Abstract
Background
Malnutrition is common at diagnosis and during treatment for sarcoma patients. Poor nutritional status is associated with increased risk of complications, particularly infections. We investigated the role of body mass index (BMI) on the incidence of surgical wound complications in patients with localized osteosarcoma treated on the Children's Oncology Group (COG) legacy trial, INT-0133.
Procedure
Patients considered in this report had localized osteosarcoma, enrolled on COG trial INT-0133, remained on protocol therapy to have definitive surgery 6–16 weeks after study entry, and had adequate height, weight, and surgical complication data for analysis. By protocol design, definitive surgical resection was planned for 10 weeks after induction chemotherapy. Wound complications within 30 days after definitive surgery were considered post-operative. BMI was calculated at the start of neoadjuvant chemotherapy and expressed as age-and gender-adjusted percentile. The incidence of wound complications was evaluated by logistic regression or Fisher's exact test.
Results
A total of 498 patients met criteria for analysis. Low BMI (≤10th percentile) was seen in 73 (14.7%), middle BMI (11th–94th percentile) in 382 (76.7%), and high BMI (≥95th percentile) in 43 (8.6%) patients. Wound infection or slough was seen in low BMI patients (OR=2.0, p=0.07) although the results did not reach statistical significance. Arterial thrombosis was more common in high BMI patients (OR=9.4, p=0.03).
Conclusions
Abnormal BMI at the start of treatment for localized osteosarcoma is associated with increased risk of post-operative wound complications such as arterial thrombosis. Future studies should evaluate whether maintenance of age-appropriate BMI reduces the risk of surgical complications.
Keywords: Osteosarcoma, Body mass index, Wound, Infection, Thrombosis
Introduction
Nutritional status plays an important role in the outcome of pediatric cancer patients. The incidence of malnutrition at diagnosis ranges from 6% to 50% and is more commonly seen in solid tumor patients such as sarcomas, neuroblastoma and Wilms tumor [1–3]. Malnutrition is associated with increased risk of infection, decreased tolerance of chemotherapy, decreased quality of life and increased risk of death [2,4]. Underweight and overweight patients with acute myeloid leukemia (based on body mass index (BMI) at diagnosis) are more likely to experience treatment-related complications and mortality compared to middle weight patients [5]. Similarly, undernourished children with acute lymphoblastic leukemia are reported to have a significantly lower 5-year overall survival compared to well nourished children [6].
Nutritional status can be assessed by several different anthropometric parameters such as weight, length or height for age, BMI, ideal body weight (IBW) and triceps skin fold measurement [7,8]. It can also be assessed by biochemical parameters such as serum prealbumin and albumin levels [9]. A recent Children’s Oncology Group (COG) nutrition committee review categorized malnutrition into underweight and overweight, based on either IBW or BMI [10].
Malnutrition is also associated with surgical complications and poor outcome in cancer patients. Underweight patients undergoing major intra-abdominal cancer surgery are at significantly higher risk for post operative complications and death [11]. Delayed wound healing is reported in malnourished non-cancer patients with lower extremity wounds and fractures, including elderly and trauma patients [12–14].
Patients with osteosarcoma (OS) routinely have major surgical intervention after intensive chemotherapy. We hypothesized that malnutrition increases the risk of pos-operative wound complications in OS patients. To test this hypothesis, we assessed the correlation between the risk of post-surgical wound related complications and BMI at diagnosis in patients with localized OS from a COG legacy clinical trial.
Methods
Eligible patients for this analysis participated in the COG legacy trial, INT-0133 (CCG-7921, POG-9351), a randomized, prospective study open to newly diagnosed OS patients from November 1993 to November 1997. This analysis was further restricted to patients with localized OS considered eligible for the study, who remained on protocol to have definitive surgery 6–16 weeks after study entry, and had height, weight, and surgical complication data available for analysis. The surgical case report forms, which specifically captured information on surgical complications within 30 days after resection of the primary tumor, were reviewed retrospectively for all patients.
INT-0133 randomized patients between the standard three drug regimen, methotrexate, doxorubicin and cisplatin (MAP) alone (Regimen A) and MAP in combination with ifosfamide (Regimen B). In addition, patients in both arms were further randomized to receive muramyl tripeptide phosphatidylethanolamine (MTP-PE) in addition to chemotherapy or chemotherapy alone (Figure 1). Induction chemotherapy was to be administered for 10 weeks prior to definitive surgery for primary tumor. Maintenance chemotherapy was to be resumed after recovery from definitive surgery. The results of the study have been reported [15,16].
