Abstract
The present study was undertaken to examine whether genetically predetermined differences in components of the endocannabinoid system were present in the brain of Sardinian alcohol-preferring (sP) and Sardinian alcohol-non preferring (sNP) rats, a pair of rat lines selectively bred for opposite alcohol preference. The effects of acquisition and maintenance of alcohol drinking, alcohol withdrawal, and alcohol re-exposure on the endocannabinoid system was also assessed in the striatum of sP rats. The findings revealed significantly higher density of the CB1 receptors and levels of CB1 receptor mRNA, CB1 receptor-mediated G-protein coupling, and endocannabinoids in the cerebral cortex, hippocampus and striatum of alcohol-naive sP rats than sNP rats. A significantly lower expression of mFAAH enzyme was evident in the hippocampus of alcohol-naive sP rats. Alcohol drinking (during both acquisition and maintenance phases) in sP rats resulted in a significant reduction in striatal CB1 receptor-mediated G-protein coupling whereas alcohol withdrawal attenuated this effect. Alcohol consumption was also associated with markedly increased levels of endocannabinoids in the striatum. Co-administration of the CB1 receptor antagonist, rimonabant (SR141716A) reduced alcohol intake, and reversed alcohol-induced changes in CB1 receptor-mediated G-protein activation. These findings provided a new insight into a potential genetic basis of excessive alcohol consumption, suggesting innate differences in the endocannabinoid system might be associated with higher alcohol preference in sP rats. The data also indicate a modulation of CB1 receptor-mediated signaling following alcohol consumption, and further strengthen the potential of the endocannabinoid system as a target for the treatment of alcohol related behaviors.
Keywords: Anandamide, Rimonabant, CB1 receptor, G-protein, FAAH
INTRODUCTION
Increasing evidence suggests a role of the endocannabinoid system in alcohol-related behaviors (Vinod and Hungund 2006). The endocannabinoid system comprises of cannabinoid (CB) receptors, endogenous agonists (endocannabinoids) and the metabolic enzymes of endocannabinoids. The CB1 receptor is one of the most widely distributed and abundant G-protein coupled receptors present in the brain (Howlett, 2005). In the CNS, endocannabinoids are synthesized in post-synaptic terminals and act as retrograde messengers to activate presynaptically localized Gi/o protein coupled CB1 receptors that in turn modulate adenylate cyclase, ion channels and extracellular signaling pathways (Howlett, 2005).
Previous studies have demonstrated a significant reduction in alcohol drinking in CB1 receptor knockout mice (Hungund et al. 2003; Poncelet et al. 2003; Wang et al. 2003; Naassila et al. 2004; Houchi et al. 2005; Thanos et al. 2005). Furthermore, pharmacological manipulation of CB1 receptor function is known to modulate alcohol-related behaviors, including alcohol drinking and self-administration, in rodents (Arnone et al. 1997; Colombo et al. 1998; Gallate et al. 1999; Rodriguez de Fonseca et al. 1999; Freedland et al. 2001; Serra et al. 2001; Colombo et al. 2002; Hungund et al. 2002; Serra et al. 2002; Colombo et al. 2004; Hansson et al. 2007; Malinen and Hyytia, 2008; Alvarez-Jaimes et al. 2009; Maccioni et al. 2009; Femenia et al. 2010). Indeed, administration of the CB1 receptor agonist (CP-55,940) has been shown to enhance alcohol drinking and motivate alcohol consumption in rodents (Gallate et al. 1999; Colombo et al. 2002). On the other hand, the CB1 receptor antagonist, rimonabant (SR141716A) has been shown to reduce alcohol consumption, relapse-like drinking, operant oral alcohol self-administration, and alcohol’s motivational properties in Sardinian alcohol-preferring (sP) rats (Colombo et al. 1998; Serra et al. 2001; Serra et al. 2002; Colombo et al. 2004; Maccioni et al. 2009). Conversely, recent preliminary clinical studies have shown no significant effect of rimonabant on alcohol consumption (Soyka et al. 2008; George et al. 2010).
In spite of this progress, the mechanism implicated in alcohol consumption involving the endocannabinoid system remains somewhat unclear. Both genetic and environmental factors have been linked to the etiology of alcohol dependence (Heath et al. 2000). To this regard, several animal models have been developed with an aim towards further understanding the genetic basis of alcoholism. One of these models, sP and Sardinian alcohol-non preferring (sNP) rat lines, has been selectively bred for opposite alcohol preference and consumption, displaying marked differences in several other alcohol-related behaviors (Colombo et al. 2006). The current study was undertaken to investigate whether genetic differences in components of the endocannabinoid system are associated to higher alcohol consumption in sP rats compared to sNP rats. The effects of various stages of alcohol drinking (under the standard, homecage two-bottle “alcohol vs water” choice regimen with unlimited access for 24 hours/day) on the endocannabinoid system were also examined in the brain of sP rats. Specifically, four different stages were evaluated: acquisition of alcohol drinking behavior (the time period during which sP rats rapidly discover alcohol’s reinforcing properties and acquire alcohol drinking behavior), long-term maintenance of alcohol drinking (a possible model of the active drinking phase of human alcoholics), withdrawal from alcohol after long-term alcohol drinking, and re-exposure to alcohol drinking after a period of deprivation [the so-called “alcohol deprivation effect”, i.e. the transient increase in alcohol intake that occurs after a period of alcohol abstinence and is thought to model relapse in human alcoholics (Spanagel, 2005)]. Since alcohol drinking behavior in sP rats is sensitive to pharmacologic manipulation of the endocannabinoid system (Colombo et al. 1998; Serra et al. 2001; Serra et al. 2002), the effect of CB1 receptor antagonism on the endocannabinoid system at different stages of alcohol drinking was also investigated.
METHODS
Animals
Adult male (9–10 week old) sP and sNP rats were used in this study. Rats were individually housed in standard plastic cages with wood chip bedding. Standard rat chow (Mucedola, Settimo Milanese, Italy) and tap water were always available. A first group of sP and sNP rats (n=8–10) was killed by decapitation prior to alcohol exposure (alcohol-naive) to allow assessment of basal differences in the endocannabinoid system between the lines. The striatum of sP rats was examined to ascertain the effects produced on the endocannabinoid system following voluntary alcohol consumption and withdrawal. Animal care and handling procedures were carried out in compliance with Institutional and NIH guidelines, as well as the Italian Law on the “Protection of animals used for experimental and other scientific reasons”.
Voluntary alcohol consumption, alcohol withdrawal, and “alcohol deprivation effect” in sP rats
sP rats were exposed to 10% (v/v) alcohol and water under the standard, homecage two-bottle free-choice regimen with unlimited access for 24 hours/day. Two groups of rats (n=8–10) were exposed to the above regimen for 10 (acquisition phase) and 60 (maintenance phase) consecutive days, and were subsequently sacrificed and their brains removed for analysis of the endocannabinoid system. Two groups of age-matched control rats (n=8–10), kept under identical conditions and given only water, were sacrificed at the same time intervals. Alcohol and water intake were recorded daily. Bottles were refilled daily with fresh alcohol solution and water, and their positions interchanged at random to avoid development of position preference. Rat body weights were recorded twice weekly.
In the alcohol withdrawal paradigm, groups of sP rats (n=8–10) were exposed to the two-bottle “alcohol (10% v/v) vs water” choice regimen for 60 consecutive days and sacrificed 1, 3, and 7 days after removal of alcohol. Comparison with a fourth group of rats exposed to the two-bottle choice regimen for 60 consecutive days (n=10) allowed us to determine whether withdrawal from alcohol, after a relatively long period of voluntary alcohol intake, is associated with changes in the endocannabinoid system.
