Abstract
Introduction
Transforming growth factor-beta 1(TGF-β1) is a regulatory protein, involved in bone fracture healing. Circulating TGF-β1 levels have been reported to be a predictor of delayed bone healing and non-union, suggesting active relationship between tissue and circulating TGF-β1 in fracture healing. The purpose of this study was to analyse TGF-β1 local and serum concentrations in fracture healing to further contribute to the understanding of molecular regulation of fracture healing.
Patients and methods
Serum samples of 113 patients with long bone fractures were collected over a period of 6 months following a standardised time schedule. TGF-β1 serum concentrations were measured using ELISA. Patients were assigned to 2 groups: Group 1 contained 103 patients with physiological healing. Group 2 contained 10 patients with impaired healing. Patients in both groups were matched. One patient of the group 2 had to be excluded because of missing match partner. In addition, fracture haematoma from 11 patients of group 1 was obtained to analyse local TGF-β1 concentrations. 33 volunteers donated serum which served as control.
Results
TGF-β1 serum concentrations increased during the early healing period and were significantly higher in patients with physiological healing compared to controls (P = 0.04). Thereafter, it decreased continuously between weeks 2 and 8 and fell again after week 8. TGF-β1 serum concentrations in patients with physiological healing were significantly higher at week 24 compared to controls (P = 0.05). In non-unions, serum concentrations differed significantly from those of controls at week 6 (P = 0.01). No significant difference in between patients with physiological and impaired fracture healing was observed. Fracture haematoma contained significantly higher TGF-β1 concentrations than peripheral serum of the patients (P = 0.017).
Conclusion
Elevated levels of TGF-β1 in haematoma and in serum after bone fracture especially during the entire healing process indicate its importance for fracture healing.
Keywords: TGF-β1, Bone fracture, Non-union, Delayed union, Human, Serum concentration
Introduction
Fracture healing is a unique process that leads to bone regeneration. This complex process has been intensively investigated during the last ten years. Many studies have focused on the roles of cytokines and growth factors in fracture healing.1–4 More than fifty cytokines, angiogenic factors, proteases and morphogens with significant roles in fracture healing, have been described.2 However, despite intensive research most of the regulation mechanisms are not well understood.1 Evidence exists that the local and systemic concentrations of certain cytokines are increased during fracture healing.2,4–6 TGF-β1, a member of TGF-β family, is a regulatory protein involved in bone remodelling and fracture healing.7–9 TGF-β1 plays a pivotal role in the process of fracture healing10–17 as it enhances the proliferation and differentiation of mesenchymal stem cells (MSCs), increases the production of extracellular matrix and is chemotactic on bone cells.18 It has a key role on the promotion of cartilage formation and increases the formation of callus and bone strength.10,17 TGF-β1 has been shown to have a stimulating effect on bone healing in several animal studies.11–16 For example systemic application of TGF-β1 seems to enhance the bone remodelling in rabbits with bone defect11 and local application of TGF-β1 has been shown to accelerate fracture healing.19 The presence of TGF-β1 in callus has been reported in human and animal fracture models.5,19–23 In addition, evidence exists that serum concentrations of TGF-β1 are increased during the process of bone healing.4 Circulating TGF-β1 levels were found to be a predictor of delayed bone healing and non-union suggesting active relationship of its circulating levels to fracture healing process.4 To our knowledge, only few data exist on systemic and local measurement of TGF-β1 with regard to fracture healing in humans so far. The aim of our study was to analyse the local and systemic levels of TGF-β1 expression after bone fracture in patients with physiological and impaired fracture healing for better understanding of the role of this cytokine in the process of human fracture healing.