Figure 1.
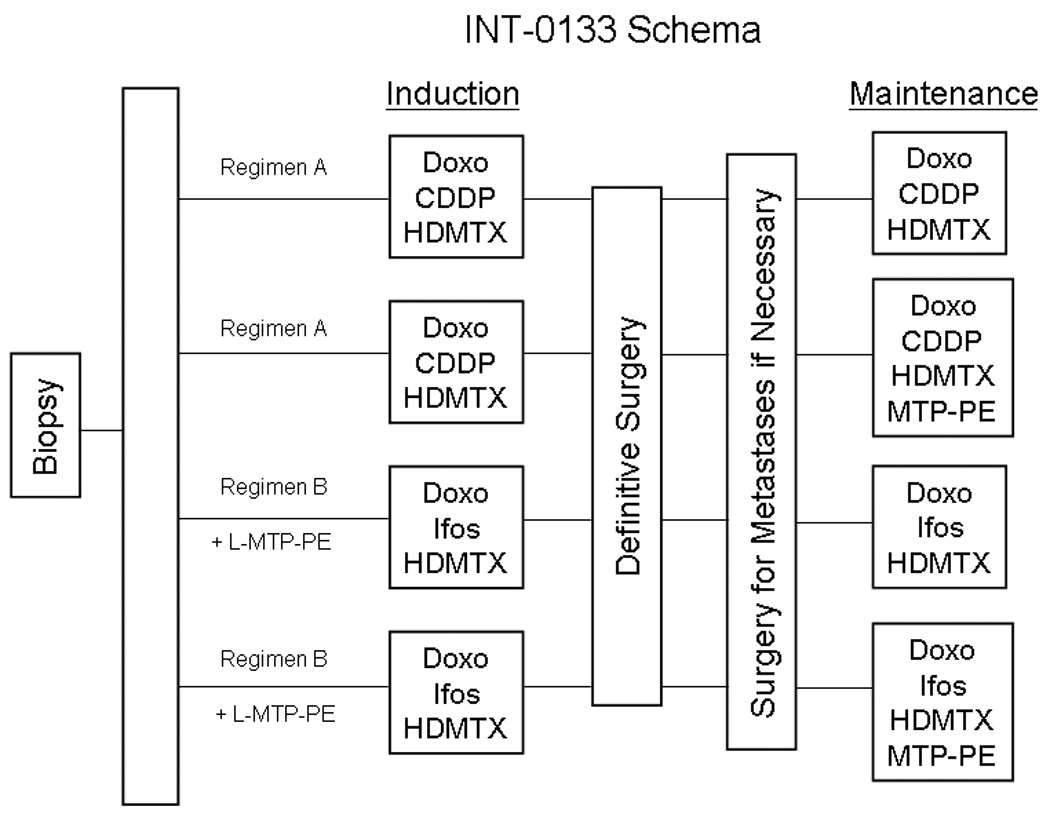
The treatment schema for patients enrolled on INT-0133. Abbreviations: High dose methotrexate (HDMTX), Cisplatin (CDDP), Doxorubicin (Doxo), Ifosfamide (Ifos), Muramyl Tripeptide Phosphatidylethanolamine (MTP-PE).
Any wound complication within 30 days of surgery was considered postoperative. These complications were coded as wound infection, wound slough, hematoma, hemorrhage, arterial thrombosis, and “other”. All “other” complications were reviewed by the investigators and classified more specifically. BMI was calculated at the start of chemotherapy as weight (kg)/ height (m2) and expressed as age and gender adjusted percentiles using the United States Growth Chart data files and formulas available through the National Center for Health Statistics on the Centers for Disease Control and Prevention website: http://www.cdc.gov/growthcharts/percentile_data_files.htm [17]. Low BMI was defined as < 10th percentile, middle BMI as 11–94th percentile and high BMI as > 95th percentile. Exact conditional testing was used to compare the proportions of subjects with postoperative wound complications for the low BMI versus middle BMI groups and for the high BMI versus middle BMI groups.
