Abstract
Cellular senescence was historically discovered as a form of cellular ageing of in vitro cultured cells. It has been under the spotlight following the evidence of oncogene-induced senescence in vivo and its role as a potent tumour suppressor mechanism. Presently, a PubMed search using keywords ‘cellular senescence and cancer’ reveals 8398 number of references (by April 2011) showing that while our knowledge of senescence keeps expanding, the complexity of the phenomenon keeps us – researchers in the field of cancer biology – fascinated and busy. In this short review, we summarise the many cellular pathways leading to cellular senescence and we discuss the latest experimental evidence and the questions emerging in the field.
Keywords: Senescence, Oncogenes, Telomeres, Reactive oxygen species, Tumour suppressor pathways
1. Introduction
1.1. The evolution of cellular senescence concept
In the early 1960s, Leonard Hayflick reported that primary human fibroblasts isolated from embryonic lung tissues cease to proliferate after a limited number of population doublings (PD 50 ± 10).1 This maximum proliferative lifespan of fibroblasts is still known as the Hayflick limit, and we use the term replicative cellular senescence, coined by Hayflick, to describe this cellular condition of ceased proliferation. Since then, for the last five decades, the original definition of cellular senescence has withstood the test of time and the subject has become more and more relevant in the contexts of ageing and cancer.
The loss of cell division potential and change in morphology of human lung and skin fibroblasts were primarily proposed as a model for ageing at the cellular level.2 Later, what Hayflick described as cellular ‘ageing’ became equally relevant to understand the events that follow oncogene activation and lead to cellular transformation.3,4 We now know that, in addition to reflecting the loss of normal tissue homoeostases as a consequence of ageing, cellular senescence acts as a natural tumour suppressor mechanism.
2. Pathways triggering the senescence programme
2.1. Telomeres: The end-replication problem appears to be more complicated than the length
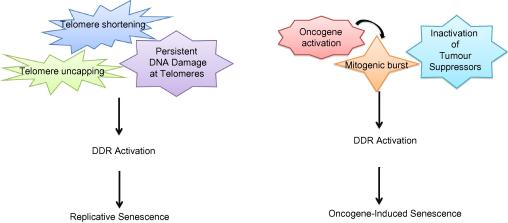
Telomeres, the physical ends of linear chromosomes, are composed of a variable number of 5′-TTAGGG-3′ repeats in vertebrates.5 These long tracts of double-stranded DNA sequences are followed by the presence of single-stranded repeats termed as G-overhangs. This structure is crucial in terms of end-protection of telomeres, since G-overhangs can serve as a primer for telomerase and contribute to the formation of a structure called T-loop (for telomere loop) which results from the invasion of G-overhang into duplex region of telomere.6 This structure together with a number of proteins that make up the so-named shelterin complex7 provides a protection to chromosome ends by blocking Homologous Recombination (HR) and Non-Homologous End Joining (NHEJ) DNA repair machineries to distinguish them from DNA double strand breaks (DSBs), as well as blocking Ataxia Telangiectasia Mutated (ATM) signalling.8–11 When telomeres shorten below a critical length they lose the functions of the shelterin complex and thus they are recognised as DSBs12 and activate the DNA damage response pathway (DDR).13 Shelterin protein complex binds and protects the chromosome ends from DDR. It also coordinates telomere maintenance by telomerase. Telomere uncapping that results from deregulation of components of the shelterin complex also initiates a DDR.14 It will be interesting to investigate whether the expression, the stability or functions of individual components of the shelterin complex are altered in physiologically relevant processes. Indeed there is already some evidence of their alteration being detected in some tumours.15
Cellular senescence can be induced by a number of exogenous or endogenous stresses and historically telomere shortening was proposed to be the main mechanism leading to replicative cellular senescence establishment.16 The inability of standard DNA polymerases to fully replicate linear DNA molecules causes the estimated loss of around 50 to 200 base pairs during each cycle of replication at the 5′ end of the lagging strand.16 Hence, the end-replication problem causes gradual shortening of chromosomes at every DNA replication round and subsequent cell division, eventually leading to DDR activation at critically short telomeres.12,13 Therefore, replicative senescence was proposed as a terminal arrest of cells with critically short telomeres lengths, thus acting as a tumour suppressive mechanism to prevent cells from being immortal. However, the exact critical length of such dysfunctional telomeres is still not fully clear, while it is clear instead that it varies widely in different species ultimately confounding our understanding of what ultimately triggers DDR activation and senescence establishment.
