Abstract
Activated T lymphocytes are abundant in the airway during lung allograft rejection. Based on respiratory viral studies, it is the current paradigm that T cells cannot divide in the airway, and that their accumulation in the lumen of the respiratory tract is the exclusive result of recruitment from other sites, such as mediastinal lymph nodes. Here, we show that CD8+ T cell activation and proliferation can occur in the airway after orthotopic lung transplantation. We also demonstrate that airway epithelium expresses major histocompatibility class I predominantly on the apical surface, both in vitro and in vivo, and initiates CD8+ T cell responses in a polarized fashion, favoring luminal activation. Our data identify a unique site for CD8+ T cell activation after lung transplantation, and suggest that attenuating these responses may provide a clinically relevant target.
Keywords: lung transplantation, mouse, airway epithelium, CD8+ T cell
CD8+ T lymphocytes are the predominant T cell subset in acutely rejecting pulmonary allografts and, unlike the case for other organs, lung rejection can occur independently of CD4+ T lymphocytes (1). CD8+ T cells also play a critical role in the clearance of respiratory viral infections and after recovery the airway contains T cells in a higher state of activation than the lung parenchyma (2–4). Such anatomic compartmentalization has been proposed to maintain responsiveness to viral reinfection by the airborne route (5). It is the currently accepted paradigm that CD8+ T cell proliferation and acquisition of antiviral effector function cannot occur in the airway. Rather, CD8+ T cells are recruited to this site after activation in regional secondary lymphoid organs (5–7). Similar to virus infection models, CD8+ T cells are abundant in the airway during episodes of acute lung allograft rejection (8).
We have previously shown that vascular endothelium can activate alloreactive CD8+ T cells (9). Airway epithelial cells (AECs) are an integral and large component of lungs but their role in regulating CD8+ T cell responses after pulmonary transplantation has not been explored. To this end, our group has recently demonstrated that, unlike in other organs, lung allograft rejection can occur independent of secondary lymphoid organs (10). Furthermore, we have also shown that CD4+ T cell–independent lung rejection does not require the presence of donor-derived hematopoietic cells, raising the possibility that antigen presentation by graft-resident nonhematopoietic cells may contribute to the activation of alloreactive T cells after lung transplantation (1).
In this article, we describe that primary cultures of differentiated mouse AECs can directly activate alloreactive CD8+ T cells. Surprisingly, T cell proliferation occurs after apical, but not basal, presentation of alloantigen. Similar to AEC primary cultures, major histocompatibility (MHC) class I molecules are expressed on the apical surface of epithelium in lung allografts and proliferative responses of alloreactive CD8+ T cells can be initiated in the airway. These studies identify the donor airway as a novel compartment for CD8+ T cell activation, and provide a mechanistic explanation for the recently reported efficacy of inhalational immunosuppression (11).
MATERIALS AND METHODS
Mice
Male inbred C57BL/6 (B6) (H-2Kb), B6 nude, and CBA/Ca (H-2Kk) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). BM3 mice (T cell receptor [TCR] transgenic H-2Kk anti–H-2Kb on a CBA/Ca background) were provided by N. Jones (University of Oxford, United Kingdom) (9), and B6 CD11c-EYFP mice were provided by M. Nussenzweig (Rockefeller University, New York). All studies were approved by the Institutional Animal Studies Committee of Washington University in St. Louis, MO.
Lung and Bone Marrow Transplantation
Lung transplants were performed as previously described (8). For bone marrow transplantation lethally irradiated B6 mice were reconstituted with T cell–depleted CBA/Ca bone marrow (9). Bone marrow chimeras are designated as recipient genotype (donor genotype), and used at least 3 months after irradiation.
AEC Cultures
B6 mouse differentiated AECs were established as previously described (12). To obtain polarized cultures, polycarbonate and polyester porous (0.4- and 3.0-μM pores) supported membranes (Transwell and Transwell Clear; Corning-Costar, Corning, NY) were coated with type I rat tail collagen, and cells were grown on the membranes until confluent. AECs were activated in vitro for 48 hours with 100 U/ml of IFN-γ before the addition of T cells.