Patients and methods
This study was approved by the Ethic Committee of the Medical University of Vienna and performed in accordance with the ethical standards in the Declaration of Helsinki. A consecutive series of 113 patients with meta-/diaphyseal fractures of long bone (humerus, femur, lower leg and forearm) treated surgically at our institution between April 2006 and April 2008, were included. Patients gave informed consent to be enrolled in the study, and were 18–90 years old. Exclusion criteria were open fractures type III according to the Gustilo classification, multiple fractures, previous bone operations, pre-existing bone diseases except for osteoporosis, renal/liver insufficiency, malignant tumours, long term steroid treatment, immunosuppression and long term treatment with non-steroidal anti-inflammatory drugs. Patients were assigned in 2 groups according to their course of fracture healing. The first group contained 103 patients (male n = 50, female n = 53, mean age: 54.2 ± 20.4) with normal fracture healing. Ten patients with impaired fracture healing (delayed- or non-union) belonged to the second group. Three of the 10 patients developed a hypertrophic type of delayed union. Seven patients developed an atrophic type of delayed union. Demographics presented in Table 1. The diagnosis of bony consolidation or delayed union was based on exercise-induced pain and conventional X-rays or computed tomography. Delayed union was defined as failed fracture healing without radiological signs of bony consolidation after 4 months postoperatively. Non union was defined as the absence of complete consolidation at 6 months after surgery. A corresponding patient with normal fracture healing and a healthy control was matched to each patient with delayed fracture healing. Table 1 presents the demographics of patients and the matching criteria. One of the 10 patients with delayed fracture healing had to be excluded, because no corresponding matching partner with adequate fracture healing could be found in our study cohort. Therefore, 9 patients with impaired and 9 patients with normal fracture healing were included in the final analyses.
Table 1.
Matching criteria and demographics of the matched patients from both groups.
| No |
Sex |
Agea |
Fracture typeb |
Location |
Soft-tissue damagec |
Fixation |
Sex |
Age |
Fracture type |
Location |
Soft-tissue damage |
Fixation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physiological healing | Impaired healing | |||||||||||
| 1 | M | 42 | 42-A | Tibia/fibula | 0 | Screw/plate | F | 52 | 42-A | Tibia/fibula | 0 | Screw/plate |
| 2 | M | 23 | 32-A | Femur | 0 | Nail | M | 24 | 32-A | Femur | 0 | Nail |
| 3 | M | 59 | 42-A | Tibia/fibula | 0 | Nail | M | 63 | 42-A | Tibia/fibula | 0 | Nail |
| 4 | M | 32 | 13-C | Humerus | 0 | Plate | M | 42 | 13-C | Humerus | 0 | Plate |
| 5 | M | 50 | 11-A | Humerus | 0 | Nail | M | 55 | 11-A | Humerus | 0 | Plate |
| 6 | M | 66 | 31-A | Femur | 0 | Nail | F | 82 | 31-A | Femur | 0 | Nail |
| 7 | M | 70 | 42-A | Tibia/fibula | 0 | External fixator | M | 63 | 42-A | Tibia/fibula | 0 | External fixator |
| 8 | F | 57 | 42-C | Tibia | II° | External fixator | M | 53 | 42-C | Tibia | II° | External fixator |
| 9 | F | 38 | 42-A | Tibia | I° | Nail | F | 37 | 42-A | Tibia | I° | Nail |
Age (±10 years).
According to ASIF classification.
Extent of soft tissue damage according to Gustilo classification.
In addition, fresh fracture haematoma was obtained from 11 patients of group 1 intra-operatively to analyse local TGF-β1 concentrations. Furthermore, 33 healthy volunteers (16 males, 17 females, mean age: 37.1 ± 11.65 years) donated one blood sample as control.
All patients were followed up for at least six months after the operation. The follow up examination was based on clinical and radiological examination at 1, 2, 4, 6, 8, 12, 24 weeks after trauma.