A causative link between DDR signalling at individual telomeres and senescence was analysed using a single-cell detection method to detect upstream DDR events and cell cycle checkpoint in HDFs at a near-senescent stage.13 Here, a significant inverse correlation between BrdU incorporation and the presence of one or few telomere dysfunction-induced foci (TIFs) was observed. These data suggest that even a single telomere-associated DNA damage event is sufficient to induce a long-term growth arrest. As the persistence of these foci seems to be the inherent feature of senescence-inducing DNA damage, this begs the question of why this DNA damage is not repaired. Answering this question will provide the essential molecular mechanism that establishes DNA damage-induced cellular senescence and ultimately why they senesce.
The realisation that telomeres are fragile sites,17 hard to replicate chromosomal regions, unveils an additional layer of complexity and suggests that telomere shortening and DDR activation may occur also during standard telomeric DNA replication. Ataxia Telangiectasia and Rad3-related protein (ATR) involved in resolving replicational stress has an important role in the suppression of telomere fragility and recombination.18,19
A recent study on DNA adducts has indicated that telomeres are more sensitive to the formation of UV-induced cyclobutane pyrimidine dimers (CPD).20 Telomeres somehow tolerate these CPDs and nucleotide excision repair is prevented at telomeres.20 The rate of oxidative DNA damage has also been correlated with the amount of telomere loss during successive rounds of DNA replication.21,22 A study explored this further in a mouse model carrying a deletion of the 8-oxoguanine DNA glycosylase (Ogg1) gene, involved in the elimination of oxidised bases via base excision repair. As telomeric repeats, being rich in guanine, are prone to oxidative damage, Ogg1-deficient mice have increased oxidative guanine lesions in telomeres in vivo and display accelerated telomere shortening under high oxygen conditions (20%) without impacting on the enzymatic activity of the telomerase.23 A further impact of the oxidative stress on telomere regulation is the nuclear export of telomerase, which has been shown to be induced by high levels of oxidative stress.24
Therefore, it appears that additional events, and not only progressive telomere shortening caused by the inherent limits of the DNA replication machinery, may contribute to telomere shortening or DNA damage generation at telomeres, with consequent replicative senescence establishment.
2.2. Oncogene activation – opening Pandora’s box
Upon exposure to various oncogenic stimuli normal mammalian cells can respond by activating DNA damage response (DDR) pathway. This response may commit cells to programmed cell death (apoptosis) in some cases25 or may induce them to enter cellular senescence.26 Hence, in addition to the first observation of cellular senescence, which was the in vitro replicative exhaustion of human fibroblasts, other diverse forms of cellular stress also lead to a cell condition in which the cells remain alive and metabolically active, yet irreversibly arrested. Oncogene-induced cellular senescence (OIS), also called premature senescence, was first observed in normal fibroblasts by the ectopic overexpression of oncogene H-RASG12V expression.26 This apparently telomere-independent type of senescence is shared with other mutant forms of RAS family proto-oncogenes and its downstream effectors. Mutations leading to the activation of oncogenes in K-RASG12V,27 BRAFV600E,28 or inactivation of the tumour suppressors, such as in PTEN29 and NF130 can trigger cellular senescence in vitro and in vivo in a variety of host tissues. Further studies into these key observations lead to the proposal of DDR activation as a critical barrier to tumourigenesis, by forcing cells to stop their aberrant proliferation.31,32 At the molecular level, OIS is regulated by two major tumour suppressor pathways, p53 and Rb. However, the requirements and dependency of these key players of both pathways, and their relative contributions seem to be dependent on the type of stress and cellular context.