Adoptive Transfers
BM3 CD8+ T cells (107), isolated from spleens and lymph nodes as previously described (9), were transferred intratracheally or intravenously. For some experiments, recipients were treated with FTY-720 (Fingolimod) (0.5 mg/kg; Cayman Chemical Co., Ann Arbor, MI), as previously described (13).
Flow Cytometry
Bronchoalveolar lavage fluid and lung tissue were prepared for flow cytometric analysis as previously described (8). Staining was performed with fluorochrome-labeled anti-CD90.2 (clone 30-H12), anti-CD8 (clone 53–6.7), or biotinylated anti-BM3 clonotypic antibody (Ti98; N. Jones), followed by a fluorochrome-conjugated streptavidin. Antibodies and their respective isotype controls were obtained from eBioscience (San Diego, CA), except Ep-CAM (CD326, clone G8.8), which was obtained from Biolegend (San Diego, CA). AECs were defined as CD326+CD45−. Intracellular staining was performed with anti–IFN-γ (clone XMG1.2) and the respective isotype control (BD Pharmingen, Bedford, MA). Intratracheal CFSE (carboxyfluorescein succinimidyl ester) administration was performed using 100 μl of 0.2 mM solution, similar to previously described methods (14). Gating strategy for identification of adoptively transferred BM3 T cells is shown in Figure E1 in the online supplement.
Immunofluorescence
Sections of cryopreserved lungs and AECs were fixed and immunostained as previously described (12) by rat anti-mouse MHC class I or isotype control antibody (1:100; BMA Biomedicals AG, August, Switzerland). Photomicroscopy was performed using a Zeiss LSM 510 META laser scanning confocal instrument (Zeiss, Thornwood, NY).
Two-Photon Imaging
Imaging of T cells, labeled with the red dye, 5-(and-6)-([(4-chloromethyl)benzoyl]amino)tetramethylrhodamine, or the blue dye, 7-amino-4-chloromethylcoumarin, in lung explants was performed using a custom-built, video-rate, two-photon microscope (10). Lung images containing 11–13 cells were analyzed based on a previously described approach using an improved analysis tool in T cell Analyzer Software (TCA; John Dempster, University of Strathclyde, Glasgow, Scotland). Neighbors were defined as cells within a centroid-to-centroid distance less than 25 μm in three-dimensional space.
Statistical Analysis
Statistical analysis was performed using Student's t test for airway versus lung-resident proliferating cells and two-way ANOVA for cluster analysis by two-photon imaging, with a P value of 0.05 considered significant. All data are representative of at least three separate experiments.
RESULTS
T-Cell Proliferation in the Lung
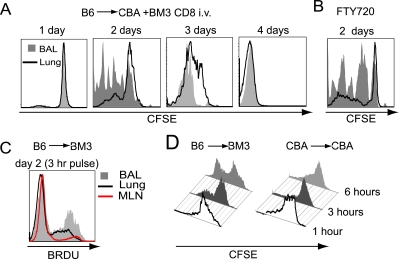
Although T cell activation after organ transplantation results in extremely rapid proliferative responses, alloreactive T cells constitute only a small portion of lymphocytes in an unprimed wild-type animal (15). Thus, to analyze rigorously the alloreactive responses after lung transplantation we decided to use the BM3 T cell receptor transgenic system (background CBA/Ca; H-2KK) where all CD8+ T cells recognize direct alloantigen presentation of a single allogeneic MHC class I molecule derived from the C57BL/6 strain (H-2Kb) (9). We thus adoptively transferred CFSE-labeled BM3 CD8+ T cells into CBA/Ca (H-2Kk) recipients of B6 (H-2Kb) lung allografts, and analyzed proliferative responses 1, 2, and 4 days later using the clonotypic anti-BM3 TCR antibody (TI-98) to detect the adoptively transferred cells (Figure E1). Although little proliferation was seen 1 day after transfer, a marked proliferative response of the BM3 T cells was detected by Day 2, and proliferation of virtually all