Abstract
Boron delivery characteristics of cis and trans isomers of a boronated unnatural amino acid, 1-amino-3-boronocyclopentanecarboxylic acid (ABCPC) were tested in B16 mouse model for human melanoma. Both ABCPC isomers delivered comparable boron to B16 melanoma tumor cells as L-p-boronophenylalanine (BPA). Secondary ion mass spectrometry (SIMS) analysis revealed the presence of boron throughout the tumor from these compounds, and a near homogeneous distribution between the nucleus and cytoplasm of B16 cells grown in vitro. These encouraging observations support further studies of these new boron carriers in BNCT.
Keywords: Boron neutron capture therapy, unnatural amino acids, SIMS, imaging mass spectrometry, boronophenylalanine, boron imaging in single cells
1. Introduction
The development of new and more effective tumor-selective boron carriers than sodium borocaptate (BSH) and boronophenylalanine (BPA) would significantly improve the efficacy of boron neutron capture therapy of cancer (Barth et al. 2005; Kabalka et al., 2006; Li et al., 2006; Zhu et al., 2010). Kabalka et al. (2004 & 2009) reported that a class of boronated unnatural cyclic amino acids had enhanced in vitro and in vivo tumor selectivity, which potentially could be far superior to BPA and BSH. One of these amino acids, 1-amino-3-boronocyclopentanecarboxylic acid (ABCPC), attained a tumor to blood ratio (T:Bl) of 8 and a tumor to normal brain ratio (T:Br) of ~ 21 in a murine melanoma model (Kabalka et al., 2004). ABCPC initially was synthesized and tested as a mixture of racemic diasteromers (cis and trans isomers) along with each of their enantiomers. Further separation of ABCPC into single enantiomers might result in compounds with enhanced selectivity for tumor cells. This study evaluates the biodistribution of cis and trans isomers of ABCPC (as racemic mixtures of L- and D- forms) in the B16 mouse model for human melanoma. Since localization of boron atoms within the nucleus results in more favorable radiobiologic microdosimetry for the 10B(n,α)7Li capture reaction (Kobayashi and Kanda, 1982; Gabel et al., 1987), we have also employed secondary ion mass spectrometry (SIMS) to study the subcellular localization of boron atoms.
2. Materials and Methods
Boron biodistribution studies in B16 mouse model for human melanoma
We have synthesized and separated the two racemic diasteriomers of ABCPC (cis and trans isomers) containing a mixture of L and D enantiomers (Kabalka et al., 2009). These compounds are water soluble and were dissolved directly in phosphate buffered saline (PBS) for studies in the B16 mouse model for human melanoma. L-p-boronophenylalanine (BPA) in the form of a fructose complex was used for a comparison of boron-delivery characteristics to ABCPC compounds.
Female BALB/c mice were injected subcutaneously with 106 B16 melanoma cells. After 8–10 days when the tumors reached a diameter of ~ 1cm, biodistribution studies were initiated. Compounds were administered intraperitoneally (i.p.) to the tumor bearing mice. The dose of each compound was equivalent to 24 mg boron/kg body weight (b.w). Mice were euthanized 2.5 hr post-injection by exposure to isofluorane following which they were bled. The tumor, liver and kidneys were collected for boron determination by means of inductively coupled plasma-optical emission spectroscopy (ICP-OES). The selection of 2.5 hr time interval between administration and euthanization was based on BPA’s optimal localization in tumor and blood concentrations in another melanoma model (Matalka et al., 1993). For SIMS studies of boron imaging, the tumor and adjacent muscle tissues were frozen and cryo-sectioned at 4 μm. The sections were attached to silicon wafers, freeze-dried, and sputter coated with a 10 Å layer of Au/Pd for enhancing their electrical conductivity for SIMS analysis with a CAMECA IMS-3f ion microscope instrument (Chandra et al., 2000).
In vitro boron imaging studies of cis and trans isomers of ABCPC with SIMS
B16 melanoma cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum, supplemented with L-glutamine and antibiotics. When the cells reached approximately 70% confluency, they were exposed to the nutrient medium containing 50 ppm boron equivalent of the racemic mixture (both L- and D-forms) of either cis or trans isomers of ABCPC for 2.5 hr. Both cis and trans isomers were soluble in the nutrient medium but a slight adjustment of the pH was required to restore the pH to 7.4. After a 2.5 hr. exposure to the test compound, the cells were cryogenically prepared with a freeze-fracture method and freeze-dried for quantitative SIMS imaging (Chandra et al., 1986; Chandra, 2010).