Although the activation of DDR by oncogene-induced hyper-replication has been shown by some groups as the key mediator of oncogene-induced senescence,31,32 other reports underline the independency of DDR in terms of senescence induction by some leukemogenic fusion proteins, such as BCR-ABL and CBFB-MYH11.33 Here, p38 kinase pathway followed by p16 induction plays a crucial role in the commitment to senescence. These data indicate that multiple pathways may be involved in senescence. While involvement of the p53 axis in human fibroblasts through the activation of DDR pathway has been well-demonstrated, in murine cells both ARF and p53 appear to be important in OIS establishment as inactivation of either p53 or ARF bypasses senescence in murine systems. Thus different mechanisms of senescence establishment and maintenance may make different contributions in different species. Murine models remain one of the best systems to prove causality of the mechanisms that in human samples can often only be proposed. However, mice retain their own specificities that occasionally must be taken into account. As an example, it is possible that the contribution of ARF in OIS may obscure the role of DDR in senescence control. Indeed, ATM in some murine models seems to display a more limited role in Ras-induced senescence compared to human systems.34 Intriguingly however, ATR suppression seems to synergise with endogenous levels of oncogenic k-Ras in the induction of a variety of tumours.35 So, it is possible that ATR is an important genetic determinant of senescence establishment and tumour suppression in mice.
The role of DDR in OIS in mouse model was probably most formidably challenged in a mouse model in which senescence is triggered by PTEN loss.36 In this system, while inactivation of single allele triggers enhanced proliferative rates, inactivation of both alleles determines abrupt senescence establishment. This occurs in the absence of DNA replication. Most importantly, DDR activation, mainly the ATM branch, is below detection. Crucially, ATM inactivation fails to rescue senescence. Although at face value these results seem to indicate that indeed OIS can be established in the absence of DDR signalling, and seem to challenge the causative role of DDR genes in OIS control, it is worth considering that ATR, rather than ATM, is likely to be the most acutely sensitive DDR kinase and may be engaged by a very early DNA replication origin firing event (which may expose DNA replication origins and single-stranded DNA even in the absence of actual DNA replication) caused by a strong mitogenic event such as PTEN loss. This hypothesis is consistent with the observation that PTEN-loss induced senescence may be rescued by a TOR inhibitor (rapamycin). Such a compound has recently been demonstrated to counteract ATR signalling in yeast.37 We thus propose that the present evidence does not yet exclude the possibility of the engagement of some branches of DDR pathways in PTEN-loss induced senescence.
A hallmark of senescent cells is the condensation of chromatin. OIS cells display senescence-associated heterochromatin foci (SAHF), which have been proposed to repress proliferative E2F-target genes38 involving lysine 9 methylation of histone H3 and complex formation with HP1 at their promoters. Initial work linked SAHFs primarily to p16/pRB functions, later studies have shown that the efficiency of SAHF formation can be dependent on p53.39 What comes as a surprise is a later stage event in SAHF development, the translocation of HIRA to PML bodies, which has been suggested to be neither p53 nor RB dependent.39 We have recently observed that SAHF are markers of oncogene activation (and likely oncogene-induced DNA replication stress) rather than specific markers of the senescence conditions. Indeed both cultured cells and human tumours can proliferate and display proliferative markers despite SAHF formation.40 This suggests that SAHF may have additional, still unanticipated functions, independent from their ability to control proliferation and expression of proliferative genes as initially proposed.38
The senescence condition is also associated with the activation of the expression of a number of inflammation-associated genes.41–43 Indeed, senescent cells have a robust secretory activity, known as a senescence-associated secretory phenotype (SASP), which has been put in relation to persistent DDR signalling.44 Senescent cells secreting matrix metalloproteinases and inflammatory cytokines alter the surrounding tissue structure and some of them, such as interleukin-6 (IL-6) and IL-8, can reinforce the senescence phenotype in an autoparacrine manner,42,43 while at the same time also stimulate their clearance by the immune system.45 This, however, is at odds with the demonstrated role of SASP in stimulating the proliferation of transformed cells.46 Probably, these observations can be reconciled in the ‘senescent cells: good citizens bad neighbours’ paradigm proposed by Judith Campisi.41