transferred cells was observed by Day 4 (Figure 1A). Interestingly, 2 days after transfer, T cells in the airway had significantly more advanced division profiles compared with those in the lung parenchyma (82.7 ± 5.2% versus 37.2 ± 3.7% proliferation, respectively; P = 0.002; n = 6). This finding suggests that proliferation was occurring in the airway, but may also represent preferential migration of activated cells to the airway from secondary lymphoid tissue, as previously described for T cell responses to viral or nominal antigen (2, 16). We then attempted to evaluate the mediastinal lymph nodes and spleen for the presence and proliferation of adoptively transferred BM3 T cells. In stark contrast to the lung or airway, BM3+ T cells were undetectable in these structures after intravenous adoptive transfer. Although this suggests that the majority of alloreactive T cells home to the lung allograft, it does not rule out the possibility that BM3 T cells receive their activation signal during the transient migration through secondary lymphoid organs before their homing to the lung. To evaluate more stringently the role of secondary lymphatic tissue in T cell priming we treated recipient mice with perioperative FTY-720, a structural analog of sphingosine, which prevents T cell egress from lymphatic tissue (13). Consistent with our previous report that alloreactive T cells can be primed in lung grafts independent of secondary lymphoid organs (10), treatment with FTY-720 resulted in proliferative profiles in both the airway and lung parenchyma comparable to those seen in untreated recipients (Figure 1B).
Figure 1.
Intra-airway CD8+ T cell proliferation. (A) Representative carboxyfluorescein succinimidyl ester (CFSE) plot of intravenous adoptively transferred BM3 CD8+ T cells in the airway (shaded gray) and lung parenchyma (solid line) of CBA/Ca recipients of B6 lungs. (B) Representative CFSE plot of FTY-720 (Fingolimod)–treated recipient. (C) Representative BRDU (5-bromo-2-deoxyuridine) incorporation histogram of bronchoalveolar lavage (BAL) (33.8 ± 3.7%), lung parenchyma (18.7 ± 5.1%), and mediastinal lymph node (11.2 ± 3.5%) of B6 to BM3 transplant recipient (n = 3). (D) Proliferative plots of CD8+ T cells, labeled via intratracheal CFSE administration, after allogeneic or syngeneic transplantation (n = 3).
To confirm these findings in a complementary model, we analyzed proliferative responses of CD8+ T cells in BM3 recipients of B6 lung grafts. Consistent with our observation of CFSE diminution, we noted significantly higher rates of DNA synthesis (after a 3-h BRDU [5-bromo-2-deoxyuridine] pulse) in the airway compared with lung parenchyma or mediastinal lymph nodes (33.8 ± 3.7% versus 18.7 ± 5.1% versus 11.2 ± 3.5%, respectively; Figure 1C). Minimal DNA synthesis was noted in syngeneic lung graft recipients (data not shown). Such data support the results of our adoptive transfer system. However, neither method could rule out the possibility that T cells proliferated within the pulmonary parenchyma before migration to the airway. To investigate this further, we labeled airway cells in situ by administering CFSE intratracheally 2 days after transplantation of B6 lungs into BM3 recipients. Unlike syngeneic controls, diminution of CD8+ T cell CFSE in the airway of allograft recipients supports the notion that proliferation was occurring in this compartment (Figure 1D). Furthermore, the rapid proliferative responses seen by CFSE diminution were corroborated by the high levels of BRDU (5-bromo-2-deoxyuridine) incorporation by intra-airway BM3 CD8+ T cells (Figure 1C).
