohydrate metabolism, and Gastric ghrelin gene expression in mice fed a high-fat diet. Metabolism. 2007;56:1635–1642. doi: 10.1016/j.metabol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo obese, insulin-resistant JCR:LA-cp rats. J Nutr. 2006;136:415–420. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- 18.Stewart LK, Wang Z, Ribnicky D, Soileau JL, Cefalu WT, Gettys TW. Failure of dietary quercetin to alter the temporal progression of insulin resistance among tissues of C57BL/6J mice during the development of diet-induced obesity. Diabetologia. 2009;52(3):514–523. doi: 10.1007/s00125-008-1252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajduch E, Alessi DR, Hemmings BA, Hundal HS. Constitutive activation of protein kinase Bα (PKBα) by membrane targeting promotes glucose and system A amino acid transport, protein synthesis and GSK3 inactivation in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 20.Philip J. Scarpace and Yi Zhang Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmet P, Hodge A, Nicolson M, Staten M, deCourten M, Moore J, Movawiecki A, Lubina J, Collier G, Alberti G, Dowse G. Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. Br Med J. 1996;313:965–969. doi: 10.1136/bmj.313.7063.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison CD, Huypens P, Stewart LK, Gettys TW. Implications of crosstalk between Leptin and insulin signaling during the development of diet induced obesity. Biochim Biophys Acta. 2009;1792 (5):409–416. doi: 10.1016/j.bbadis.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez C, Fernandez-Galaz C, Fernandez-Agullo T, Arribas C, Andres A, Ros M, Carrascosa JM. leptin impairs insulin signaling in rat adipocytes (19) and 2004. Diabetes. 53:347–353. doi: 10.2337/diabetes.53.2.347. [DOI] [PubMed] [Google Scholar]
- 24.Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, Huh JY, Moon KH. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. 2006;55:716–724. doi: 10.2337/diabetes.55.03.06.db05-0917. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, dedousis N, Bhatt BA, O’Doherty RM. Impaired activation of Phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Bio Chem. 2004;279:21695–21700. doi: 10.1074/jbc.M401546200. [DOI] [PubMed] [Google Scholar]
- 26.Zhou QL, Park JG, Jiang ZY, Holik JJ, Mitra P, Semiz S, Guilherme A, Powelka AM, Tang X, Virbasius J, Czech MP. Analysis of insulin signalling by RNAi-based gene silencing. Biochem Soc Trans. 2004;32:817–21. doi: 10.1042/BST0320817. [DOI] [PubMed] [Google Scholar]
- 27.Tan K, Kimber WA, Luan J, Soos MA, Semple RK, Wareham NJ, O’Rahilly S, Barroso I. Analysis of genetic variation in Akt2/PKB-β in severe insulin resistance, lipodystrophy, type 2 diabetes, and related metabolic phenotypes. Diabetes. 2007;56:714–719. doi: 10.2337/db06-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphonositide 3-kinase-dependent steps in actitivation of protein kinase B. Molecular Cellular Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNAmediated gene silencing. Proc Natl Acad Sci U S A. 2003;100:7569–74. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holman GD, Kasuga M. From receptor to transporter: insulin signaling to glucose transport. Diabetologia. 1997;40:991–1003. doi: 10.1007/s001250050780. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, He J, Menshikova EV, et al. Dysfunction in mitochondria in human Skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 32.Petersen KF, Dufour S, Befroy D, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 35.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma Acyl carnitine Profiles Suggest Incomplete Fatty Acid β-Oxidation and Altered Tricarboxylic Cycle Activity in Type 2 Diabetic African-American Women. J Nutr. 2009;139(6):1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su X, Han X, Mancuso DJ, Abendschein DR, Gross RW. Accumulation of long-Chain acyl carnitine and 3-hydroxy acyl carnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry. 2005;44(13):5234–5245. doi: 10.1021/bi047773a. [DOI] [PubMed] [Google Scholar]
- 38.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition Compromises mitochondrial performance and metabolism control. J Biol Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koves TR, Ussher JR, slentz D, Mosedale M, Ilkayeva O, Bain JR, Stevens R, Dyck J, Newgard CB. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metabolism. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed I, Adeghate E, Cummings E, Sharma AK, Singh J. Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol Cell Biochem. 2004;261(1–2):63–70. doi: 10.1023/b:mcbi.0000028738.95518.90. [DOI] [PubMed] [Google Scholar]