2.3. Reactive oxygen species – a cause or a consequence?
Reactive Oxygen Species (ROS) have been linked to cellular ageing since the postulation of ‘The Free Radical Theory of ageing’,47 based on the idea that free radicals may cause damage, including DNA damage, leading to mutations, cancer and ageing. The production of ROS is an inevitable biochemical consequence of oxygen metabolism, which is essential for the life of aerobic species. Antioxidant systems, via both enzymatic (superoxide dismutases, catalases, peroxidases) and non-enzymatic (small molecules like vitamin C, glutathione) defenses, maintain a controlled balance against oxidative stress within the cell by conversion of such oxidants into harmless more reduced molecular species. It has been proposed that both the rates of telomere shortening and replicative senescence can be modulated by simply modifying the amount of oxidative stress,48 which leads to DNA breaks accumulating at telomeres.
ROS have been suggested to induce cellular senescence by causing direct oxidative DNA damage.49–51 However, ROS are also known to positively modulate cell proliferation and act as second messenger molecules involved in mitogenic signal transduction.52,53 As an example, NAPDH oxidases are membrane-associated enzymes that produce superoxide (O2−) and/or hydrogen peroxide (H2O2), implicated in regulation of cytoskeletal remodelling, gene expression, proliferation, differentiation, migration and cell death.54–56 Superoxide is chemically one electron reduced form of oxygen. The chemical conversions of highly reactive superoxide species to a less reactive but more soluble hydrogen peroxide make it easier to penetrate through membranes quickly working as a secondary messenger signalling molecule.57
Depending on the concentration, exogenous H2O2 was shown to affect positively or negatively cells’ growth and cell cycle kinetics.58 Growth factors stimulate the production of ROS59 and expression of oncogenic Ras also has been demonstrated to induce ROS generation.52 Indeed, H2O2 can mimic the activity of growth factors.60 The dual role of ROS in DNA damage inducers and mitogenic mediators is further complicated by the recent indication that ROS control DDR signalling.61 Indeed, using a systems biology approach, it was shown that there is a dynamic feedback loop, which is triggered by DDR and p21, leading to mitochondrial dysfunction and ROS production in order to reinforce DDR activation.62 Oxidative stress can be additionally modulated also by cellular genes such as the seladin-1, an oxidative stress sensor and a regulator of p53, contributing to p53-mediated Ras-induced senescence.63 Lately, DDB2 joined the list of genes induced by ROS leading to OIS by repressing the antioxidant system and reinforcing persistent ROS accumulation within the cells.64 These studies show that ROS may be instrumental to trigger p53-dependent OIS by regulating gene expression and functions rather than merely generating DNA damage. However, the precise mechanism and downstream paths will need further investigative efforts. Oxidation of nucleotide pools by ROS has also been recently proposed to control senescence induction.65
Apparently, ROS is a mediator of OIS and participates both in the initiation and further maintenance of the senescence status of the cells. However, there are many unanswered questions. Which is the biologically relevant source of ROS in OIS? Does the source of ROS that is activated upon oncogene activation and the one that locks the cells in the senescent state remain the same? Most importantly, how do ROS impact on cell-cycle progression?
A limitation to the study of ROS in cells is our ability to detect and measure ROS and to monitor their targets. Most of the techniques to visualise or quantify ROS are limited by several restrictive factors, one of which is their short half-life. Moreover, most of the probes used for detection or quantification also promote further free radical production increasing background noise to signal ratio.66 Fortunately, the emerging studies in the field of live imaging and the usage of specialised reporter probes, such as reduction–oxidation sensitive GFP,67,68 may help us to get more insight information about how ROS are regulated within cells or whole animals.