3. Results and Discussion
Boron biodistribution studies in B16 melanoma mouse model
ICP-OES data shown in Table 1 reveal that both ABCPC compounds delivered boron concentrations to tumor cells that were equivalent to that of BPA. Although the mean boron concentrations in the blood of animals were higher for ABCPC compounds than BPA, these differences were not statistically significant (p < 0.05). Hepatic uptake in animals that received BPA had significantly less boron (p = 0.01) than those that received cis-ABCPC. No significant differences between the compounds were observed for boron concentrations in the kidney. In general, these observations indicate that the ABCPC compounds seem to be comparable to BPA in delivering boron to tumor cells but their blood clearance (or metabolism in the liver) may be somewhat longer than that of BPA.
Table 1.
Biodistribution of boron from BPA and ABCPC compounds in B16 melanoma mouse model.
| Compound | n | Boron concentration (μg/g tissue) (mean ± SD) |
|||
|---|---|---|---|---|---|
| Blood | Tumor | Liver | Kidney | ||
| BPA | 4 | 5.1 ± 2.7 | 19.6 ± 5.5 | 5.5 ± 3.7a | 15.1 ± 12.4 |
| Trans-ABCPC | 6 | 9.3 ± 4.1 | 21.3 ± 8.9 | 15.1 ± 11.7 | 17.1 ± 9.3 |
| Cis-ABCPC | 4 | 9.9 ± 3.3 | 26.5 ± 4.9 | 18.9 ± 8.1b | 30.3 ± 20.2 |
Different superscript letters “a” and “b” indicate statistically significant difference in liver boron concentrations between the compounds as determined by means of Student’s t-test.
SIMS imaging of subcellular boron distribution in B16 melanoma mouse model
To determine the microdistribution of boron within the tumor, we analyzed tumor tissues prepared for SIMS imaging. The CAMECA IMS-3f SIMS instrument used in this study is capable of imaging the distribution of any elements from H to U (via isotopic detection) at 500 nm spatial resolution with ppm to ppb sensitivity. Figure 1 shows B16 tumor morphology in a cryosection that was stained with hematoxylin & eosin (H&E). The tumor was composed of a monomorphic population of cells with large, hyperchromatic nuclei and cytoplasmic melanin. SIMS analysis of an adjacent cryosection shows typical observations of boron distribution for both cis and trans ABCPC compounds in B16 tumor cells (Fig. 2). The positive secondary ion images of 39K and 11B show the potassium and boron distributions in tumor cells. In 39K SIMS image, some tumor cell nuclei are discernible. The boron from ABCPC compounds is distributed throughout the tumor with some degree of heterogeneity. Quantitative observations from SIMS images of boron distribution in the tumor tissue revealed that there were no significant differences between BPA and the ABCPC compounds (not shown).
Figure 1.

H&E stained section of the B16 melanoma.
Figure 2.
SIMS images revealing the distribution of potassium-39 and boron-11 atoms in a B16 mouse melanoma tumor tissue section from trans-ABCPC treated animals.
SIMS boron imaging of individual B16 melanoma cells grown in culture
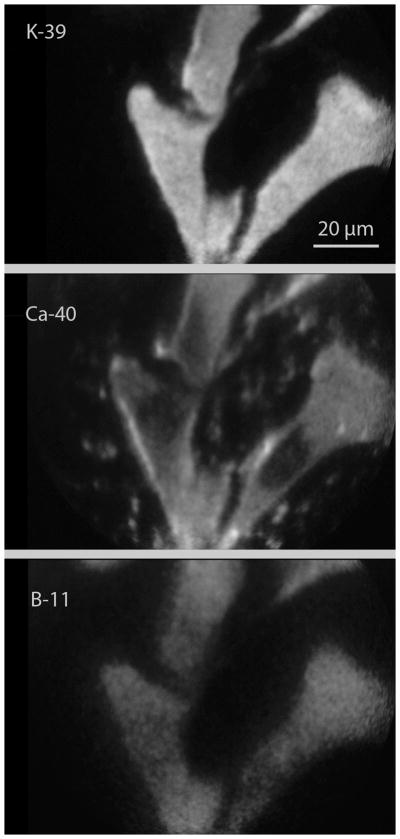
The B16 cells grown in vitro served as a useful model for SIMS imaging studies for observing the subcellular distribution of boron in single cells delivered by cis or trans ABCPC compounds. Figure 3 shows a typical example of boron distribution imaged by SIMS in B16 cells after 2.5 hr. exposure to cis or trans ABCPC compounds. Figure 3 shows SIMS imaging analysis of the same three B16 melanoma cells reveal the subcellular distribution of 39K, 40Ca, and 11B in cis-ABCPC treated cells. These cells had high-K and low-Na signatures (23Na image not shown) representing viable tumor cells. In the 40Ca SIMS image, the location of the cell nucleus is discernible in each cell due to its lower total calcium content in comparison to the cell cytoplasm which contains the calcium storing organelle, endoplasmic reticulum. The 11B SIMS image reveals the subcellular distribution of 11B atoms delivered by cis-ABCPC to individual cells in the field of view. The 11B is distributed throughout the cell, including the nucleus. No significant differences were observed in boron delivery to the nucleus (or cytoplasm) of B16 melanoma cells between the cis-ABCPC and the trans-ABCPC compounds. The boron partitioning of approximately 4:1, between the cell interior to the nutrient medium, was observed after 2.5 hr. exposure of cis or trans ABCPC compounds.