Blood samples
Peripheral venous blood was obtained from each patient at 1, 2, 4, 6, 8, 12, 24 weeks after surgery and stored at −80 °C until analysis. TGF-β1 serum concentration was measured in 11 patients immediately after trauma at hospital admission. Each control individual donated one blood sample. Fracture haematoma was obtained at surgery. Haematoma was removed manually before any manipulation or irrigation, avoiding contamination by blood in the operating field, and placed in sterile containers. These specimens were centrifuged immediately and the resulting supernatant was stored at −80 °C until assayed.
Measurement of TGF-β1
TGF-β1 concentrations were measured by a commercially available antibody (Quantikine, RD Systems, Minneapolis, MN, USA) in enzyme-linked immuno sorbent assay (ELISA). All analytical steps were performed according to the manufacturer's recommended protocol. The TGF-β1 assay detects specifically the biologic active form of the protein. Concentrations are presented as mean of duplicate measurements. To avoid interassay variability, samples of the corresponding matching partner were analysed with the same assay. The comparison of the measurements utilising different Kits for the same time points of the study measurements indicates the low range of variability of the assays.
Statistical analysis
Comparisons between groups of continuous variables were performed by using non-parametric ANOVA (Wilcoxon rank-sum test for two variables or Kruskal–Wallis-Test for more than two variables). For statistical comparison of a serum value at a certain time point between the non-union group and the matched unions nonparametric Mann–Whitney U test for unpaired samples were used. Statistical analyses were performed using the SAS system for Windows, v 9.1 and the Enterprise Guide, v 4.1 (SAS Institute, Inc., Cary, NC). Data are presented as means ± SEM. The statistical significance level was set at P < 0.05.
Results
TGF-β1 serum concentrations in patients with physiological and impaired fracture healing
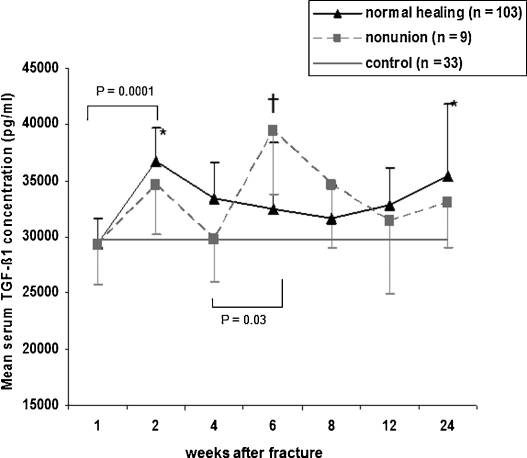
TGF-β1 serum concentration immediately after injury (measured in 11 patients) was 14,171.2 ± 5642.66 pg/ml. TGF-β1 serum concentrations were at a minimum level at 1 week (29,178.0 ± 1364.29 pg/ml) and increased to reach a maximum level (36,334.0 ± 1688.3 pg/ml) at 2 weeks after trauma (P = 0.0001). Serum concentrations decreased continuously after week 2 and reached another minimum concentration (31,932.4 ± 1397.0 pg/ml) at week 8 after trauma. After week 8, a continuous increase of the TGF-β1 serum concentrations was observed. A second peak of the TGF-β1 serum concentration was observed at week 24 after trauma (35,267.7 ± 2220.3 pg/ml) (Fig. 1). In patients with impaired fracture healing TGF-β1 serum concentrations were at a minimum level (27,339.7 ± 2973.45 pg/ml) at week 1 and increased to reach a first peak at week 2 (34,265.8 ± 4337.3 pg/ml), which was followed by a clear decline at week 4 after trauma (27,939.6 ± 3327.7 pg/ml). Between week 4 and 6 a significant increase of the TGF-β1 serum concentrations were observed (P = 0.03). TGF-β1 serum concentrations were highest at week 6 after fracture (43,294.1 ± 4949.5 pg/ml). Thereafter, a continuous decline of the serum levels was observed for the rest of the observation period (Fig. 1).
Fig. 1.