3. Conclusions and perspectives
From the discovery of cellular senescence in cultured cells to the observation of its in vivo accumulation in various human pre-malignant lesions,69 there is mounting evidence suggesting that senescence is a powerful natural anti-tumour mechanism. However, the proper clinical use of therapy-induced ‘accelerated senescence’70 both in terms of prevention, and progression, or recurrence of human cancers is still not completely understood. PTEN-loss-induced cellular senescence (PICS) has been proposed to provide an alternative ‘pro-senescence’ therapy approach.36 Combination of pharmacological inhibitors against PTEN and Mdm2 may promise a novel cancer therapy. However, the cautionary marks that need to be considered are that the proposed Mdm2 inhibitor and p53 activator Nutlin3 may, reportedly, trigger a DNA damage response upon treatment in cancer cells by slowing DNA repair.71 Thus, while the activation of DDR by Nutlin3 may lead to a positive feedback loop to elevate p53 activity, it may also result in further mutagenic events.
The mechanisms underlying the bypass of senescence response in the progression of tumours as well as the identification of multiple biomarkers in tissues will pave the road for successful clinical decisions. In a recent study, researchers introduced a ‘senescence scoring’, combining a DNA damage associated and a modified secretory senescence signature as a network of senescence-associated gene interactions rather than individual identified biomarkers.72 Therefore, they were able to identify differential expression of damage associated and secretory senescence pathways in a context-dependent manner. Such bioinformatic analysis could improve our understanding in tumour prognosis or response to the treatment.
On the other hand, recent studies reveal a dark side of cellular senescence, which is associated with the secreted inflammatory factors, and may alter the microenvironment in the favour of tumour progression.73 Therefore, it would be beneficial to modulate the SASPs.
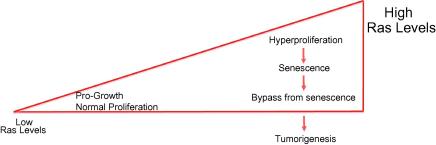
DDR appears to be instrumental not only in the establishment, but also in the maintenance of several aspects of the senescence programme (Fig. 1). Since widely used traditional cancer therapy relies on destruction of tumours by cytotoxic treatment, understanding the details of OIS within the context of DDR signalling may provide us invaluable information for translating our basic research knowledge into successful clinical outcomes. According to the three-stage carcinogenesis model proposed previously for Ras-induced tumours using a dose-dependent mouse model, low levels of Ras activation promote cellular proliferation and are neither sufficient for cellular transformation nor senescence. The second stage leads to increasing the levels of Ras, and turns hyperplasic lesions to the oncogenic threshold signal, which activates DDR providing senescence barrier74 The third stage is the bypass of senescence as a result of inactivation of tumour suppressor pathways (Fig. 2). In line with this model, two recent studies showed that the p53- a well-known downstream target of DDR pathway- is activated only when oncogene activation signalling reaches a critical threshold in these activated-kras mice model settings.75,76 Hence, restoring p53 has no effect in benign tumours because the activity is not enough to engage the p53 system. However, in the later stage malignant tumour cells, reactivated p53 eliminates cells. Previous analysis of physiological levels of expression of kRasG12D mouse models questioned the presence of OIS as an artefact of kRas overexpression.77 However, two new studies demonstrated that senescence does occur in MEFs expressing physiological levels of oncogenic kRas in mice, either in the absence of WT178 or suppression p16 tumour suppressors79 in lung and pancreatic mouse models, respectively. At low levels of oncogenic signalling, either the signal amplification of the oncogene or the loss of negative regulators of the oncogene is 77 necessary for the development of the senescence phenotype. Combination targeted therapy, which keeps the senescence associated secretory phenotype under control and restores tumour suppressors, could be a promise for future.