Results
INT-0133 enrolled seven hundred and ninety-three patients of which 498 patients met all eligibility criteria for analysis. Table I outlines the patient demographics and treatment assignment. Table II lists the reasons for exclusion from analysis. At study entry, seventy-three patients (14.7%) had a low BMI, 382 patients (76.7%) had middle BMI and 43 patients (8.6%) had a high BMI. Table III lists the number and proportion of subjects with each of the different wound complications. Wound slough was the most common complication. Hemorrhage and hematoma were not associated with BMI at diagnosis. Although when considered together, wound infection or slough were more common in patients with low BMI (OR=2.0, p=0.07), the results did not reach statistical significance at the p ≤0.05 level. Arterial thrombosis was more common in high BMI patients (OR=9.4, p=0.03) than middle and low BMI patients (Table IV). For wound complication types that were frequent enough to attempt multivariable modeling, we evaluated whether including additional variables for randomized treatment arm assignment or surgery site in the model was warranted. Neither treatment arm nor surgery site was statistically significant, so they were not included in the final model.
Table I.
Patient Characteristics
| Characteristics | No. of Patients | % | ||
|---|---|---|---|---|
| Age, years | ||||
| Mean | 14 | |||
| Range | 3.7–30 | |||
| Sex | ||||
| Male | 274 | 55 | ||
| Female | 224 | 45 | ||
| Primary site | ||||
| Bones | 1 | 0.2 | ||
| Jaw | 6 | 1.2 | ||
| Arm | 1 | 0.2 | ||
| Arm-humerus | 56 | 11.24 | ||
| Arm-radius | 7 | 1.41 | ||
| Arm-ulna | 2 | 0.4 | ||
| Pelvis | 1 | 0.2 | ||
| Pelvis-ilium | 3 | 0.6 | ||
| Leg | 2 | 0.4 | ||
| Leg-femur | 278 | 55.82 | ||
| Leg-tibia | 128 | 25.7 | ||
| Leg-fibula | 13 | 2.61 | ||
| Assigned therapy | ||||
| Reg A without MTP-PE | 125 | 25.1 | ||
| Reg A withMTP-PE | 117 | 23.49 | ||
| Reg B without MTP-PE | 127 | 25.5 | ||
| Reg B with MTP-PE | 129 | 25.9 | ||
Table II.
List of exclusion criteria for the current study analysis
| Reasons for Exclusion | Excluded |
|---|---|
| Metastatic disease | 92 |
| No record of definitive surgery | 78 |
| Erroneous height data* | 36 |
| Definitive surgery too late** | 23 |
| Definitive surgery too early*** | 17 |
| Ineligible for INT-0133 | 16 |
| No wound complication data | 13 |
| Missing height or weight data | 9 |
| Disease progression before surgery | 6 |
| Withdrawal from study prior to surgery | 4 |
| Start treatment date is unknown | 1 |
| Total | 295 |
height either >99.5th percentile or <0.5th percentile height for age;
definitive surgery was after the end of 16th week after start of course 1 of chemotherapy;
definitive surgery was before the 6th week after start of course 1 of chemotherapy
Table III.
Incidence of all post-operative wound complications (note: some patients had more than one post-operative complication)
| Complications | Number of patients (%) |
|---|---|
| Any | 76 (15.3%) |
| Wound slough | 52 (10.4%) |
| Wound infection | 15 (3.0%) |
| Hemorrhage | 4 (0.8%) |
| Hematoma | 17 (3.4%) |
| Arterial thrombosis | 6 (1.2%) |
Table IV.
Incidence of all complications based on the BMI of the patients
| Wound Complication | BMI | Number of patients | Statistical Analyses |
|---|---|---|---|
| Wound infection | Low | 4 (5.5%) | OR=2.7, p=0.22 |
| Middle | 8 (2.1%) | Reference | |
| High | 3 (7.0%) | OR=3.5, p=0.18 | |
| Wound slough | Low | 11 (15.1%) | OR=1.8, p=0.19 |
| Middle | 35 (9.2%) | Reference | |
| High | 6 (14.0%) | OR=1.6, p=0.44 | |
| Wound infection or slough | Low | 14 (19.2%) | OR=2.0, p=0.07 |
| Middle | 40 (10.5%) | Reference | |
| High | 7 (16.3%) | OR=1.7, p=0.36 | |
| Arterial thrombosis | Low | 0 (0%) | NA, p=1.00* |
| Middle | 3 (0.8%) | Reference | |
| High | 3 (7.0%) | OR=9.4, p=0.03 | |
| Hemorrhage | Low | 0 (0%) | NA, p=1.00 |
| Middle | 3 (0.8%) | Reference | |
| High | 1 (2.3%) | OR=3.0, p=0.70 | |
| Hematoma | Low | 2 (2.7%) | OR=0.7, p=0.94 |
| Middle | 15 (3.9%) | Reference | |
| High | 0 (0%) | p=0.39* | |
OR, odds ratio;
Odds ratio estimate not provided for comparisons involving a group that had zero complications.