In the “alcohol deprivation effect” experiment, sP rats were exposed to the two-bottle “alcohol (10% v/v) vs water” choice regimen for 60 consecutive days. Subsequently, rats were divided into two groups (n=8–10), one of which was allowed unlimited access to alcohol and water (alcohol-non deprived rats), whilst in the other alcohol was removed for 14 consecutive days, with water being the only fluid available (alcohol-deprived rats). On the 15th day, alcohol was presented again at the start of the dark phase. One hour later, alcohol intake was recorded in both groups. Rats were then sacrificed and the striatum isolated for analysis. The time point chosen corresponds to a period during which sP rats display the largest “alcohol deprivation effect” (Colombo et al. 2006). This experiment allowed us to determine whether the increase in alcohol intake manifested by sP rats following a period of alcohol deprivation is associated with changes in the endocannabinoid system.
Effect of rimonabant on the endocannabinoid system during acquisition and maintenance of alcohol drinking and “alcohol deprivation effect”
Procedures for acquisition and maintenance of alcohol drinking and “alcohol deprivation effect” were similar to those described in earlier experiments, with the exception of administration of rimonabant (Sanofi-Aventis, Montpellier, France). During the acquisition phase, sP rats underwent initial treatment with rimonabant (1 and 3 mg/kg, i.p.) or vehicle 30 min prior to alcohol presentation on day 1, and subsequently twice daily for 10 consecutive days (at 6 hr intervals during the dark phase). Rats were then sacrificed at the end of alcohol exposure period. The effect of rimonabant treatment alone for 10 days (twice daily) on endocannabinoid levels in alcohol-naive sP rats was assessed.
During the maintenance phase, rats were exposed to the two-bottle choice regimen for 55 consecutive days before the start of rimonabant treatment. Treatment with rimonabant (1 and 3 mg/kg, i.p.) was administered twice daily (30 min before lights off and 6 hrs later) for 5 consecutive days. Rats were sacrificed at the end of 5th day of rimonabant treatment.
In the “alcohol deprivation effect” paradigm, all rats were first exposed to a two-bottle choice regimen for 60 consecutive days. Subsequently, rats were divided into two groups: alcohol-non deprived rats continued to have unlimited access to alcohol and water, while alcohol-deprived rats were given water only for 14 consecutive days. On the 15th day, both groups of rats received rimonabant (1 and 3 mg/kg, i.p.) 30 min before the start of dark phase; alcohol was presented again at the start of the dark phase. One hour later the rats were sacrificed and the striatum isolated for analysis of the endocannabinoid system.
Rimonabant doses were chosen on the basis of the findings of previous experiments (Serra et al. 2001; Serra et al. 2002; Colombo et al. 2004; Maccioni et al. 2009). In all experiments, rimonabant was suspended in saline containing 0.1% (w/v) Tween 80 and injected at a volume of 2 ml/kg.
CB1 receptor assay
The radioligand binding assay was performed using crude synaptic membranes isolated from brain regions as previously described (Vinod et al. 2006) using a saturating concentration (1–10 nM) of radiolabeled CB1 agonist, [3H]CP-55,940. All ligands were prepared in an assay buffer (50 mM Tris-HCl, 3 mM MgCl2,1 mM EDTA) containing 0.1% fatty acid-free BSA. Assay solutions were incubated in silicone-treated test tubes for 1 hr at 37°C with membrane fractions (50–100 μg protein). The non-specific binding was determined by addition of 10 μM unlabeled CP-55,940. The reaction was terminated by addition of 2 ml of ice-cold buffer (50 mM Tris-HCl, and 0.1% BSA) and was rapidly filtered through the GF/B filters (pretreated with 0.1% polyethylenimine) using a Brandel cell harvester. Filters were washed three times with ice-cold buffer, transferred to scintillation vials containing 5 ml of scintillation cocktail and incubated overnight at room temperature. Radioactivity was measured using a liquid scintillation counter at an efficiency of 50% for 3H.
Agonist-stimulated [35S]GTPγS binding assay
CB1 receptor-stimulated [35S]GTPγS activation was measured in crude synaptic membrane as previously described with minor modifications (Vinod et al. 2006). Briefly, an aliquot of membrane (20–50 μg protein) was incubated in Tris-MgCl2-EDTA buffer containing 0.1% fatty acid free BSA, 100 mM NaCl, 30 μM GDP and 0.05 nM [35S]GTPγS in silicone-treated test tubes for 1 hr at 37°C. The CB1 receptor agonist, CP-55,940 (1.5 μM) was used to examine CB1 receptor-stimulated [35S]GTPγS binding to G-protein. Basal activity was measured in absence of CP-55,940. The reaction mixture was rapidly filtered through the GF/B glass filters. Radioactivity was measured using a liquid scintillation counter at an efficiency of 95% for 35S.
In situ hybridization histochemistry (ISHH)
Brain sections were cut as previously described for [35S]GTPγS autoradiography (Ortiz et al. 2004; Vinod et al. 2008). ISHH was performed using synthetic oligonucleotide probes complementary to CB1 receptor mRNA (bases 4–51, 349–396, 952–999). Oligonucleotide probes were labeled using terminal deoxytransferase to add a [35S]-labeled deoxyATP (1000 Ci mmol−1; Amersham, Madrid, Spain) tail to the 3′ end of the probes. The probe in hybridization buffer was applied to each section and left overnight at 37°C for hybridization. Sections were subsequently washed four times for 15 min each in 0.15 M NaCl, 0.015 M sodium citrate (saline sodium citrate, SSC, pH 7.2) at 55°C followed by two 30 minute washes in SSC at room temperature, one brief water dip and were then air dried. To check for imaging enhancement variables each set of slides was exposed to the film (Kodak BioMax MR-1, Amersham, Madrid, Spain) for 15–20 days.
Immunoblot analysis
Brain tissue was homogenized in Tris-buffer (pH 7.4) containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). An aliquot of tissue homogenate (30 μg) was separated by 10% or 12% polyacrylamide gel and electrophoretically transferred to nitrocellulose membrane. The membrane was treated with blocking buffer (TTBS, [10mM Tris, 0.9% NaCl; 1% Tween 20 containing 5% milk powder] of pH 7.4) for 1hr at room temperature. The membrane was then incubated with either polyclonal anti-CB1 receptor (1:1000 dilution; Invitrogen, Carlsbad, CA) or monoclonal anti-FAAH (1;2000 dilution; Abnova, Taipei City, Taiwan) overnight at 4°C. The blot was washed 3 times with TTBS and then incubated with HRP conjugated secondary anti-IgG for 1hr at room temperature. After washing the blot 3 times with TTBS, the immunoreactive band was visualized using ECL reagent (GE HealthCare, Piscataway, NJ). X-ray films were developed and scanned using HP ScanJet. Band intensities were measured using ImageJ software (NIH, Bethesda, MD) and analyzed using GraphPad Prism software. Blots were reprobed with α-tubulin antibody (1:1000 dilution, Santa Cruz Biotech, Santa Cruz, CA) to ensure equal protein loading.
Endocannabinoid assay
The endocannabinoid assay was performed by LC-MS method using the isotopic dilution procedure as described previously with minor modifications (Vinod et al. 2005). Briefly, brain tissue was homogenized in 4 ml of chloroform-methanol-Tris buffer (2:1:1, pH 7.4) containing 0.25 mM PMSF, 20 μl of 1% BHT, AEA-d8 (50 ng) and 2-AG-d8 (500 ng). Tissue homogenate was centrifuged and the organic layer dried under nitrogen. The residue was dissolved in 0.3 ml ethyl acetate, recentrifuged and the supernatant dried under nitrogen. The residue was redissolved in ethyl alcohol (40 μl) and transferred to a vial for measurement of AEA and 2-AG by LC-MS (Agilent 1100 series mass LC-MSD). Separation was achieved on a supelcosil LC-8 column using methanol-ammonium acetate-acetic acid (85:15:0.05) as a mobile phase.
