Abstract
Mucus is a protective gel that lines respiratory tract surfaces. To identify potential roles for secreted gel–forming mucins in lung development, we isolated murine lungs on embryonic days (E) 12.5–18.5, and postnatal days (PN) days 5, 14, and 28. We measured the mucin gene expression by quantitative RT-PCR, and localization by histochemical and immunohistochemical labeling. Alcian blue/periodic acid–Schiff–positive cells are present from E15.5 through PN28. Muc5b transcripts were abundant at all time points from E14.5 to PN28. By contrast, transcript levels of Muc5ac and Muc2 were approximately 300 and 85,000 times lower, respectively. These data are supported by immunohistochemical studies demonstrating the production and localization of Muc5ac and Muc5b protein. This study indicates that mucin production is prominent in developing murine lungs and that Muc5b is an early, abundant, and persistent marker of bronchial airway secretory cells, thereby implicating it as an intrinsic component of homeostatic mucosal defense in the lungs.
Keywords: mouse, lung, mucin, Muc5ac, Muc5b
CLINICAL RELEVANCE.
The lungs are exposed to millions of particles and potential pathogens daily from early life to adulthood. Mucus is an intrinsic defensive component in the airways that acts as a barrier to inhaled materials. This study provides important insights into the composition of mucin glycoprotein and the timing of its production in murine lungs.
At approximately 4 weeks of gestation in humans (embryonic days 9.0–9.5 in mice), tracheal and lung-bud formation initiates as a pocket of endoderm evaginates from the ventral floor of the embryonic foregut and invades the underlying mesoderm. The developing lungs undergo repeated branching steps, giving rise to the complex of tubes that form the conducting airways and alveolar saccules. During this process of branching and morphogenesis, the endoderm changes from an undifferentiated mass to a pseudostratified squamous layer, and then to a differentiated layer comprised of heterogeneous progenitor and mature cells (1).
Mature respiratory epithelial cells possess many unique functions that can be attributed to their structural features or their secretory products. Structural features such as the long, apical projections of tracheobronchial ciliated cells and the thin sheet-like squamous morphologies of alveolar Type I cells are examples of obvious morphological traits that are specifically linked to cellular function (ciliary clearance and gas exchange, respectively). Secreted products also demarcate the functions of individual respiratory epithelial subtypes. The tracheobronchial epithelium produces secreted products, such as mucins, that serve defensive functions by preventing the accumulation of particles and pathogens in the distal lung (2–4), whereas Type II pneumocytes produce surfactant lipids that reduce surface tension and prevent alveolar collapse, thereby permitting effective gas exchange (5). Additional anatomically selective markers include the secretaglobin (Scgb) family, with members such as Scgb1a1 (or Clara cell secretory protein) in the airways, and surfactant proteins A, B, and C in the alveoli. The expression of many of these markers is low early during lung development, when the bronchial epithelium is poorly differentiated, and becomes more abundant as epithelial maturation occurs late in embryonic gestation and throughout postnatal life (6).
The importance of mucins as defensive molecules in adult airways is suggested by their abundance during homeostasis and their up-regulation during inflammation. We previously showed that Muc5b is the predominant secreted gel-forming mucin expressed in healthy adult murine lungs (7, 8), whereas Muc5ac is the most predominantly induced gel-forming mucin in allergic murine lungs (7). To determine whether the baseline expression patterns of Muc5ac and Muc5b reflect secretory cell differentiation during lung development, we measured their production prenatally and postnatally.
MATERIALS AND METHODS
Mice
C57BL6/J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were housed in accordance with the Institutional Animal Care and Use Committee of the M.D. Anderson Cancer Center. To initiate timed pregnancies, mice were mated for a single night. Females manifesting a vaginal plug were separated and housed in isolation for an additional 9–20 days. At specific postcoital times (days 10–18), some females were killed, and their fetuses were isolated surgically. Three to eight pups were collected at various time points for histological and expression analyses.