Discussion
The results from our study show that an abnormal BMI was seen in about 25% of patients with localized OS at the start of neoadjuvant chemotherapy. Because wound slough may represent a more serious manifestation of a wound infection, we combined the two post-operative complication categories for the purposes of analysis. Patients who have a low BMI at diagnosis showed increased risk for developing post- operative wound infection or slough after their delayed definitive surgery with an OR of 2.0 and a p value of 0.07. Patients with high BMI are at increased risk of arterial thrombosis in the post operative period. Our data show an association between nutritional status and an increased incidence of post operative wound complications in patients with OS.
Prior literature has established the impact of nutrition supplementation in patients with cancer to improve outcomes such as tolerance to chemotherapy and radiation therapy, quality of life, incidence of post operative wound complications and overall survival. For example, early nutritional intervention improved tolerance to therapy and outcomes in head and neck cancer patients undergoing concurrent chemo-radiotherapy [18]. Malnutrition influenced the rate of recovery and incidence of post operative complications after surgery for gastrointestinal cancers. Early assessment of nutritional status and intervention improved outcome and decreased post-operative wound healing complications [11,19–21]. The American Society for Parenteral and Enteral Nutrition has issued guidelines for nutrition support in surgical oncology based on an extensive review of the literature [22]. This report suggested that peri-operative nutritional support may be beneficial in moderately to severely malnourished patients if administered 7–14 days preoperatively but routine use of nutritional support is not recommended in all patients undergoing major cancer surgery. Enteral nutrition has decreased risk of complications when compared to parenteral nutrition in these patients.
Since our results show that low BMI at diagnosis might be a risk factor for a major post-operative complication later in treatment, early nutritional intervention could correct poor nutritional status and potentially reduce the risk of wound complications. The INT-0133 dataset did not collect information about nutritional intervention, nor did it collect weight data immediately prior to surgery. Therefore, it is not possible to determine whether interventions to correct low BMI such as enteral or parenteral supplementation were attempted, if intervention corrected low BMI prior to surgery, or if correction reduced the risk of post-operative complications. Further, the lack of other measures such as anthropometric or biochemical analysis limits the capability of this study to assess the true nutitional status of these patients as BMI alone might not be an accurate predictor of overall nutritional status. A recent COG Nutrition Committee survey revealed a lack of standardized methods of evaluation of nutritional status and criteria for intervention amongst the COG institutions across the country [23]. Based upon a literature review and expert consensus, the COG Nutrition Committee established the categories of malnutrition using either the BMI or IBW and an algorithm for nutritional intervention in pediatric oncology patients [10]. It is possible that greater institutional standardization of nutritional support could reduce the risk of post-operative complications in patients with OS.
Given our retrospective analysis, it is not possible to draw a clear cause and effect relationship between low BMI at diagnosis and post-operative complications based on this study. INT-0133 was not designed to assess the relationship between nutritional status and patient outcomes in a prospective manner. Our results, though not statistically significant for the low BMI group, provide the hypothesis for future prospective trials to validate the results of the current study. This analysis also delineates the additional questions that need to be answered to address this hypothesis directly. These include measuring other anthropometric or biochemical parameters of malnutrition, such as prealbumin, in addition to the BMI as a more comprehensive measure of nutritional status, collecting more precise information regarding the grade or severity of post operative complications such as wound infection, and evaluating the effect of correcting malnutrition on the incidence of postoperative wound complications in patients with OS.