TGF-β1 serum concentrations (mean ± SEM) in controls and in patients with long-bone fractures and physiological (unions) or impaired fracture healing (non-unions). Asterisks indicate significant differences in TGF-β1 concentrations between unions and controls (P = 0.04 at week 2 and P = 0.05 at week 24). †Significant differences in TGF-β1 concentrations between non-unions and controls (P = 0.01).
Comparison of TGF-β1 serum concentrations in patients with normal/impaired healing
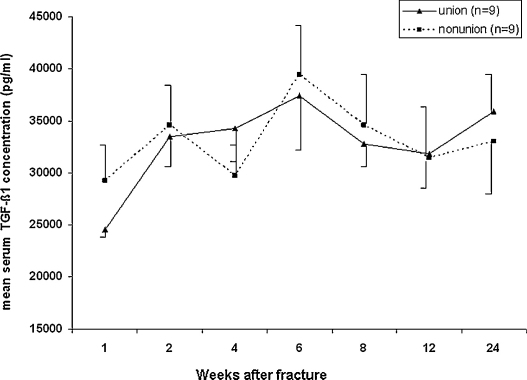
Comparison of TGF-β1 serum concentrations between the matched patients with normal and impaired healing revealed no statistically significant difference for the entire observation period (Fig. 2).
Fig. 2.
Time course of TGF-β1 concentrations (mean ± SEM) in matched patients with physiological (unions) or impaired fracture healing (non-unions) after surgery for long-bone fractures.
Comparison of TGF-β1 serum concentrations between controls and patients
Comparison of TGF-β1 serum concentrations of patients with normal fracture healing and controls (29,735.3 ± 1328.4 pg/ml) revealed significant differences at weeks 2 (P = 0.04) and 24 (P = 0.05). At these time points significantly higher TGF-β1 concentration were observed in patients with normal healing compared to controls. Comparison between the TGF-β1 serum concentrations of patients with impaired fracture healing and controls showed significantly higher TGF-β1 serum concentrations in patients with impaired healing at week 6 (P = 0.01).
To exclude that the differences in TGF-β1 serum levels between the patients and the controls are related to the age difference between the both groups (mean age: 35.6 vs. 54.2) an additional analysis with an age matched group was performed. This analysis revealed no age related difference in the TGF-β1 serum level between both matched groups and confirmed the reported results.
Comparison of TGF-β1 concentration in fracture haematoma and in serum of patients
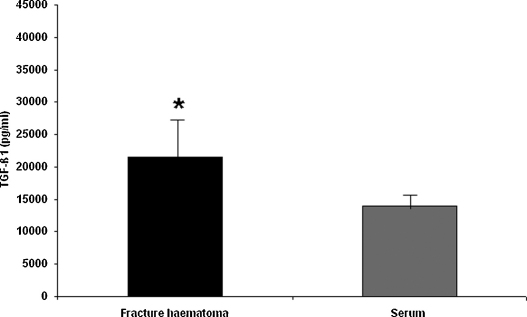
Mean TGF-β1 concentration measured in fracture haematoma was 28,157 ± 6282.6 pg/ml. Mean TGF-β1 serum concentrations was 14,171.2 ± 2132.7 pg/ml. Fracture haematoma contained significantly higher TGF-β1 concentration than serum (P = 0.017) (Fig. 3).
Fig. 3.
TGF-β1 concentrations (mean ± SEM) in fracture haematoma and serum of patients. Asterisk indicates significant difference in TGF-β1 concentrations (P = 0.017).