Fig. 1.
Main roads to cellular senescence. While telomere maintenance is a critical regulator of replicative senescence, multiple pathways can lead to DDR activation and oncogene-induced cellular senescence.
Fig. 2.
Dose-dependent oncogenic signalling outcomes. While acquired Ras mutations are pro-growth signals that promote proliferation, the level of activation is an important predictor in terms of the outcome in vivo.
Conflict of interest statement
None declared.
Acknowledgements
F.d’A.d.F laboratory is supported by AIRC (Associazione Italiana per la Ricerca sul Cancro), the European Community’s 7th Framework Programme (FP7/2007-2013) under grant agreement n° 202230, acronym ‘GENINCA’, HFSP (Human Frontier Science Program), AICR (Association for International Cancer Research), EMBO Young Investigator Program, Telethon and Progetto Ricerca Finalizzata RF-IRE-2007-672847.
References
- 1.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 3.d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8(7):512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 4.Collado M., Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10(1):51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakian V.A. Telomeres: beginning to understand the end. Science. 1995;270(5242):1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- 6.Griffith J.D., Comeau L., Rosenfield S. Mammalian telomeres end in a large duplex loop. Cell. 1999;97(4):503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 7.Lange Td. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):11. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 8.Celli G.B., Denchi E.L., de Lange T. Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat Cell Biol. 2006;8(8):885–890. doi: 10.1038/ncb1444. [DOI] [PubMed] [Google Scholar]
- 9.Smogorzewska A., Karlseder J., Holtgreve-Grez H., Jauch A., de Lange T. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr Biol. 2002;12(19):1635–1644. doi: 10.1016/s0960-9822(02)01179-x. [DOI] [PubMed] [Google Scholar]
- 10.Denchi E.L., de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448(7157):1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 11.Karlseder H.K., Mirzoeva O.K., Bakkenist C. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2004;2(8):e240. doi: 10.1371/journal.pbio.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d’Adda di Fagagna F., Reaper P.M., Clay-Farrace L. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 13.Herbig U., Jobling W.A., Chen B.P., Chen D.J., Sedivy J.M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14(4):501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 14.Palm W d.L.T. How shelterin protects mammalian telomeres? Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 15.Martinez P., Blasco M.A. Role of shelterin in cancer and aging. Aging Cell. 2010;9(5):653–666. doi: 10.1111/j.1474-9726.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 16.Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 17.Sfeir A., Kosiyatrakul S.T., Hockemeyer D. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138(1):90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNees CJ T.A., Martínez P., Murga M. ATR suppresses telomere fragility and recombination but is dispensable for elongation of short telomeres by telomerase. J Cell Biol. 2010;188(5):639–652. doi: 10.1083/jcb.200908136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez P., Thanasoula M., Munoz P. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23(17):2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rochette P.J., Brash D.E. Human telomeres are hypersensitive to UV-induced DNA Damage and refractory to repair. PLoS Genet. 2010;6(4):e1000926. doi: 10.1371/journal.pgen.1000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitte N., Saretzki G., von Zglinicki T. Accelerated telomere shortening in fibroblasts after extended periods of confluency. Free Radic Biol Med. 1998;24(6):885–893. doi: 10.1016/s0891-5849(97)00363-8. [DOI] [PubMed] [Google Scholar]
- 22.von Zglinicki T., Saretzki G., Docke W., Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220(1):186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Rhee D.B., Lu J. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010;6(5):e1000951. doi: 10.1371/journal.pgen.1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haendeler J., Hoffmann J., Diehl J.F. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94(6):768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 25.Zindy F., Eischen C.M., Randle D.H. Myc signaling via the ARF tumour suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12(15):2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 27.Collado M., Gil J., Efeyan A. Tumour biology: senescence in premalignant tumours. Nature. 