Abstract
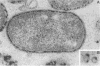
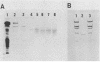
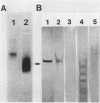
Cell-associated alkaline phosphatase (ALPase) of Bacteroides gingivalis 381 was found in the outer part of the periplasmic space by using an ultracytochemical procedure. Cell-associated ALPase was solubilized by extraction with 1% Triton X-100, and the solubilized enzyme was purified 904-fold with 5.6% recovery by using affinity column chromatography for mammalian intestinal-form ALPase. The purified enzyme gave a single protein band that corresponded to the enzyme activity band on polyacrylamide gel electrophoresis preparations. A single protein band at a molecular weight of 61,000 was observed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis preparations. The molecular weight of the native enzyme was estimated to be 130,000 by gel filtration with TSK-gel G3000SW. These findings indicate that B. gingivalis ALPase is a homodimer. The optimal pH of the enzyme was between 9.1 and 9.3 in the absence of divalent metal ions and was between 10.1 and 10.3 in the presence of manganese or zinc ions. The apparent km for p-nitrophenylphosphate was 0.037 +/- 0.003 mM (mean +/- standard deviation) at pH 9.2 in the absence of divalent metal ions and 0.22 +/- 0.02 mM at pH 10.2 in the presence of 1 mM manganese ions. Under both of the conditions described above, the purified enzyme was able to hydrolyze casein and O-phosphoserine, suggesting that B. gingivalis ALPase can act as a phosphoprotein phosphatase. ALPase that immunologically cross-reacted with the purified enzyme was found in the extracellular soluble fraction. This means that ALPase is released from the periplasmic space into the culture supernatant as a soluble form.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder T. A., Goodson J. M., Socransky S. S. Gingival fluid levels of acid and alkaline phosphatase. J Periodontal Res. 1987 Jan;22(1):14–19. doi: 10.1111/j.1600-0765.1987.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenna O., Perrella M., Pace M., Pietta P. G. Affinity-chromatography purification of alkaline phosphatase from calf intestine. Biochem J. 1975 Nov;151(2):291–296. doi: 10.1042/bj1510291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Localization of alkaline phosphatase in three gram-negative rumen bacteria. J Bacteriol. 1973 Oct;116(1):424–440. doi: 10.1128/jb.116.1.424-440.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. M., Voegel J. C. Bacterial bone resorption in advanced cases of human periodontitis. J Periodontal Res. 1978 May;13(3):251–261. doi: 10.1111/j.1600-0765.1978.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., McBride B. C. Isolation of a membrane-associated Bacteroides gingivalis glycylprolyl protease. Infect Immun. 1987 Dec;55(12):3131–3136. doi: 10.1128/iai.55.12.3131-3136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Fukasawa K., Hiraoka B. Y., Fukasawa K. M. Similarity between alkaline phosphatases from bovine dental pulp and liver. J Dent Res. 1984 Jan;63(1):28–31. doi: 10.1177/00220345840630010501. [DOI] [PubMed] [Google Scholar]
- Harkness E. R. Studies on human placental alkaline phosphatase. I. Purification and crystallization. Arch Biochem Biophys. 1968 Aug;126(2):503–512. doi: 10.1016/0003-9861(68)90435-9. [DOI] [PubMed] [Google Scholar]
- Heijl L., Wennström J., Lindhe J., Socransky S. S. Periodontal disease in gnotobiotic rats. J Periodontal Res. 1980 Jul;15(4):405–419. doi: 10.1111/j.1600-0765.1980.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Kreitzman S. N., Fritz M. E., Saffir A. J. Enzymatic destruction of bone in vitro. Nature. 1970 Nov 7;228(5271):575–576. doi: 10.1038/228575a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Mayahara H., Hirano H., Saito T., Ogawa K. The new lead citrate method for the ultracytochemical demonstration of activity of non-specific alkaline phosphatase (orthophosphoric monoester phosphohydrolase). Histochemie. 1967;11(1):88–96. doi: 10.1007/BF00326615. [DOI] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C. Exological relationships of bacteria involved in a simple, mixed anaerobic infection. Infect Immun. 1980 Jan;27(1):44–50. doi: 10.1128/iai.27.1.44-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas T., Greenman J. Production of cell-bound and vesicle-associated trypsin-like protease, alkaline phosphatase and N-acetyl-beta-glucosaminidase by Bacteroides gingivalis strain W50. J Gen Microbiol. 1989 Mar;135(3):557–564. doi: 10.1099/00221287-135-3-557. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Carlsson J., Sundqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985 Nov;50(2):467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Yamashita Y. The rabbit differs from other mammalian in the tissue distribution of alkaline phosphatase isoenzymes. Biochem Biophys Res Commun. 1987 Feb 27;143(1):15–19. doi: 10.1016/0006-291x(87)90622-x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Yamamoto A., Naito Y., Takazoe I., Slots J., Genco R. J. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986 Dec;54(3):659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Okuda K., Takazoe I. Purification and characterization of a thiol-protease from Bacteroides gingivalis strain 381. Oral Microbiol Immunol. 1987 Jun;2(2):77–81. doi: 10.1111/j.1399-302x.1987.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Slots J. Enzymatic characterization of some oral and nonoral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981 Sep;14(3):288–294. doi: 10.1128/jcm.14.3.288-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A., Hayduk S. E., Minah G. E., Krywolap G. N. Black-pigmented Bacteroides from clinically characterized periodontal sites. J Periodontal Res. 1979 Sep;14(5):376–382. doi: 10.1111/j.1600-0765.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Kinouchi T., Wakano Y., Ohnishi Y. Purification and characterization of a protease from Bacteroides gingivalis 381. Infect Immun. 1987 Feb;55(2):420–427. doi: 10.1128/iai.55.2.420-427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Hanada N., Takehara T. Purification of a fourth glucosyltransferase from Streptococcus sobrinus. J Bacteriol. 1989 Nov;171(11):6265–6270. doi: 10.1128/jb.171.11.6265-6270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981 Apr;32(1):198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenbergen T. J., Kastelein P., Touw J. J., de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontal Res. 1982 Jan;17(1):41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]