Footnotes
Conflict of Interest: none
References
- 1.Ladas EJ, Sacks N, Meacham L, et al. A multidisciplinary review of nutrition considerations in the pediatric oncology population: a perspective from children's oncology group. Nutr Clin Pract. 2005;20(4):377–393. doi: 10.1177/0115426505020004377. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson SS, Wesley MN, DeWys WD, et al. A study of the nutritional status of pediatric cancer patients. Am J Dis Child. 1981;135(12):1107–1112. doi: 10.1001/archpedi.1981.02130360015007. [DOI] [PubMed] [Google Scholar]
- 3.van Eys J. Malnutrition in children with cancer: incidence and consequence. Cancer. 1979;43(5 Suppl):2030–2035. doi: 10.1002/1097-0142(197905)43:5+<2030::aid-cncr2820430711>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Marin Caro MM, Laviano A, Pichard C. Impact of nutrition on quality of life during cancer. Curr Opin Clin Nutr Metab Care. 2007;10(4):480–487. doi: 10.1097/MCO.0b013e3281e2c983. [DOI] [PubMed] [Google Scholar]
- 5.Lange BJ, Gerbing RB, Feusner J, et al. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293(2):203–211. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]
- 6.Lobato-Mendizabal E, Lopez-Martinez B, Ruiz-Arguelles GJ. A critical review of the prognostic value of the nutritional status at diagnosis in the outcome of therapy of children with acute lymphoblastic leukemia. Rev Invest Clin. 2003;55(1):31–35. [PubMed] [Google Scholar]
- 7.Oguz A, Karadeniz C, Pelit M, et al. Arm anthropometry in evaluation of malnutrition in children with cancer. Pediatr Hematol Oncol. 1999;16(1):35–41. doi: 10.1080/088800199277579. [DOI] [PubMed] [Google Scholar]
- 8.Mei Z, Grummer-Strawn LM, Pietrobelli A, et al. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75(6):978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 9.Elhasid R, Laor A, Lischinsky S, et al. Nutritional status of children with solid tumors. Cancer. 1999;86(1):119–125. doi: 10.1002/(sici)1097-0142(19990701)86:1<119::aid-cncr17>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Rogers PC, Melnick SJ, Ladas EJ, et al. Children's Oncology Group (COG) Nutrition Committee. Pediatr Blood Cancer. 2008;50(2 Suppl):447–450. doi: 10.1002/pbc.21414. discussion 451. [DOI] [PubMed] [Google Scholar]
- 11.Mullen JT, Davenport DL, Hutter MM, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. 2008;15(8):2164–2172. doi: 10.1245/s10434-008-9990-2. [DOI] [PubMed] [Google Scholar]
- 12.Dwyer AJ, John B, Mam MK, et al. Nutritional status and wound healing in open fractures of the lower limb. Int Orthop. 2005;29(4):251–254. doi: 10.1007/s00264-004-0629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo JJ, Yang H, Qian H, et al. The effects of different nutritional measurements on delayed wound healing after hip fracture in the elderly. J Surg Res. 159(1):503–508. doi: 10.1016/j.jss.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Patel GK. The role of nutrition in the management of lower extremity wounds. Int J Low Extrem Wounds. 2005;4(1):12–22. doi: 10.1177/1534734605274574. [DOI] [PubMed] [Google Scholar]
- 15.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children's Oncology Group. J Clin Oncol. 2008;26(4):633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 18.Cady J. Nutritional support during radiotherapy for head and neck cancer: the role of prophylactic feeding tube placement. Clin J Oncol Nurs. 2007;11(6):875–880. doi: 10.1188/07.CJON.875-880. [DOI] [PubMed] [Google Scholar]
- 19.Attili VS, Bapsy PP, Ramachandra C, et al. Influence of postoperative complications on relapse-free survival in gastrointestinal malignancies. Gastrointest Cancer Res. 2009;3(5):179–182. [PMC free article] [PubMed] [Google Scholar]
- 20.Nishi M, Hiramatsu Y, Hioki K, et al. Risk factors in relation to postoperative complications in patients undergoing esophagectomy or gastrectomy for cancer. Ann Surg. 1988;207(2):148–154. doi: 10.1097/00000658-198802000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozzetti F. Rationale and indications for preoperative feeding of malnourished surgical cancer patients. Nutrition. 2002;18(11–12):953–959. doi: 10.1016/s0899-9007(02)00988-7. [DOI] [PubMed] [Google Scholar]
- 22.Huhmann MB, August DA. Nutrition support in surgical oncology. Nutr Clin Pract. 2009;24(4):520–526. doi: 10.1177/0884533609335375. [DOI] [PubMed] [Google Scholar]
- 23.Ladas EJ, Sacks N, Brophy P, et al. Standards of nutritional care in pediatric oncology: results from a nationwide survey on the standards of practice in pediatric oncology. A Children's Oncology Group study. Pediatr Blood Cancer. 2006;46(3):339–344. doi: 10.1002/pbc.20435. [DOI] [PubMed] [Google Scholar]