2005;436(7051):642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 28.Dhomen N., Reis-Filho J.S., da Rocha Dias S. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15(4):294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z., Trotman L.C., Shaffer D. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumourigenesis. Nature. 2005;436(7051):725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Courtois-Cox S., Genther Williams S.M., Reczek E.E. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10(6):459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Micco R., Fumagalli M., Cicalese A. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444(7119):638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 32.Bartkova J., Rezaei N., Liontos M. Oncogene-induced senescence is part of the tumourigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444(7119):633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 33.Wajapeyee N., Wang S.Z., Serra R.W. Senescence induction in human fibroblasts and hematopoietic progenitors by leukemogenic fusion proteins. Blood. 2010;115(24):5057–5060. doi: 10.1182/blood-2009-09-245928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efeyan A., Murga M., Martinez-Pastor B. Limited role of murine ATM in oncogene-induced senescence and p53-dependent tumour suppression. PLoS One. 2009;4(5):e5475. doi: 10.1371/journal.pone.0005475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilad O., Nabet B.Y., Ragland R.L., Schoppy Combining ATR suppression with oncogenic Ras synergistically increases genomic instability, causing synthetic lethality or tumourigenesis in a dosage-dependent manner. Cancer Res. 2010;70(23):9693–9702. doi: 10.1158/0008-5472.CAN-10-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alimonti A., Nardella C., Chen Z. A novel type of cellular senescence that can be enhanced in mouse models and human tumour xenografts to suppress prostate tumourigenesis. J Clin Invest. 2010;120(3):681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robert T., Vanoli F., Chiolo I. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature. 2011;471(7336):74–79. doi: 10.1038/nature09803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narita M., Krizhanovsky V., Nunez S. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126(3):503–514. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 39.Ye X., Zerlanko B., Zhang R. Definition of pRB- and p53-dependent and -independent steps in HIRA/ASF1a-mediated formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27(7):2452–2465. doi: 10.1128/MCB.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Micco R., Sulli G., Dobreva M. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat Cell Biol. 2011;13(3):292–302. doi: 10.1038/ncb2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campisi J. Senescent cells, tumour suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Acosta J.C., O’Loghlen A., Banito A. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 43.Kuilman T., Michaloglou C., Vredeveld L.C. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 44.Rodier F., Coppe J.P., Patil C.K. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue W ZL, Miething C, Dickins RA, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007;445(7128):656–60. [DOI] [PMC free article] [PubMed]
- 46.Coppe J.P., Patil C.K., Rodier F. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumour suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 48.Martin-Ruiz C., Saretzki G., Petrie J. Stochastic variation in telomere shortening rate causes heterogeneity of human fibroblast replicative life span. J Biol Chem. 2004;279(17):17826–17833. doi: 10.1074/jbc.M311980200. [DOI] [PubMed] [Google Scholar]
- 49.Bertram C., Hass R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol Chem. 2008;389(3):211–220. doi: 10.1515/BC.2008.031. [DOI] [PubMed] [Google Scholar]
- 50.Wei Y.H., Lu C.Y., Lee H.C., Pang C.Y., Ma Y.S. Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann N Y Acad Sci. 1998;854:155–170. doi: 10.1111/j.1749-6632.1998.tb09899.x. [DOI] [PubMed] [Google Scholar]