Intra-Airway T-Cell Activation
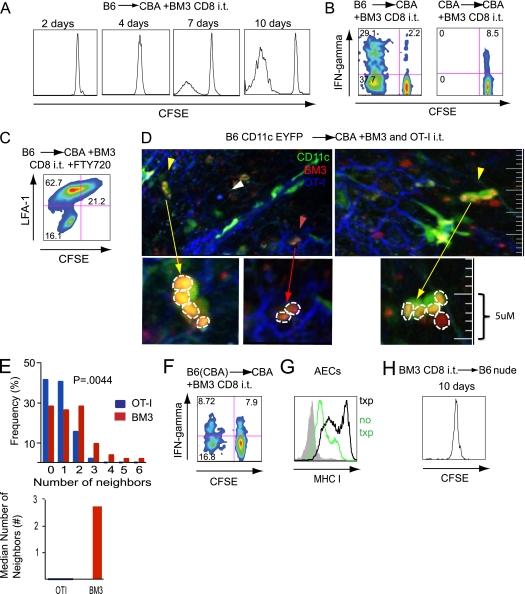
Intratracheal instillation of T cells, followed by their removal by bronchoalveolar lavage, has been described as a reliable method to study their behavior in the airway (14). We next set out to evaluate the fate of BM3 CD8+ T cells after intratracheal transfer at the time of transplantation. When transferred into the airway of CBA/Ca recipients of B6 lungs, the majority of these cells underwent progressive division, albeit at a lower rate than cells injected intravenously (Figure 2A). Furthermore, BM3 CD8+ T cells transferred intratracheally into CBA/Ca recipients of B6 lung grafts up-regulated CD69 (data not shown), and, unlike those transferred into syngeneic controls, had the capacity to produce IFN-γ (Figure 2B). Flow cytometric evaluation of mediastinal lymph nodes 2, 4, 7, and 10 days after intratracheal adoptive transfer revealed a small population (≈5,000 cells) of BM3 CD8+ T cells on Day 2. After that time point, the adoptively transferred cells were undetectable in the mediastinal lymph nodes. Because this finding raised the possibility that intratracheally transferred cells receive the signal to proliferate in secondary lymphoid organs, before migration back to the airway, we treated intratracheal transplant recipients of BM3 CD8+ T cells with FTY 720 and documented similar levels of proliferation (74.3 ± 5% versus 68.1 ± 17% for treated and untreated animals, respectively; P = 0.72). Furthermore, lymphocyte function-associated antigen 1 (LFA-1) is down-regulated on airway-resident BM3 CD8+ T cells that have undergone proliferation (Figure 2C) (14). Thus, although different kinetics of proliferation between intravenously (Figure 1A) and intratracheally (Figure 2A) transferred cells suggest that transmigration of T lymphocytes through the lung parenchyma may lower their threshold for activation, collectively, these data support the notion that division of alloreactive T cells can occur in the airway (10).
Figure 2.
Intratracheal CD8+ T cell administration. (A) Representative proliferative profiles of BM3 CD8+ T cells 2, 4, 7, and 10 days (n = 3) after intratracheal administration into CBA/Ca recipients of B6 lung grafts. (B) Proliferation and IFN-γ production by BM3 T cells delivered intratracheally into B6 (left panel) or CBA (right panel) grafts. (C) Representative plot demonstrating proliferation and lymphocyte function-associated antigen 1 (LFA-1) down-regulation in BM3 CD8+ T cells 10 days after intratracheal adoptive transfer into FTY-720 treated CBA/Ca recipient of a B6 lung graft (n = 4). (D) Two separate sections demonstrating two-photon imaging of the alveolar space of B6 CD11c-EYFP+→CBA/Ca graft showing BM3 T cells (5-(and-6)-([(4-chloromethyl)benzoyl]amino)tetramethylrhodamine [CMTMR], red), OT-I T cells (7-amino-4-chloromethylcoumarin [CMAC], blue), CD11c-EYFP+ cells (green), and collagen fibers (second harmonic signal, blue) (left graph). The yellow arrows point to multiple red BM3 T cells clustering around a green CD11c+ alveolar macrophage. Red T cells directly overlying a green macrophage appear yellow. Red arrow points to two BM3 T cells clustering within the airway next to airway epithelial cells (AECs), as seen by the blue second harmonic signal of supporting collagen fibers in the alveolar space. White arrowhead points to a solitary OT-I T cell in the alveolar space. The row below represents higher magnification of clustering with individual BM3 T cells outlined by white dashed lines. (E) Neighboring distribution analysis (top graph) was performed on 21 representative z-stacks from two independent experiments evaluating clustering of BM3 (red bars) and OT-I (blue bars) T cells. The median number of neighbors was 0 for OT-I and 2.75 for BM3 T cells (bottom graph). The analysis is based on evaluation of 103 OT-I and 91 BM3 T cells. (F) Proliferation and IFN-γ production by BM3 CD8+ T cells after intratracheal administration into CBA/Ca recipients of B6(CBA) lung grafts (n = 6). (G) Major histocompatibility (MHC) class I expression on AECs in untransplanted B6 control mice (green) or transplanted lungs (black) (n = 6). (H) Proliferation of BM3 CD8+ T cells 10 days after intratracheal administration into B6 nude recipients (n = 5).