Enzyme assay
FAAH activity was determined in the post-membrane supernatant as previously described (Vinod et al. 2007 Vinod et al. 2009). Samples (25–50 μg of protein) were incubated for 30 min at 37°C in a solution containing 50 mM Tris-HCl, pH 8.0, 0.1% BSA, and 30 μM anandamide (ethanolamine1-3H) (10–20 Ci/mmol; Radiolabeled Chemicals, St. Louis, MO). For the MGL assay, the supernatant (30 μg protein) was incubated with 2-oleoyl-[3H]glycerol (0.1 mCi; American Radiolabeled Chemicals, St. Louis, MO) and 2-oleoyl glycerol (70 μM) in Tris-HCl buffer (50 mM, pH 7.4) containing 0.1% BSA, for 30 min at 37°C. After incubation, samples were extracted by methanol and chloroform mixture (1:1) and subjected to liquid scintillation counting. The protein concentration of tissue homogenates was determined using BCA method.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software. Data relating to the effect of treatment with rimonabant on alcohol intake during acquisition and maintenance of alcohol drinking and “alcohol deprivation effect” were analyzed by one or two-way ANOVAs followed by the Newman-Keuls test for post hoc comparison. The levels of CP-55,940 stimulated [35S]GTPγS binding, mCB1 receptor and mFAAH were expressed as percentage over the basal or control. Autoradiograms were analyzed using NIH Image software and data were expressed as 100% over mean control values. Statistical significance was evaluated by ANOVA followed by Newman-Keuls test for post hoc comparison or, when appropriate, independent-sample t-test. All data are presented as mean±SEM, and statistical significance was set at p<0.05.
RESULTS
Status of the endocannabinoid system in the brain of alcohol-naive sP and sNP rats
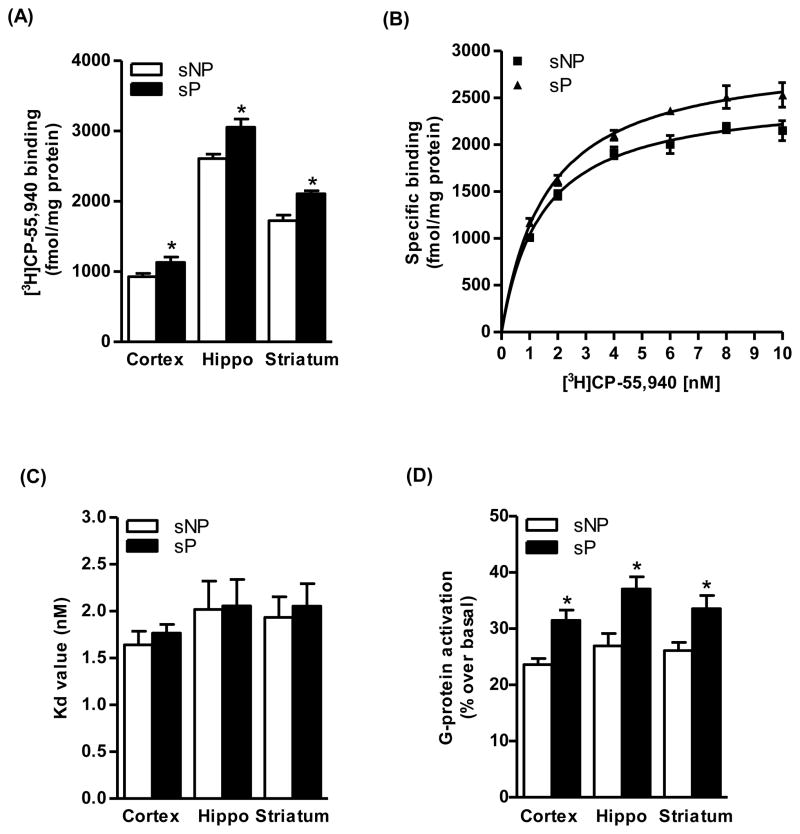
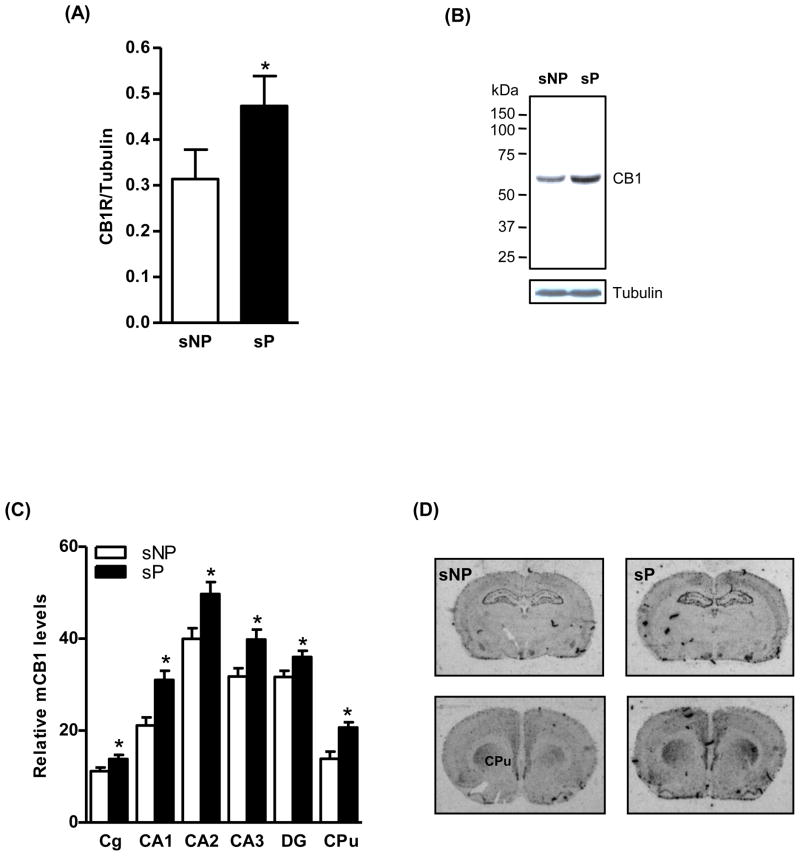
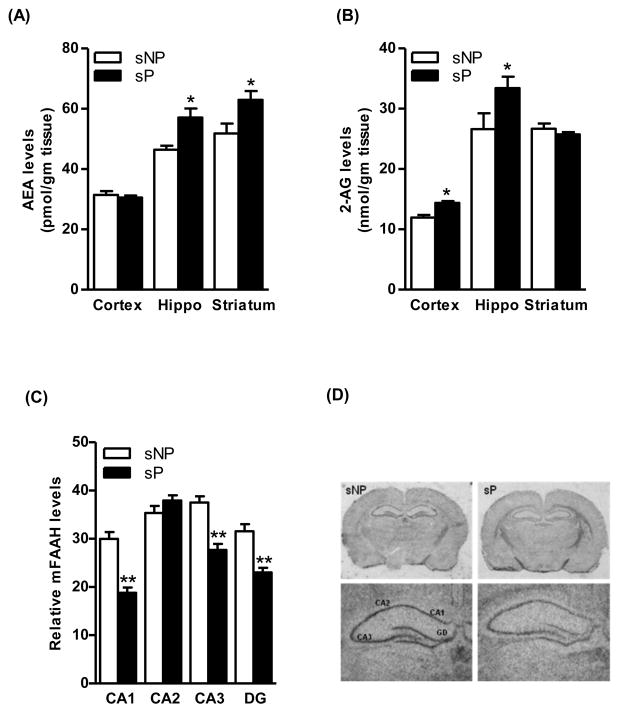
Saturation analysis of [3H]CP-55,940 binding revealed slightly higher Bmax values in the cerebral cortex (22%, p<0.05), hippocampus (18%, p<0.05) and striatum (22%, p<0.05) of sP rats compared to sNP rats (Fig. 1A). A representative graph of saturation isotherm of [3H]CP-55,940 binding to hippocampal membranes is shown in figure 1B. However, no significant differences were observed in the affinity of CB1 receptor for the radioligand in the brain of sP and sNP rats (Fig. 1C). Similarly, higher levels of CB1 receptor-mediated G-protein coupling were observed in these brain regions of sP rats in comparison to sNP rats (29–38%; Fig. 1D). Immunoblot analysis confirmed a higher level of CB1 receptor immunoreactivity in the hippocampus (49%, p<0.05; Fig. 2A) of sP rats compared to sNP rats. A representative immunoblot is shown in figure 2B. In agreement with the results of the radioligand assay, in situ hybridization analysis showed a higher CB1 receptor mRNA expression in the cingulate cortex (24%, p<0.05; Fig. 2C and 2D), CA1 (47%, p<0.01), CA2 (24%, p<0.05), CA3 (25%, p<0.05), and DG (14%, p<0.05) of hippocampus (Fig. 2C and 2D), and striatum (49%, p<0.01; Fig. 2C and 2D) of sP rats compared to sNP rats. LC-MS analysis revealed a significantly higher level of AEA in the hippocampus (23%, p<0.05; Fig. 3A) and striatum (21%, p<0.05; Fig. 3A) of sP than sNP rats. Significantly higher levels of 2-AG were found in the cerebral cortex (21%, p<0.05; Fig. 3B) and hippocampus (26%, p<0.05; Fig. 3B) of sP rats compared to sNP rats. In situ hybridization analysis revealed a significantly lower expression of mFAAH enzyme in the CA1 (37%, p<0.001), CA3 (26%, p<0.001) and DG (27%, p<0.001) of sP rats compared to sNP rats (Fig. 3C and 3D).