Discussion
Various studies demonstrated that fracture repair is not a local process but is rather associated with systemic reactions that might partly be attributable to the uptake of bioactive molecules from the fracture site.12,24,25 In the present study, local and systemic concentrations of TGF-β1 in patients with long bone fractures were analysed to elucidate the role of this osteogenic cytokine in the bone healing process. Previous studies showed characteristic alterations in serum concentrations of numerous enzymes and growth factors during fracture healing.3–5,26 Consistently, we found significant alterations in local and systemic distribution of TGF-β1 at certain time points after fracture of long bones. TGF-β1 concentrations in fracture haematoma were significantly higher than serum concentrations within the first hours after trauma indicating local release of TGF-β1 during the immediate response in concert with previous studies.2,4,8,16,25 Our data show a considerable fluctuation of the systemic TGF-β1 concentration within the first weeks of fracture healing. Whilst the mean post-traumatic serum TGF-β1 level in those 11 patients with immediate post-traumatic measurement was 14,171 pg/ml, the mean TGF-β1 serum concentration of the rest of the patients as well as half of the control-baseline of the healthy volunteers was almost twofold high at one week after trauma. Due to the fact that TGF-β1 is released from granulas of platelets during the clotting process, high local TGF-β1 concentrations in fracture haematoma of our patients do not appear surprisingly.27 An earlier study showed that TGF-β1 is released by platelets into the fracture haematoma, and then synthesised by osteoblasts and chondrocyts throughout the healing process.27 This explains the following increase of serum TGF-β1 concentrations within the first 2 weeks after trauma in our patients. Increased expression of TGF-β1 as well as other cytokines early after fracture was reported in other studies.6,28–32 This increase may partly be attributable to the absorption of cytokines from the fracture site into the circulation.12,33,34 On the other hand, the significant increase of serum TGF-β1 concentrations together with other cytokines such as PDGF, VEGF and M-CSF might indicate a systemic response to fracture. Supporting influence of systemic parameters on bone formation is well known and was demonstrated in previous studies.35,36 Maximum serum TGF-β1 concentrations during the intramembranous bone formation phase might give evidence for the chemotactic effect of TGF-β1 on bone cells. It is well known that an increasing number of osteoblasts, chondroblasts and immature progenitor cells invade the fracture area during the phase of intramembranous bone formation.33 Moreover, TGF-β1 is reported to stimulate bone formation by inducing differentiation of subperosteal mesenchymal cells into osteoblasts,9,37 which synthesise and release TGF-β1 by themselves at the proliferations stage and again exert stimulating effects on osteoblasts in an autocrine fashion.8,38,39
TGF-β1 concentrations started to decrease and reached a plateau between weeks 4 and 8. We believe that this continuous decrease of TGF-β1 serum concentration might be due to the increasing gain of mechanical stability of the fracture, as suggested by other clinical studies.3,33,34 For the later course, our data demonstrate that TGF-β1 serum concentrations in patients with bone fractures remain elevated during the remodelling phase; and this seems to be necessary to activate osteoblasts during the remodelling phase. On the other hand, osteoblasts activate TGF-β1 during the remodelling phase which might explain significantly high TGF-β1 serum concentration at week 24.40 TGF-β1 expression pattern observed in our patients is in agreement with previous animal and human studies.4,30 Moreover, the expression pattern of TGF-β1 was very similar to that of M-CSF and VEGF. In previously published studies, our group observed an almost identical course of TGF-β1 levels compared to M-CSF for the entire observation period and a very similar course compared to VEGF until week 8 after fracture.31,32 These findings indicate that not only the local presence of the osteogeneic growth factors but also their systemic presence is necessary to support fracture healing. Therefore, we suggest that whenever osteogenic growth factors are clinically or experimentally utilised for the enhancement of fracture healing, they should be used locally and systemically.