Two-photon microscopy has significantly broadened our understanding of cellular immunity, and detection of T cell clustering using this method has been described as a sensitive readout for lymphocyte priming that temporally precedes other stages of T cell activation, such as proliferation (10, 17). We next used two-photon imaging of whole-lung explants to evaluate the behavior of intratracheally administered T cells. We transplanted B6 CD11c-EYFP lungs into CBA/Ca recipients, injected 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine–labeled BM3 and control 7-amino-4-chloromethylcoumarin–labeled OT-I CD8+ T cells intratracheally at the time of transplantation, and then quantitatively assessed T cell clustering by comparing the neighboring distribution of adoptively transferred cells 2 days later (Figure 2D). The BM3 neighboring distribution was significantly different from that observed for OT-I cells (P = 0.004), with BM3 T cells having up to six neighbors within 25 μm and a higher median number of neighbors (2.75 for BM3 and 0 for OT-I; Figure 2E). Whereas, similar to T cell priming in secondary lymphoid organs, clustering precedes proliferative responses in the airway, the kinetics of progression of clustering to proliferation differs in this compartment (Figures 2A and 2D) (17). Combined with the well established finding that the strength of TCR stimulation directly correlates with the rate of cell cycle progression (18), these findings suggest that the slower kinetics of proliferation after intratracheal transfer might be due to lower levels of TCR engagement in the airway compared with other sites of T cell activation, such as lung parenchyma or secondary lymphoid organs (10, 17). Notably, two-photon imaging revealed that some BM3 T cells clustered around CD11c+ alveolar macrophages, whereas some clustered independently of CD11c+ cells (Figure 2D). This raised the possibility that, in addition to hematopoietic cells, nonhematopoietic cells, such as AECs, can activate CD8+ T cells in the airway. To evaluate this further, we adoptively transferred BM3 CD8+ T cells intratracheally to CBA/Ca recipients of lung grafts derived from B6(CBA/Ca) bone marrow chimeras, thereby limiting alloantigen presentation to nonhematopoietic cells (9). Here, we also observed proliferation and IFN-γ production by BM3 T cells, albeit at a lower rate than after B6→CBA/Ca transplantation (26.4 ± 10.7% versus 68.1 ± 17% proliferation, respectively; P = 0.01; Figure 2F). Thus, contrary to viral immune responses (5, 14, 16, 19), our data collectively show that T cell priming can occur in the airway after lung transplantation, and that luminal alloantigen presentation by epithelial cells is sufficient to initiate T cell activation.
Because some degree of ischemia–reperfusion injury is unavoidable after organ transplantation, we next set out to evaluate the role of such injury on T cell responses. Indeed, 36 hours after transplantation, MHC class I was up-regulated on AECs of transplanted B6 lungs compared with untransplanted controls (Figure 2G). Consistent with this finding, little proliferation of adoptively transferred BM3 CD8+ T cells was detectable 10 days after intratracheal transfer into untransplanted B6nu/nu recipients (3.9 ± 0.9%), which was significantly lower than the proliferation observed in the transplant recipients (68.1 ± 17%; P = 0.002; Figure 2H).