- 51.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 52.Irani K., Xia Y., Zweier J.L. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275(5306):1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 53.D’Autréaux B T.M. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 54.Reddy M.M., Fernandes M.S., Salgia R. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25(2):281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lassegue B., Griendling K.K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30(4):653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim K.S., Choi H.W., Yoon H.E., Kim I.Y. Reactive oxygen species generated by NADPH oxidase 2 and 4 are required for chondrogenic differentiation. J Biol Chem. 2010;285(51):40294–40302. doi: 10.1074/jbc.M110.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forman H.J., Maiorino M., Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burdon R.H., Gill V., Rice-Evans C. Cell proliferation and oxidative stress. Free Radic Res Commun. 1989;7(3–6):149–159. doi: 10.3109/10715768909087937. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee S.P., Lane R.H., Lynn W.S. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27(22):2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- 60.Czech M.P. Differential effects of sulfhydryl reagents on activation and deactivation of the fat cell hexose transport system. J Biol Chem. 1976;251(4):1164–1170. [PubMed] [Google Scholar]
- 61.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330(6003):517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 62.Passos JF N.G., Wang C., Richter T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6 doi: 10.1038/msb.2010.5. (347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu C., Miloslavskaya I., Demontis S., Maestro R., Galaktionov K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature. 2004;432(7017):640–645. doi: 10.1038/nature03173. [DOI] [PubMed] [Google Scholar]
- 64.Roy N., Stoyanova T., Dominguez-Brauer C. DDB2, an essential mediator of premature senescence. Mol Cell Biol. 2010;30(11):2681–2692. doi: 10.1128/MCB.01480-09. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Rai P O.T., Young J.J., McFaline J.L. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc Natl Acad Sci USA. 2009;106(1):169–174. doi: 10.1073/pnas.0809834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tollefson K.E., Kroczynski J., Cutaia M.V. Time-dependent interactions of oxidant-sensitive fluoroprobes with inhibitors of cellular metabolism. Lab Invest. 2003;83(3):367–375. doi: 10.1097/01.lab.0000059934.53602.4f. [DOI] [PubMed] [Google Scholar]
- 67.Gutscher M., Pauleau A.L., Marty L. Real-time imaging of the intracellular glutathione redox potential. Nat Methods. 2008;5(6):553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 68.Maulucci G LV, Mele M, Panieri E, et al. High-resolution imaging of redox signaling in live cells through an oxidation-sensitive yellow fluorescent protein. Sci Signal. 2008;1(43):pl3. [DOI] [PubMed]
- 69.Bartkova J., Horejsi Z., Koed K. DNA damage response as a candidate anti-cancer barrier in early human tumourigenesis. Nature. 2005;434(7035):864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 70.Gewirtz D.A., Holt S.E., Elmore L.W. Accelerated senescence. An emerging role in tumour cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76(8):947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 71.Verma R., Rigatti M.J., Belinsky G.S., Godman C.A., Giardina C. DNA damage response to the Mdm2 inhibitor nutlin-3. Biochem Pharmacol. 2010;79(4):565–574. doi: 10.1016/j.bcp.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lafferty-Whyte K., Bilsland A., Cairney C.J. Scoring of senescence signalling in multiple human tumour gene expression datasets, identification of a correlation between senescence score and drug toxicity in the NCI60 panel and a pro-inflammatory signature correlating with survival advantage in peritoneal mesothelioma. BMC Genomics. 2010;11:532. doi: 10.1186/1471-2164-11-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gorgoulis VG H.T. Oncogene-induced senescence. The bright and dark side of the response. Curr Opin Cell Biol. 2010;22(6):816–827. doi: 10.1016/j.ceb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Sarkisian C.J., Keister B.A., Stairs D.B. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumourigenesis. Nat Cell Biol. 2007;9(5):493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 75.Junttila M.R., Karnezis A.N., Garcia D. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature. 2010;468(7323):567–571. doi: 10.1038/nature09526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feldser D.M., Kostova K.K., Winslow M.M. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010;468(7323):572–575. doi: 10.1038/nature09535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez-Mancera P.A., Tuveson D.A. Physiological analysis of oncogenic K-ras. Methods Enzymol. 2006;407:676–690. doi: 10.1016/S0076-6879(05)07053-9. [DOI] [PubMed] [Google Scholar]
- 78.Vicent S., Chen R., Sayles L.C. Wilms tumour 1 (WT1) regulates KRAS-driven oncogenesis and senescence in mouse and human models. J Clin Invest. 2010;120(11):3940–3952. doi: 10.1172/JCI44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee K.E., Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell. 2010;18(5):448–458. doi: 10.1016/j.ccr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]