Figure 1.
Saturation analysis of [3H]CP-55,940 binding indicated a higher Bmax values in the cerebral cortex (22%, p<0.05), hippocampus (18%, p<0.05) and striatum (22%, p<0.05) of alcohol-naive sP than sNP rats (A). A representative graph of saturation isotherm of [3H]CP-55,940 binding to hippocampal membranes is shown in figure B. There were no significant differences in Kd values of the receptor for the radioligand between the groups (C). Basal levels CB1 receptor-mediated G-protein coupling were found to be higher in the cerebral cortex (33%, p<0.05), hippocampus (38%, p<0.01) and striatum (29%, p<0.05) of alcohol-naive sP than sNP rats (D).
Figure 2.
Western blot analysis showed a higher level of the CB1 receptor immunoreactivity in the hippocampus of the alcohol-naive sP than sNP rats (49%, p<0.05; A). A representative immunoblot is shown in figure B. In situ hybridization study demonstrated higher level of the CB1 receptor mRNA expression in the cingulate cortex (Cg; 24%, p<0.05) and CA1 (47%, p<0.01), CA2 (24%, p<0.05), CA3 (25%, p<0.05) and DG (14%, p<0.05) of hippocampus, and striatum (49%, p<0.01) of alcohol-naive sP than sNP rats (C and D).
Figure 3.
There was a significant higher level of AEA in the hippocampus (23%, p<0.05; A) and striatum (21%, p<0.05; A) of alcohol-naive sP than sNP rats. The level of 2-AG was found to be higher in the cerebral cortex (21%, p<0.05; B) and hippocampus (26%, p<0.05; B) of alcohol-naive sP than sNP rats. In situ hybridization analysis revealed a lower expression of mRNA of FAAH enzyme in the CA1 (37%, p<0.001), CA3 (26%, p<0.001) and DG (27%, p<0.001) of alcohol-naive sP than sNP rats (C and D).
Effect of voluntary alcohol consumption, alcohol withdrawal and “alcohol deprivation effect” in sP rats
In the acquisition experiment, all 8 sP rats had acquired alcohol drinking behavior by the 2nd day of exposure to the two-bottle choice regimen, as indicated by individual daily alcohol intakes exceeding 4 g/kg [i.e., the selection criterion adopted in the breeding program of sP rats (Colombo et al. 2006)]; daily alcohol intake averaged approximately 4 g/kg on the first day of exposure and rose progressively to 6–7.5 g/kg on continuing exposure. In the maintenance experiment (in which rats were exposed to alcohol for 60 consecutive days), alcohol drinking behavior was rapidly acquired by all rats; after acquisition, alcohol intake averaged 6–7 g/kg/day throughout the entire period of exposure. These data were comparable to those repeatedly recorded in sP rats exposed to alcohol and water under the two-bottle choice regimen (Colombo et al. 2006).
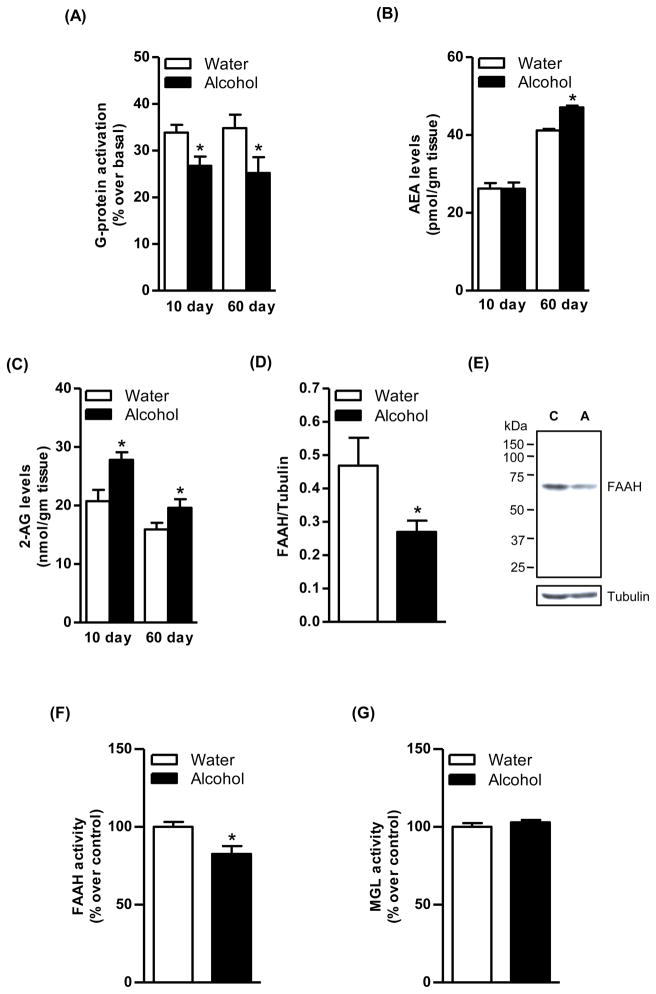
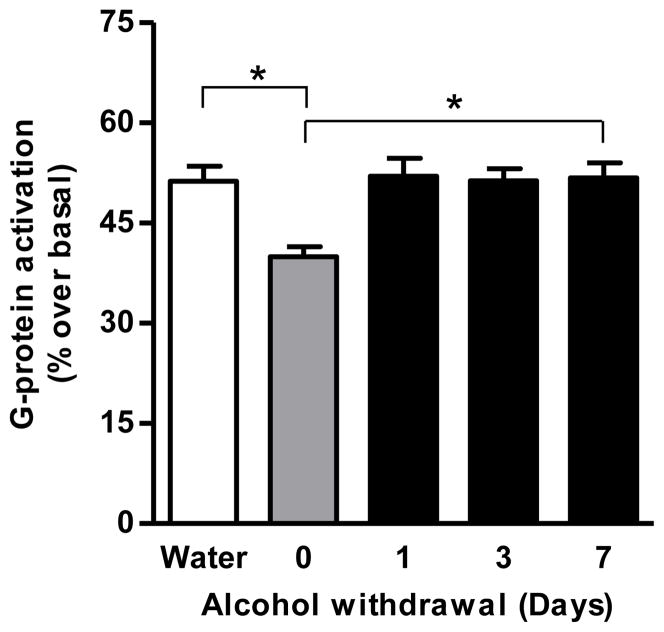
Agonist-stimulated [35S]GTPγS binding assay revealed a significant decrease in the CB1 receptor-mediated G-protein activation in the striatum of sP rats following acquisition of alcohol drinking for 10 days compared to the corresponding water control group (20%, p<0.05; Fig. 4A). This effect was further enhanced at the end of the 60-day maintenance phase of alcohol drinking (28%, p<0.05; Fig. 4A). In addition, alcohol drinking during the 10-day acquisition phase increased only 2-AG level (34%, p<0.01; Fig. 4C) whereas alcohol drinking during the 60-day maintenance phase elevated both AEA (15%, p<0.05; Fig. 4B) and 2-AG (24%, p<0.05; Fig. 4C) in the striatum of sP rats compared to control rats. Conversely, FAAH enzyme levels were significantly reduced in the striatum of sP rats following 60 days (39%, p<0.05; Fig. 4D) alcohol drinking compared to water group. A representative immunoblot is shown in figure 4E. Alcohol exposure for 60 days decreased the FAAH activity in the striatum of sP rats compared to water control rats (18%, p<0.05; Fig. 4F) without significant alterations in the MGL activity (Fig. 4G). A significant increase in CB1 receptor-mediated G-protein signaling was observed in the striatum following withdrawal from alcohol maintenance on day 1 (26%, p<0.05), day 3 (29%, p<0.05), and day 7 (30%, p<0.05; Fig. 5).