AECs Express MHC Class I Apically and Induce Polarized CD8+ T Cell Responses
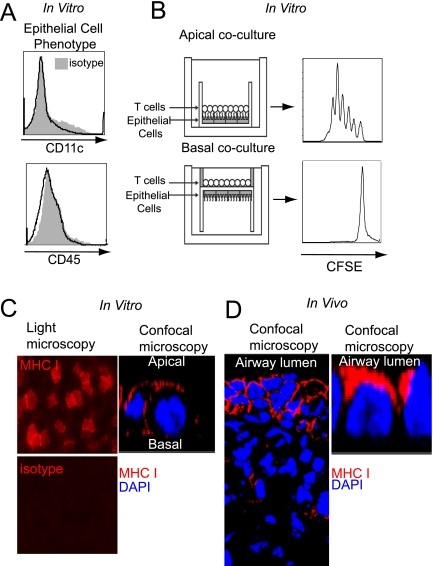
Next, we set out to evaluate further the alloantigen presentation by airway nonhematopoietic cells. Here, we took advantage of our previously established method for the isolation and in vitro culture of primary mouse AECs (12). When cultured at an air–liquid interface, these preparations develop apical–basolateral orientation resembling AECs in vivo, and are free of contaminating CD45+ or CD11c+ hematopoietic cells by flow cytometry (Figure 3A). Coculture of BM3 CD8+ T cells on the apical surface of B6 AECs, which corresponds to the airway lumen, results in their robust proliferation. Alternatively, CD8+ T cells do not divide when exposed to the basal surface of the AECs (Figure 3B). Immunohistochemical staining of AECs demonstrated that MHC class I was primarily expressed apically (Figure 3C). Notably, this observation closely mirrored MHC class I expression patterns on AECs in vivo (Figure 3D). Collectively, our results identify a previously unrecognized polarized expression of MHC molecules on AECs, which contributes to their ability to initiate alloreactive CD8+ T cell responses in the airway.
Figure 3.
Polarized antigen presentation by AECs. (A) AECs grown in vitro are free of CD45+ or CD11c+ hematopoietic cells. (B) Proliferation of BM3 CD8+ T cells after 5 days of coculture with the apical (top panel) or basal surface (bottom panel) of differentiated B6 AECs, as depicted by a diagram of polarized AEC-T cell cocultures (left) and CFSE proliferation plots (right) (n ≥ 4). (C) MHC class I expression on B6 AECs in vitro as depicted by light microscopy (left panels) and confocal microscopy of a single cell (right panel). (D) MHC class I expression on transplanted B6 lungs in vivo as imaged by confocal microscopy (left panel), and in magnified detail for a single cell (right panel).
DISCUSSION
Based on viral studies, a paradigm has been formulated that T cell activation cannot occur within the airway (3, 14). Rather, after viral infection T cell priming depends on their encounter of antigen either in draining lymphoid tissue or pulmonary parenchyma, with subsequent recruitment of these activated T cells to the airway (4, 6, 20). In contrast to this model, we now show that the airway provides a suitable environment for alloreactive CD8+ T cell activation, and that AECs are sufficient to prime such responses. Potential reasons for differences in airway responses between alloreactive and virus-specific CD8+ T lymphocytes include larger numbers of cells expressing allo–MHC class I when compared with professional bone marrow–derived antigen-presenting cells, such as CD8α+ dendritic cells, that can elicit efficient virus-specific immune responses (21). It is possible that activation of CD8+ T lymphocytes by CD8α+ dendritic cells is dependent on antigen presentation in secondary lymphoid organs. Here we show however, that nonhematopoietic cells are sufficient to initiate CD8+ T cell–mediated alloimmune responses independently of bone marrow–derived antigen-presenting cells, and may thus facilitate local antigen presentation in the airway (9). Such intra-airway proliferation may be further facilitated by antigen-presenting cell activation associated with ischemia–reperfusion injury inherent to organ transplantation.
Our findings set airway epithelium apart from nonhematopoietic cells of other mucosal surfaces, such as uterine and intestinal epithelium, where MHC expression is restricted to the basolateral surface (22, 23). It has been hypothesized that basolateral expression of MHC facilitates antigen presentation to T lymphocytes transported by the bloodstream (23). It is possible that apical epithelial expression of MHC regulates the survival of virus-specific T cells, which are maintained in the airway for prolonged periods after resolution of infection. Such polarized expression, however, may inadvertently favor luminal proliferation of alloreactive T cells in the setting of lung transplantation. Our results provide a rationale for targeting intra-airway T cell activation after lung transplantation (11).