Another question addressed in our study was whether TGF-β1 expression differs in patients with impaired fracture healing from those with physiological fracture healing. Since previous studies showed decreased serum concentrations of TGF-β1 with increasing age and in females6 we generated 2 homogenous groups with 9 patients to reduce the influence of treatment modalities, gender and age. Therefore, to each patient with impaired fracture healing a patient with physiological fracture healing was matched. As previously mentioned continuous decline of TGF-β1 serum concentrations during the plateau phase in patients with normal fracture healing was assumed to be caused by an increase of the mechanical stability of the fracture. Elevated TGF-β1 serum concentrations at week 6 in patients with impaired healing reflects the opposite course compared to normally healed patients and might be due to a lack of mechanical stability at that time. TGF-β1 serum concentrations of both groups were very similar for the rest of the observation period in our study. In contrast to results reported by Zimmermann et al4 we observed no significant differences in the TGF-β1 concentrations of patients with physiological and disturbed fracture healing. To exclude the only possible explanation for this discrepancy another analysis, i.e. only in patients with atrophic type of non union, was performed. However, the results did not reveal a significant difference.
Finally, this study provides prospectively collected data on systemic levels of TGF-β1 over the entire period of fracture healing in a large collective of patients and data on local TGF-β1 concentrations in a smaller collective of patients with physiological fracture healing which may contribute to the understanding of molecular regulation of fracture healing. One limitation of this study is the small number of patients with impaired fracture healing. However, strictly chosen matching criteria enabled us to compare the data of patients with impaired fracture healing with those who had physiological healing.
Elevated levels of TGF-β1 in haematoma and in serum after bone fracture indicate its involvement in the human fracture healing. Significant differences in TGF-β1 levels of patients with physiological and impaired fracture healing could not be observed. Definitely further studies with higher number of patients with impaired fracture healing are needed to clarify the role of TGF-β1 in fracture healing.
Conflict of interest statement
All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence this work.
Acknowledgements
This study was supported by grants from the Lorenz Böhler Foundation and the Austrian Science Fund (FWF grant #: P19188-B09) to K. Sarahrudi.
Footnotes
Authors indicate that they have no financial relationship with the organization that sponsored the research. They further state that they have full control of all primary data and that they agree to allow the journal to review their data if requested. This study was approved by the Ethic Committee of the Medical University of Vienna and performed in accordance with the ethical standards in the Declaration of Helsinki.
Contributor Information
Kambiz Sarahrudi, Email: kambiz.sarahrudi@meduniwien.ac.at.
Anita Thomas, Email: anita.thomas@meduniwien.ac.at.
Mehdi Mousavi, Email: mehdi.mousavi@wienkav.at.
Georg Kaiser, Email: georg.kaiser@meduniwien.ac.at.
Julia Köttstorfer, Email: julia.koettstorfer@meduniwien.ac.at.
Mathias Kecht, Email: mathias.kecht@meduniwien.ac.at.
S. Hajdu, Email: Stefan.hajdu@meduniwien.ac.at.
S. Aharinejad, Email: seyedhossein.aharinejad@meduniwien.ac.at.
References