Figure 4.
The CB1 receptor-mediated G-protein activation was found to be significantly reduced in the striatum of sP rats exposed to the two-bottle “alcohol vs water” choice regimen for 10 consecutive days (20%, p<0.05; A) and 60 consecutive days (28%, p<0.05; A) compared to water control rats. Alcohol drinking during the acquisition phase increased 2-AG level (34%, p<0.01; C) whereas alcohol drinking during the maintenance phase elevated both AEA (15%, p<0.05; B) and 2-AG (24%, p<0.05; C) in the striatum compared to water control rats. Level of the FAAH enzyme was significantly reduced in the striatum of sP rats following alcohol drinking during the maintenance phase (39%, p<0.05; D). A representative immunoblot is shown in figure E (C; Control and A; Alcohol). Alcohol exposure for 60 days decreased the FAAH activity in the striatum of sP rats compared to water control rats (18%, p<0.05; F) without significant effect on the MGL activity (G).
Figure 5.
The downregulation of CB1 receptor-mediated G-protein signaling observed in the striatum of sP rats after chronic (60 day) alcohol drinking returned to normal levels following 1-, 3-, and 7-days of withdrawal from alcohol (26–30%, p<0.05).
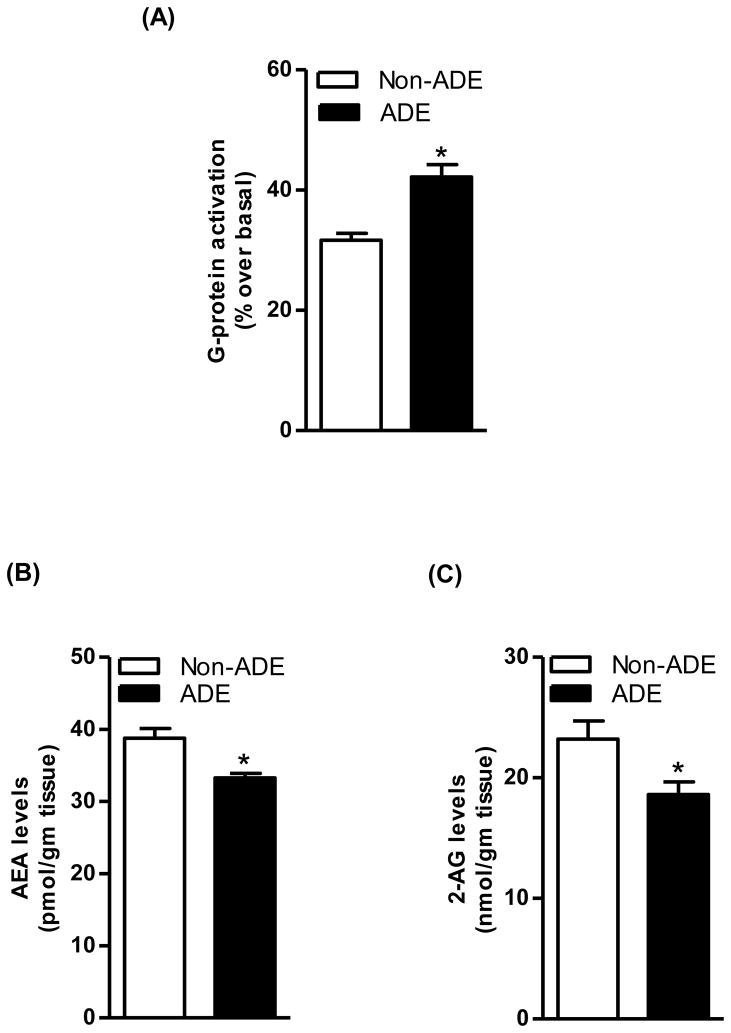
In the “alcohol deprivation effect” experiment, over the first hour of re-access alcohol intake averaged 0.70±0.04 and 1.06±0.07 g/kg in the alcohol-non deprived and -deprived rat groups, respectively (51%, p<0.0005; two-tailed Mann-Whitney test). This increase in alcohol intake following a period of deprivation reflects the “alcohol deprivation effect”, and is comparable to alcohol drinking behavior repeatedly observed in sP rats exposed to this alcohol deprivation protocol (Colombo et al., 2006). Moreover, “alcohol deprivation effect” was associated with an upregulation of CB1 receptor-mediated G-protein coupling in the striatum of sP rats, as shown by data collected in alcohol-deprived and -non deprived rats (25%, p<0.05; Fig. 6A). However, AEA levels (17%, p<0.05; Fig. 6B) and 2-AG (20%, p<0.05; Fig. 6C) were lower in alcohol-deprived than -non deprived rats.
Figure 6.
“Alcohol deprivation effect” resulted in upregulation of the CB1 receptor-mediated G-protein signaling in the striatum of sP rats, as shown by the comparison between alcohol-deprived (ADE) and -non deprived (Non-ADE) rat groups (25%, p<0.05; A). There was a significant reduction in the levels of AEA (17%, p<0.05; B) and 2-AG (20%, p<0.05; C) following “alcohol deprivation effect” in the striatum of sP rats, as shown by the comparison between alcohol-deprived and -non deprived rat.
Effect of rimonabant on the endocannabinoid system in the striatum of sP rats during acquisition and maintenance of alcohol drinking and “alcohol deprivation effect”
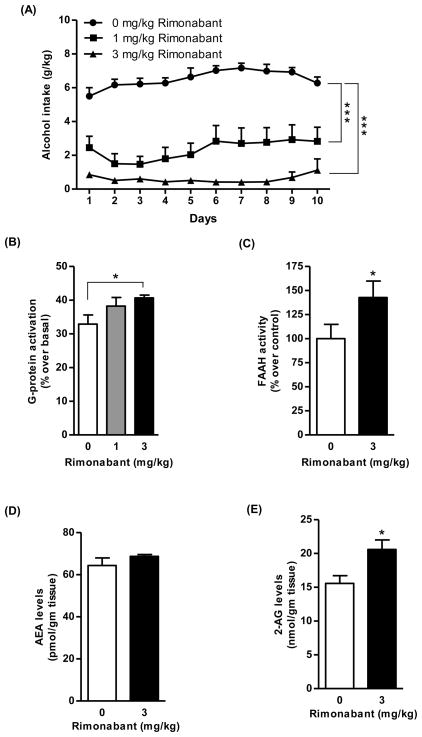
In the rat group exposed to alcohol for 10 days, all vehicle-treated rats rapidly acquired alcohol drinking behavior and their mean daily alcohol intake rose to 6–7 g/kg; treatment with rimonabant resulted in a dose-dependent reduction in daily alcohol intake (Fig. 7A) [Fdose (2,21)=58.14, p<0.0001; Fday (9,189)=2.65, p<0.01; Finteraction (18,189)=1.60, p>0.05]. Notably, in the rat group treated with 3 mg/kg rimonabant, mean daily alcohol intake was steadily lower than 1 g/kg/day, indicative of a complete blockade of acquisition of alcohol drinking behavior (Fig. 7A).