Figure 3.
SIMS images revealing the subcellular distribution of potassium, calcium, and boron atoms in B16 melanoma cells grown in cultures. The cells were treated with 50 ppm boron equivalent concentration of cis-ABCPC compound for 2.5 hrs.
4. Conclusions
Boronated unnatural amino acids are a class of compounds that are currently under development as potential delivery agents for BNCT. This study provides support for previously published data (Kabalka et al., 2009) suggesting that further studies with these compounds are warranted. Observations indicate that cis or trans ABCPC compounds, even when administered as racemic mixtures of their enantiomers (L and D isomers), are comparable to BPA in delivering boron to B16 melanoma cells both in vitro and in vivo. Separation of the L and D isomers of these compounds may provide even better boron targeting of tumor cells. The water solubility of these compounds is a valuable feature for their potential use as delivery agents for BNCT. Studies are underway for testing these compounds in the F98 rat and GL261 mouse glioma models.
Acknowledgments
This study was funded by a NIH grant R01CA129326 (GWK, RFB, SC). Cornell SIMS Laboratory (PI- S. Chandra) is affiliated with New York State Foundation for Science, Technology, and Innovation (NYSTAR). Asha Duhan and Syed A. Haider are acknowledged for their help in processing of images.
References
- Barth RF, Coderre JA, Vicente MGH, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- Chandra S. Quantitative imaging of chemical composition in single cells by secondary ion mass spectrometry: cisplatin affects calcium stores in renal epithelial cells. Methods Mol Biol. 2010;656:113–130. doi: 10.1007/978-1-60761-746-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Morrison GH, Wolcott CC. Imaging intracellular elemental distribution and ion fluxes in cultured cells using ion microscopy: A freeze-fracture method. J Microsc. 1986;144:15–37. doi: 10.1111/j.1365-2818.1986.tb04670.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Smith DR, Morrison GH. Subcellular imaging by dynamic SIMS ion microscopy. Anal Chem. 2000;72:104A–114A. doi: 10.1021/ac002716i. [DOI] [PubMed] [Google Scholar]
- Gabel D, Foster S, Fairchild RG. The Monte Carlo simulation of the biological effect of the 10B(n,α)7Li reaction in cells and tissue and its implications for boron neutron capture therapy. Radiat Res. 1987;111:14–25. [PubMed] [Google Scholar]
- Kabalka GW, Wu ZZ, Yao ML, Natarajan N. The synthesis and in vivo biodistribution of novel boronated unnatural amino acids. Appl Radiat Isotopes. 2004;61:1111–1115. doi: 10.1016/j.apradiso.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Yao M-L. The synthesis and use of boronated amino acids for boron neutron capture therapy. Anti-cancer Agents Med Chem. 2006;6:111–125. doi: 10.2174/187152006776119144. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Yao M-L, Marepally SR, Chandra S. Biological evaluation of boronated unnatural amino acids as new boron carriers. Appl Radiat Isot. 2009;67:S374–S379. doi: 10.1016/j.apradiso.2009.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kanda K. Analytical calculations of boron-10 dosage in cell nucleus for neutron capture therapy. Radiat Res. 1982;91:77–94. [PubMed] [Google Scholar]
- Li T, Hamdi J, Hawthorne MF. Unilamellar liposomes with enhanced boron content. Bioconjugate Chem. 2006;17:15–20. doi: 10.1021/bc0501350. [DOI] [PubMed] [Google Scholar]
- Matalka KZ, Bailey MQ, Barth RF, et al. Boron neutron capture therapy of intracereberal melanoma using borono-phenylalanine as a capture agent. Cancer Res. 1993;53:3308–3313. [PubMed] [Google Scholar]
- Zhu Y, Lin Y, Zhu YZ, et al. Boron drug delivery via encapsulated magnetic nanocomposites: A new approach for BNCT in cancer treatment. J Nanomaterials. 2010:8. doi: 10.1155/2010/409320. Article ID 409320. [DOI] [Google Scholar]