- 1.Einhorn T.A. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77:940–956. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 3.Weiss S., Baumgart R., Jochum M., Strasburger C.J., Bidlingmaier M. Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res. 2002;17:1280–1289. doi: 10.1359/jbmr.2002.17.7.1280. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann G., Henle P., Kusswetter M. TGF-beta1 as a marker of delayed fracture healing. Bone. 2005;36:779–785. doi: 10.1016/j.bone.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Giannoudis P.V., Pountos I., Morley J., Perry S., Tarkin H.I., Pape H.C. Growth factor release following femoral nailing. Bone. 2008;42:751–757. doi: 10.1016/j.bone.2007.12.219. [DOI] [PubMed] [Google Scholar]
- 6.Street J.T., Wang J.H., Wu Q.D., Wakai A., McGuinness A., Redmond H.P. The angiogenic response to skeletal injury is preserved in the elderly. J Orthop Res. 2001;19:1057–1066. doi: 10.1016/S0736-0266(01)00048-1. [DOI] [PubMed] [Google Scholar]
- 7.Bostrom M.P. Expression of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res. 1998:S116–123. doi: 10.1097/00003086-199810001-00013. [DOI] [PubMed] [Google Scholar]
- 8.Joyce M.E., Jingushi S., Bolander M.E. Transforming growth factor-beta in the regulation of fracture repair. Orthop Clin North Am. 1990;21:199–209. [PubMed] [Google Scholar]
- 9.Joyce M.E., Roberts A.B., Sporn M.B., Bolander M.E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes G.L., Kostenuik P.J., Gerstenfeld L.C., Einhorn T.A. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805–1815. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 11.Beck L.S., Amento E.P., Xu Y. TGF-beta 1 induces bone closure of skull defects: temporal dynamics of bone formation in defects exposed to rhTGF-beta 1. J Bone Miner Res. 1993;8:753–761. doi: 10.1002/jbmr.5650080614. [DOI] [PubMed] [Google Scholar]
- 12.Gazit D., Karmish M., Holzman L., Bab I. Regenerating marrow induces systemic increase in osteo- and chondrogenesis. Endocrinology. 1990;126:2607–2613. doi: 10.1210/endo-126-5-2607. [DOI] [PubMed] [Google Scholar]
- 13.Lind M., Schumacker B., Soballe K., Keller J., Melsen F., Bunger C. Transforming growth factor-beta enhances fracture healing in rabbit tibiae. Acta Orthop Scand. 1993;64:553–556. doi: 10.3109/17453679308993691. [DOI] [PubMed] [Google Scholar]
- 14.Park S.H., O’Connor K.M., McKellop H. Interaction between active motion and exogenous transforming growth factor Beta during tibial fracture repair. J Orthop Trauma. 2003;17:2–10. doi: 10.1097/00005131-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Schmidmaier G., Wildemann B., Heeger J. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone. 2002;31:165–172. doi: 10.1016/s8756-3282(02)00798-6. [DOI] [PubMed] [Google Scholar]
- 16.Tieline L., Puolakkainen P., Pohjonen T., Rautavuori J., Tormala P., Rokkanen P. The effect of transforming growth factor-beta1, released from a bioabsorbable self-reinforced polylactide pin, on a bone defect. Biomaterials. 2002;23:3817–3823. doi: 10.1016/s0142-9612(02)00105-9. [DOI] [PubMed] [Google Scholar]
- 17.Wildemann B., Schmidmaier G., Ordel S., Stange R., Haas N.P., Raschke M. Cell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-beta1: comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res B Appl Biomater. 2003;65:150–156. doi: 10.1002/jbm.b.10512. [DOI] [PubMed] [Google Scholar]
- 18.Bauer D.C., Rosen C., Cauley J., Cummings S.R. Low serum IGF-1 but not IGFBP-3 predicts hip and spine fracture: the study of osteoporotic fracture. Bone. 1998;23:561. [Google Scholar]
- 19.Blumenfeld I., Srouji S., Lanir Y., Laufer D., Livne E. Enhancement of bone defect healing in old rats by TGF-beta and IGF-1. Exp Gerontol. 2002;37:553–565. doi: 10.1016/s0531-5565(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 20.Bostrom M.P., Lane J.M., Berberian W.S. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13:357–367. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- 21.He X.B., Lu W.Z., Tang K.L. [Effects of bone morphogenetic protein and transforming growth fractor-beta on biomechanical property for fracture healing in rabbit ulna] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2003;17:185–188. [PubMed] [Google Scholar]