Figure 7.
Treatment with the CB1 receptor antagonist, rimonabant (1 and 3 mg/kg, i.p.) suppressed the acquisition of alcohol drinking behavior in sP rats (*p<0.0001 with respect to vehicle-treated rats; A) Treatment with rimonabant (3 mg/kg) during the acquisition phase of alcohol drinking led to an increase in the CB1 receptor-mediated G-protein activation (23%, p<0.05; B) and elevated the activity of FAAH enzyme (42%, p<0.05; C) in the striatum compared to vehicle-treated rats. Treatment with rimonabant alone to alcohol-naive sP rats significantly elevated 2-AG (32%, p<0.05; E) but not AEA (D) in the striatum compared to vehicle treated group.
Co-administration of rimonabant (3 mg/kg) led to a marked increase in CB1 receptor-mediated G-protein activation in the striatum of sP rats exposed to the acquisition phase for 10 days compared to vehicle treated group (23%, p<0.05; Fig. 7B). However, an elevation in FAAH activity was observed in the striatum of sP rats when rimonabant (3 mg/kg) was co-administered during alcohol acquisition phase compared to vehicle treated group (42%, p<0.05; Fig. 7C). Treatment of alcohol-naive sP rats with rimonabant per se led to a significant elevation in levels of 2-AG (32%, p<0.05; Fig. 7E) but not AEA (Fig. 7D) in the striatum compared to vehicle treated group.
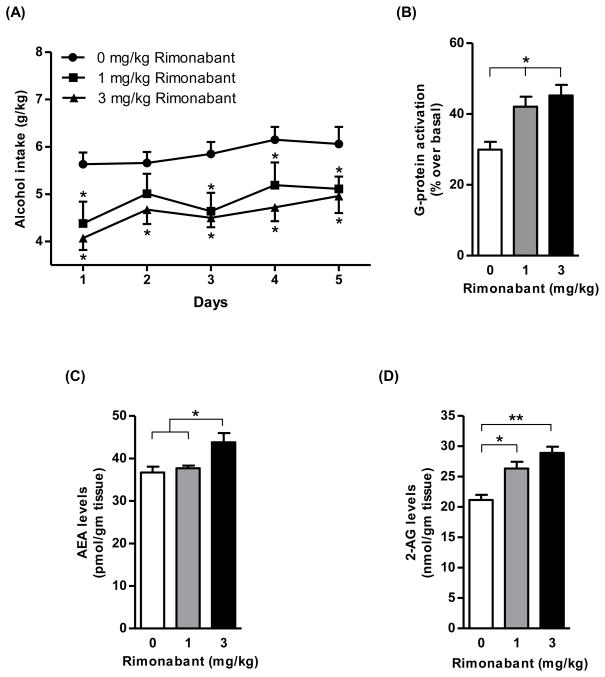
Treatment with rimonabant over the last 5 days of the 60-day maintenance phase resulted in a significant reduction in daily alcohol intake (Fig. 8A) [Fdose (2,21)=6.66, p<0.01, Fday (4,84)=4.84, p<0.005, Finteraction (8,84)=0.45, p>0.05]. On all five days of treatment, the magnitude of rimonabant-induced reduction in daily alcohol intake averaged 15–30%, with a relatively modest dose-dependence relationship (Fig. 8A). In this experiment, an upregulation of CB1 receptor-mediated G-protein activation was observed in the striatum of sP rats following co-administration of 1 mg (41%; p<0.05; Fig. 8B) or 3 mg/kg rimonabant (51%; p<0.01; Fig. 8B) compared to vehicle-treated rats. Treatment with rimonabant (3 mg/kg) during the alcohol maintenance phase resulted in elevation of AEA (20%, p<0.05; Fig 8C) and 2-AG (37%, p<0.01; Fig 8D) levels in the striatum of sP rats compared to vehicle-treated rat group.
Figure 8.
Treatment with rimonabant (1 and 3 mg/kg, i.p.) significantly reduced daily alcohol intake during the last 5 days of the maintenance phase of alcohol drinking in sP rats (*p<0.05 with respect to vehicle-treated rats; A). Treatment with rimonabant at 1 mg/kg (41%, p<0.05) and 3 mg/kg (51%, p<0.01) during the maintenance phase of alcohol drinking led to an enhancement of the CB1 receptor-mediated G-protein activation in the striatum in comparison to vehicle-treated rats (B). Treatment with 3 mg/kg rimonabant during the maintenance phase of alcohol drinking elevated the levels of AEA (20%, p<0.05; C) and 2-AG (37%, p<0.01; D) in the striatum in comparison to vehicle-treated rats.
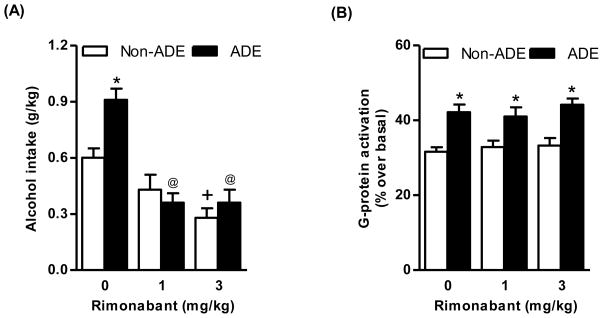
ANOVA revealed significant effects of both deprivation [F(1,54)=4.31, p<0.05] and treatment with rimonabant [F(2,54)=28.00, p<0.0001], as well as a significant interaction between the two factors [F(2,54)=4.87, p<0.05] on alcohol intake in the first hour of the post-deprivation phase. Alcohol intake was higher (approximately 50%) in vehicle-treated alcohol-deprived rats than in vehicle-treated alcohol-non deprived rats (Fig. 9A), indicative of development of the “alcohol deprivation effect” (Colombo et al. 2006). Post hoc analysis revealed that treatment with 3 mg/kg rimonabant reduced alcohol intake in alcohol-non deprived rats (Fig. 9A) and both doses of rimonabant suppressed the “alcohol deprivation effect”. Indeed, alcohol intake in both rimonabant-treated alcohol-deprived rat groups was significantly lower than that recorded in vehicle-treated alcohol-deprived rats (Fig. 9A). Rimonabant treatment produced no significant effect on increase in CB1 receptor-mediated G-protein coupling in the striatum induced by the “alcohol deprivation effect” (Fig. 9B).
Figure 9.
Treatment with rimonabant (1 and 3 mg/kg, i.p.) significantly suppressed “alcohol deprivation effect” in alcohol-deprived sP rats and alcohol intake in alcohol-non deprived sP rats (*p<0.01 with respect to alcohol-non deprived rats receiving 0 mg/kg rimonabant; +p<0.01 with respect to alcohol-non deprived rats receiving 0 mg/kg rimonabant; @p<0.0005 with respect to alcohol-deprived rats receiving 0 mg/kg rimonabant; A). “Alcohol deprivation effect” was associated with a significant increase in CB1 receptor-mediated G-protein activation in the striatum (25%, *p<0.05 with respect to alcohol-non deprived rats; B). Treatment with rimonabant did not alter increased CB1 receptor-mediated G-protein activation associated to “alcohol deprivation effect” (B).