Supplementary Material
Acknowledgments
The authors thank Arlene Ligori (Aligori Studios, St. Louis, MO; http://www.aligori.com/) for her help with the illustrations, and the Children's Discovery Institute Airway Epithelial Core (St. Louis Children's Hospital, Washington University, St. Louis) for airway epithelial cells.
This work was supported by the National Cancer Institute (NCI) and Thoracic Surgery Foundation for Research and Education (TSFRE) grant KO8CA131097 and American Association of Thoracic Surgeons and American Cancer Society Internal Research Grant from the Alvin Siteman Cancer Center, Washington University in St. Louis (A.S.K.), by National Heart, Lung, and Blood Institute (NHLBI) grant 1R01HL094601 (D.K. and A.E.G.), and by NHLBI/TSFRE grant K08HL083983 (D.K.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0099OC on September 20, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, Sugimoto S, Tietjens JR, Patterson GA, Krupnick AS, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol 2008;180:4754–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan RJ, Cauley LS, Ely KH, Cookenham T, Roberts AD, Brennan JW, Monard S, Woodland DL. Long-term maintenance of virus-specific effector memory CD8+ T cells in the lung airways depends on proliferation. J Immunol 2002;169:4976–4981. [DOI] [PubMed] [Google Scholar]
- 3.Ely KH, Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol 2003;171:3338–3342. [DOI] [PubMed] [Google Scholar]
- 4.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med 2001;193:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodland DL, Randall TD. Anatomical features of anti-viral immunity in the respiratory tract. Semin Immunol 2004;16:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol 2003;170:1423–1429. [DOI] [PubMed] [Google Scholar]
- 7.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 1998;8:683–691. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, Huang HJ, Das NA, Patterson GA, Gelman AE, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant 2007;7:1672–1679. [DOI] [PubMed] [Google Scholar]
- 9.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med 2002;8:233–239. [DOI] [PubMed] [Google Scholar]
- 10.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, Kornfeld CG, Kreisel FH, Sugimoto S, Tietjens JR, et al. Cutting edge: acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol 2009;182:3969–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacono AT, Johnson BA, Grgurich WF, Youssef JG, Corcoran TE, Seiler DA, Dauber JH, Smaldone GC, Zeevi A, Yousem SA, et al. A randomized trial of inhaled cyclosporine in lung-transplant recipients. N Engl J Med 2006;354:141–150. [DOI] [PubMed] [Google Scholar]
- 12.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321. [DOI] [PubMed] [Google Scholar]
- 13.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med 2009;179:344–355. [DOI] [PubMed] [Google Scholar]
- 14.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol 2006;176:537–543. [DOI] [PubMed] [Google Scholar]
- 15.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol 2001;166:973–981. [DOI] [PubMed] [Google Scholar]
- 16.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med 2002;195:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med 2004;200:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid DA, Irving MB, Posevitz V, Hebeisen M, Posevitz-Fejfar A, Sarria JC, Gomez-Eerland R, Thome M, Schumacher TN, Romero P, et al. Evidence for a TCR affinity threshold delimiting maximal CD8+ T cell function. J Immunol 2010;184:4936–4946. [DOI] [PubMed] [Google Scholar]
- 19.Kohlmeier JE, Woodland DL. Memory T cell recruitment to the lung airways. Curr Opin Immunol 2006;18:357–362. [DOI] [PubMed] [Google Scholar]
- 20.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol 2001;166:1813–1822. [DOI] [PubMed] [Google Scholar]
- 21.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008;322:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershberg RM, Framson PE, Cho DH, Lee LY, Kovats S, Beitz J, Blum JS, Nepom GT. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest 1997;100:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Eijkeren MA, Peters PJ, Geuze HJ. Polarized expression of major histocompatibility complex class I molecules in human endometrial and endocervical epithelial cells. Eur J Immunol 1991;21:3049–3052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.