- 22.Si X., Jin Y., Yang L., Tipoe G.L., White F.H. Expression of BMP-2 and TGF-beta 1 mRNA during healing of the rabbit mandible. Eur J Oral Sci. 1997;105:325–330. doi: 10.1111/j.1600-0722.1997.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura Y., Nomura S., Kawasaki S., Tsutsumimoto T., Shimizu T., Takaoka K. Colocalization of noggin and bone morphogenetic protein-4 during fracture healing. J Bone Miner Res. 2001;16:876–884. doi: 10.1359/jbmr.2001.16.5.876. [DOI] [PubMed] [Google Scholar]
- 24.Einhorn T.A., Simon G., Devlin V.J., Warman J., Sidhu S.P., Vigorita V.J. The osteogenic response to distant skeletal injury. J Bone Joint Surg Am. 1990;72:1374–1378. [PubMed] [Google Scholar]
- 25.Nunamaker D.M. Experimental models of fracture repair. Clin Orthop Relat Res. 1998:S56–65. doi: 10.1097/00003086-199810001-00007. [DOI] [PubMed] [Google Scholar]
- 26.Weiss S., Zimmermann G., Baumgart R., Kasten P., Bidlingmaier M., Henle P. Systemic regulation of angiogenesis and matrix degradation in bone regeneration – distraction osteogenesis compared to rigid fracture healing. Bone. 2005;37:781–790. doi: 10.1016/j.bone.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Joyce M.E., Jingushi S., Scully S.P., Bolander M.E. Role of growth factors in fracture healing. In: Barbule A., Caldwell M.D., Eaglstein W.H., editors. Clinical and Experimental Approaches to Dermal and Epidermal Repair: Normal and Chronic Wounds. Wiley-Liss; 1991. pp. 391–416. [Google Scholar]
- 28.Dimitriou R., Tsiridis E., Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Henle P., Zimmermann G., Weiss S. Matrix metalloproteinases and failed fracture healing. Bone. 2005;37:791–798. doi: 10.1016/j.bone.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Lammens J., Liu Z., Aerssens J., Dequeker J., Fabry G. Distraction bone healing versus osteotomy healing: a comparative biochemical analysis. J Bone Miner Res. 1998;13:279–286. doi: 10.1359/jbmr.1998.13.2.279. [DOI] [PubMed] [Google Scholar]
- 31.Sarahrudi K., Mousavi M., Thomas A., Eipeldauer S., Vecsei V., Pietschmann P. Elevated levels of macrophage colony-stimulating factor in human fracture healing. J Orthop Res. 2010;28:671–676. doi: 10.1002/jor.21048. [DOI] [PubMed] [Google Scholar]
- 32.Sarahrudi K., Thomas A., Braunsteiner T., Wolf H., Vecsei V., Aharinejad S. VEGF serum concentrations in patients with long bone fractures: a comparison between impaired and normal fracture healing. J Orthop Res. 2009;27:1293–1297. doi: 10.1002/jor.20906. [DOI] [PubMed] [Google Scholar]
- 33.Einhorn T.A. The science of fracture healing. J Orthop Trauma. 2005;19:S4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 34.Holbein O., Neidlinger-Wilke C., Suger G., Kinzl L., Claes L. Ilizarov callus distraction produces systemic bone cell mitogens. J Orthop Res. 1995;13:629–638. doi: 10.1002/jor.1100130420. [DOI] [PubMed] [Google Scholar]
- 35.Maes C., Carmeliet P., Moermans K. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 36.Tatsuyama K., Maezawa Y., Baba H., Imamura Y., Fukuda M. Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur J Histochem. 2000;44:269–278. [PubMed] [Google Scholar]
- 37.Mueller M., Schilling T., Minne H.W., Ziegler R. A systemic acceleratory phenomenon (SAP) accompanies the regional acceleratory phenomenon (RAP) during healing of a bone defect in the rat. J Bone Miner Res. 1991;6:401–410. doi: 10.1002/jbmr.5650060412. [DOI] [PubMed] [Google Scholar]
- 38.Oursler M.J., Riggs B.L., Spelsberg T.C. Glucocorticoid-induced activation of latent transforming growth factor-beta by normal human osteoblast-like cells. Endocrinology. 1993;133:2187–2196. doi: 10.1210/endo.133.5.8404670. [DOI] [PubMed] [Google Scholar]
- 39.Stein G.S., Lian J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993;14:424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 40.Silver I., Murrills R., Etherington D. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]