DISCUSSION
The first aim of the present study was to examine whether the opposite alcohol preference and consumption displayed by sP and sNP rats was associated with innate differences in components of the brain endocannabinoid system. Accordingly, the density of CB1 receptor and levels of mCB1 receptors, CB1 receptor-mediated G-protein coupling, and endocannabinoids (AEA and 2-AG) were found to be higher in the brains of alcohol-naive sP compared to sNP rats. In addition, in situ hybridization analysis revealed a lower expression of mFAAH, the enzyme that degrades AEA, in sP rat brain. The changes observed may be capable of enhancing endocannabinoid-mediated CB1 receptor signaling, which is one of the potential mechanisms underlying an increased alcohol consummatory behavior in sP rats. This assumption is based on the fact that activation of the CB1 receptor enhances alcohol drinking behavior in rodents (Gallate 1999; Vinod et al. 2007; Vinod et al. 2008) including sP rats (Colombo et al. 2002). A correlation between age-dependent decrease in alcohol preference and decline in limbic CB1 receptor signaling (Wang et al. 2003) also supports this view. An elevation of AEA either through the genetic deletion or pharmacological inhibition of FAAH has also been shown to increase alcohol self-administration (Basavarajappa et al. 2006; Blednov et al. 2007; Vinod et al. 2008). A comparison of expression of endocannabinoid-related genes in alcohol-preferring (AA) and alcohol-non preferring (ANA) rats - selectively bred using the same breeding procedure, and selection criteria subsequently used to breed sP and sNP rats - revealed lower expression and activity of FAAH in the prefrontal cortex of AA rats (Hansson et al. 2007). On the contrary, a lower CB1 receptor expression and CB1 receptor-mediated G-protein coupling in the brain has been linked to a greater alcohol consummatory behavior in Fawn Hooded than in Wistar rats (Ortiz et al. 2004). Although the reason for this discrepancy is unclear, the use of different rat strains and co-existence of depressive-like behavior (Rezvani et al. 2002) in Fawn Hooded rats may potentially account for these changes. The endocannabinoid system in other reward-mediating brain areas in sP and sNP rats should be further investigated to better understand its role played in alcohol-related behaviors.
The second aim of this study was to examine the effect of various phases of alcohol drinking and withdrawal on the striatal endocannabinoid system in sP rats exposed to the homecage two-bottle “alcohol vs water” choice regimen. The effect of acquisition of alcohol drinking on the endocannabinoid system was examined in sP rats following exposure to alcohol for 10 consecutive days. This time frame features a period during which sP rats fully acquire alcohol drinking behavior, reaching pharmacologically relevant levels of alcohol intake. A reduction in CB1 receptor-mediated G-protein activation was observed in the striatum of sP rats following a 10-day exposure to alcohol compared to controls. To model an active drinking phase resembling human alcoholism and investigate possible changes in the endocannabinoid system, sP rats were exposed to alcohol for 60 consecutive days during which a steady alcohol consumption of approximately 6 g/kg daily was achieved. This paradigm led to a significant decrease in CB1 receptor-mediated G-protein activation in the striatum of sP rats. To assess alcohol withdrawal effects, following the exposure of sP rats to alcohol for 60 days, the latter was subsequently withdrawn for 1, 3, or 7 days. During the withdrawal phase, a reversal of the effect was manifested, leading to normalization of CB1 receptor-mediated G-protein activation. Similarly, “alcohol deprivation effect” (i.e., the transient increase in alcohol intake following a period of alcohol deprivation) resulted in upregulation of CB1 receptor-mediated G-protein signaling in the striatum of sP rats. The above attenuations were probably due to a reduction in endocannabinoid levels (Vinod et al. 2006).
The reduction observed in CB1 receptor-mediated G-protein coupling in the striatum secondary to alcohol consumption would appear to be a response to elevated endocannabinoid levels. This finding is in agreement with previous studies showing how chronic exposure to alcohol increases endocannabinoid content and concomitant downregulation of CB1 receptors in the limbic region (Gonzalez et al. 2002b, Ortiz et al. 2004, Vinod et al. 2006; Oliva et al. 2008). Notably, the reduction in CB1 receptor-mediated G-protein coupling is associated with decreased levels of FAAH. These findings are consistent with recent results obtained in the ventral striatum of alcoholic subjects post-mortem (Vinod et al. 2009) and an elevation in endocannabinoids in the brain of AA rats following long-term exposure to alcohol (Malinen et al. 2009). Accordingly, reduced FAAH activity might result in increased AEA levels in key brain areas linked to reward processes. Although there was no significant effect of alcohol maintenance on the activity of MGL, elevation in 2-AG level could be due to alterations in other metabolic enzymes.
Higher level of CB1 receptor-mediated G-protein activation has been demonstrated in the whole brain (Basavarajappa and Hungund, 2001) and striatum (Vinod et al. 2008) of alcohol-preferring C57BL/6J mice compared to alcohol non-preferring DBA/2J mice. Although the mechanism(s) underlying different alcohol drinking behavior in sP and sNP lines remain to be elucidated, the higher endocannabinoid-mediated CB1 receptor signaling observed in the striatum seems to play a critical role in determining susceptibility towards increased consumption of alcohol in sP rats. Conversely, blockade of CB1 receptor-mediated signaling by microinjection of rimonabant into the striatum (NAc) has been shown to suppress alcohol drinking behavior in AA rats (Malinen et al. 2008). In addition, alcohol intake has been shown to elevate limbic AEA levels (Weiss et al. 1994; Cheer et al. 2007; Gonzalez et al. 2002a; Hungund et al. 2003) leading to activation of the mesolimbic dopaminergic system (Gessa et al. 1998; Solinas et al. 2006; Malinen et al. 2008). This activation appears to be mediated by the CB1 receptor in the NAc (Hungund et al. 2003; Cheer et al. 2007). Thus, a functional interaction between the endocannabinoid and dopamine systems might be associated with the reinforcing effects of alcohol.
The third and last aim of the present study was to evaluate the effect of treatment with the prototypic CB1 receptor antagonist, rimonabant, and alcohol intake on the endocannabinoid system in the striatum of sP rats. Our findings confirm previous data suggesting that acute or repeated administration of rimonabant (a) prevented acquisition of alcohol drinking behavior (Serra et al. 2001), (b) reduced alcohol intake during the maintenance phase of alcohol drinking (Colombo et al. 1998), and (c) suppressed “alcohol deprivation effect” (Serra et al. 2002) in sP rats. These data are also in line with previous observations showing a reduction in alcohol consumption and preference in CB1 receptor knockout mice (Hungund et al. 2003; Poncelet et al. 2003; Wang et al. 2003; Naassila et al. 2004; Houchi et al. 2005; Thanos et al. 2005). In the present study, the suppressant effect of rimonabant on alcohol drinking behavior in sP rats was associated with changes in the endocannabinoid system. Interestingly, there was an elevation in the endocannabinoids by rimonabant treatment during alcohol maintenance. The attenuation of alcohol-induced decrease in CB1 receptor-mediated G-protein coupling appears to be due to neuroadaptation in response to the pharmacological blockade of endocannabinoid action on CB1 receptor. This suggests that both endocannabinoids and CB1 receptors are important in mediating alcohol drinking behavior. Similarly, alcohol withdrawal and “alcohol deprivation effect” resulted in an upregulation of CB1 receptor-mediated G-protein coupling in the striatum of sP rats. This could be due to a reduction of endocannabinoid levels, which had previously been raised following chronic alcohol intake. A single dose of rimonabant (1 or 3 mg/kg), however, did not affect increased CB1 receptor-mediated G-protein activation in alcohol-deprived rats. The acute dose of rimonabant likely produces a transient effect through blocking the action of endocannabinoids on the CB1 receptor during “alcohol deprivation effect”, whilst producing no significant effects on CB1 receptor levels. Taken together, these results reveal that alcohol consumption elevates endocannabinoids, while rimonabant reduces alcohol consumption through blockade of an endocannabinoid-mediated effect on CB1 receptors resulting in attenuation of alcohol-induced changes in the endocannabinoid system. Interestingly, rimonabant treatment per se for a period of 10 days produced an increase in 2-AG levels in the striatum of sP rats. A recent study reported a significant decrease in the functional activity of CB1 receptor following chronic treatment with rimonabant, without effect on the density of the CB1 receptor (Martin-Garcia et al. 2010). Further studies are necessary to assess whether rimonabant per se produces any effect on other components of the endocannabinoid system, and to provide further insight into the mechanism of action involved.
To conclude, the findings of the present study, conducted in an animal model mimicking the various stages of alcohol addiction, provide evidence to support the critical role played by the endocannabinoid system. Genetically determined differences in the endocannabinoid system might contribute towards the increased alcohol consummatory behavior displayed by sP rats. Moreover, alcohol-induced endocannabinoid release might be implicated in the rewarding effect of alcohol and the development of alcohol dependence. Alcohol exposure downregulates CB1 receptor-mediated G-protein signaling and alcohol withdrawal attenuates this effect in the striatum of sP rats. Co-treatment with rimonabant reversed alcohol-induced dysfunctions in the endocannabinoid system. The sP rat appears to be an interesting model for use in studying the mechanisms underlying the involvement of the endocannabinoid system in alcohol-related behaviors. These findings therefore suggest the importance of the endocannabinoid system in the development of excessive alcohol drinking, constituting a potential therapeutic target for the treatment of alcohol-related behaviors.
Acknowledgments
This study was supported by grants from NIH (AA015525) to BLH and RETICS (Red de Trastornos Adictivos; RD06/0001/1004, Spain) to JM. MSG was supported by a fellowship from the Spanish Ministry of Health. We thank Patricia Rodriguez and Analía Rico for excellent technical assistance and Carla Acciaro for animal breeding and care.
Footnotes
Authors Contribution
The authors KYV, BLH and GC contributed to the study concept and design. MP, MAMC, and GC were involved in in vivo studies. KYV, MSG, JM, SX, TBC and TF contributed to neurochemical studies. KYV, BLH, JM, MAMC and GC provided comments on the draft. All authors critically reviewed content and approved the final version of the manuscript for publication. There is no conflict of interest to declare.
References
- 1.Alvarez-Jaimes L, Polis I, Parsons LH. Regional influence of cannabinoid CB1 receptors in the regulation of ethanol self-administration by Wistar rats. Open Neuropsychopharmacol J. 2009;2:77–85. doi: 10.2174/1876523800902020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnone M, Maruani J, Chaperon F, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacol. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 3.Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacol. 2006;7:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Blednov Y, Cravatt BF, Boehm SL, II, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: Studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacol. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- 5.Cheer JF, Wassum KM, Sombers LA, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo G, Agabio R, Fa M, et al. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol and alcoholism. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 7.Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL. Different sensitivity to ethanol in alcohol-preferring sP and -nonpreferring sNP rats. Alcohol Clin Exp Res. 2000;24:1603–8. [PubMed] [Google Scholar]
- 8.Colombo G, Vacca G, Serra S, Carai MAM, Gessa GL. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR 141716, on alcohol’s motivational properties in alcohol-preferring rats. Eur J Pharmacol. 2004;498:119–123. doi: 10.1016/j.ejphar.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 9.Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–38. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- 10.Femenia T, García-Gutiérrez MS, Manzanares J. CB1 receptor blockade decreases ethanol intake and associated neurochemical changes in fawn-hooded rats. Alcohol Clin Exp Res. 2010;34:131–41. doi: 10.1111/j.1530-0277.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 11.Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–82. [PubMed] [Google Scholar]
- 12.Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacol. 1999;142:302–8. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- 13.Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 14.George DT, Herion DW, Jones CL, et al. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacol. 2010;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002a;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB1 receptor binding and mRNA levels. Drug Alcohol Depend. 2002b;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- 18.Hansson AC, Bermudez-Silva FJ, Malinen H, et al. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacol. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- 19.Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacol. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- 20.Howlett AC. Cannabinoid receptor signaling. Handbook Exp Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 21.Hungund BL, Basavarajappa BS, Vadasz C, et al. Ethanol, Endocannabinoids And Cannabinoidergic Signaling System. Alcohol Clin Exp Res. 2002;26:565–574. [PubMed] [Google Scholar]
- 22.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 Receptor Knockout Mice Exhibit Markedly Reduced Voluntary Alcohol Consumption and Lack Alcohol-Induced Dopamine Release in the Nucleus Accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 23.Maccioni P, Fantini N, Carai MAM, Gessa GL, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, rimonabant, on alcohol self-administration in alcohol-preferring rats. Open Neuropsychopharmacol J. 2009;2:40–44. [Google Scholar]
- 24.Maccioni P, Colombo G, Carai MAM. Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets. 2010;9:55–59. doi: 10.2174/187152710790966623. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–32. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Malinen H, Hyytia P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–83. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 27.Malinen H, Lehtonen M, Hyytia P. Modulation of brain endocannabinoid levels by voluntary alcohol consumption in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2009;33:1711–20. doi: 10.1111/j.1530-0277.2009.01008.x. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Garcia E, Burokas A, Martín M, et al. Central and peripheral consequences of the chronic blockade of CB1 cannabinoid receptor with rimonabant or taranabant. J Neurochem. 2010;112:1338–13351. doi: 10.1111/j.1471-4159.2009.06549.x. [DOI] [PubMed] [Google Scholar]
- 29.Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacol. 2004;46:243–53. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Oliva JM, Ortiz S, Pérez-Rial S, Manzanares J. Time dependent alterations on tyrosine hydroxylase, opioid and cannabinoid CB1 receptor gene expressions after acute ethanol administration in the rat brain. Eur Neuropsychopharmacol. 2008;18:373–82. doi: 10.1016/j.euroneuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz S, Oliva JM, Perez-Rial S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcoholism. 2004;39:88–92. doi: 10.1093/alcalc/agh036. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz S, Oliva JM, Pérez-Rial S, Palomo T, Manzanares J. Differences in basal cannabinoid CB1 receptor function in selective brain areas and vulnerability to voluntary alcohol consumption in Fawn Hooded and Wistar rats. Alcohol Alcohol. 2004;39:297–302. doi: 10.1093/alcalc/agh063. [DOI] [PubMed] [Google Scholar]
- 33.Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–8. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- 34.Rezvani AH, Overstreet DH, Cleves M, Parsian A. Further genetic characterization of the fawn-hooded (FH Wjd) rat, an animal model of comorbid depression and alcoholism. Psychiatr Genet. 2007;17:77–83. doi: 10.1097/YPG.0b013e328012d7c3. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, MN Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Acta Pharmacol Sin. 1999;20:1109–1114. [PubMed] [Google Scholar]
- 36.Serra S, Carai MAM, Brunetti G, et al. The cannabinoid receptor antagonist SR 141716 prevents acquisition of drinking behaviour in alcohol-preferring rats. Eur J Pharmacol. 2001;430:369–371. doi: 10.1016/s0014-2999(01)01379-6. [DOI] [PubMed] [Google Scholar]
- 37.Serra S, Brunetti G, Pani M, et al. Blockade by the cannabinoid CB1 receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol. 2002;443:95–97. doi: 10.1016/s0014-2999(02)01594-7. [DOI] [PubMed] [Google Scholar]
- 38.Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- 39.Soyka M, Koller G, Schmidt P, et al. Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trial. J Clin Psychopharmacol. 2008;28:317–24. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- 40.Spanagel R. How to measure relapse in animals. In: Spanagel R, Mann K, editors. Drugs for Relapse Prevention of Alcoholism. Birkäuser Verlag; Basel: 2005. pp. 13–21. [Google Scholar]
- 41.Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:233–43. doi: 10.1111/j.1471-4159.2007.04956.x. 2007 J. Neurochem. [DOI] [PubMed] [Google Scholar]
- 43.Vinod KY, Yalamanchili R, Thanos PK, et al. Genetic and pharmacological manipulations of the CB1 receptor alter ethanol consumption and dependence in ethanol-preferring and ethanol non-preferring mice. Synapse. 2008;62:574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vinod KY, Yalamanchili R, Shan X, Cooper T, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Nat Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]