Histological Preparation
Because of their small size, whole embryos from embryonic days (E) 10.5 to E16.5 were fixed in 10% neutral buffered formalin. For samples isolated from E17.5 to postnatal day (PN) 28, lungs were isolated and inflated with formalin. Tissues were dehydrated, paraffin-embedded, and cut into 5-μm sections that were collected on positively charged slides and stored for subsequent histochemical and immunohistochemical staining.
Histochemistry and Immunohistochemistry
Dewaxed and rehydrated tissue sections were stained histochemically using alcian blue/periodic acid–Schiff (AB-PAS), as described previously (9), to detect acidic sulfated mucins (AB), O-glycosides (PAS), and sialic acid (PAS). AB-PAS is not mucin-specific, because it also labels glycosaminoglycan and other polysaccharide moieties. Thus, for the immunolocalization of Muc5ac, tissues were exposed to an anti-mouse Muc5ac peptide chicken polyclonal antibody (1:2,000) at room temperature for 1 hour (10). For Muc5b, tissues were incubated with a polyclonal rabbit anti-mouse Muc5b peptide antibody (1:10,000) at room temperature for 1 hour (8). Specific staining for both antibodies was confirmed by comparing experimental samples with preimmune and immune sera–treated adult lung, stomach, and sublingual gland samples (data not shown). After washing, biotinylated secondary antibodies, followed by a horseradish peroxidase (HRP)–streptavidin binding complex (Vector Laboratories, Burlingame, CA), were then applied. HRP reactions were visualized using 3–3′ diaminobenzadine. Nuclei were counterstained with methyl green. Slides were imaged using an Olympus DP-71 CCD camera, mounted on an Olympus BX-60 upright microscope (Olympus America, Center Valley, PA).
RNA Isolation and Quantitative RT-PCR
To measure the expression of specific mucin transcripts and Scgb1a1, quantitative PCR was performed on reverse-transcribed total RNA isolated from lungs (E17.5–PN28) or microdissected thoraces (E14.5–E16.5). Isolated tissues were placed in a Petri dish, and minced in 1 ml Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA was isolated according to the manufacturer's instructions. Complementary DNA was synthesized using Superscript III Reverse Transcriptase (Invitrogen), with random 9-mer oligonucleotide primers (Sigma, St. Louis, MO). Volumes of cDNA reaction mix, corresponding to an initial total RNA input mass of 100 ng, were amplified using gene-specific fluorescein amidate/Black Hole Quencher (FAM/BHQ)–labeled fluorescent reporter probes, as reported previously for the gel-forming mucins Muc2, Muc5ac, Muc5b, and Muc19 (7). The expression patterns of Scgb1a1 and of the membrane-associated mucins Muc1, Muc4, and Muc16 were also examined. Primer and probe sequences are listed in Table E1 in the online supplement. Data were normalized to γ-actin expression levels, and differences were compared by ANOVA, with a P-value cutoff of 0.05.
RESULTS
Detection of Mucin-Producing Cells in the Lungs during Embryonic and Postnatal Development
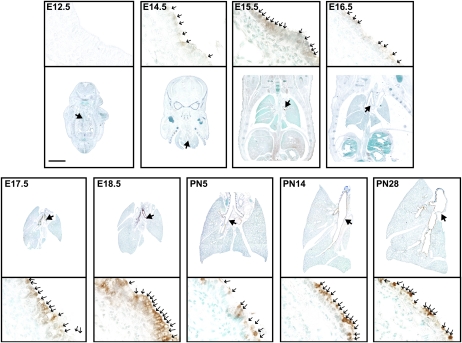
Cells containing apical AB-PAS–stained materials that were morphologically indicative of mucous cells were detectable in developing murine lungs from E15.5 through PN28 (Figure 1). They were present predominantly in the cartilaginous (extrapulmonary) and axial (intrapulmonary) bronchial airways, and they were rare in the bronchioles from E15.5 onward (Table E2). These data suggest that a mucous phenotype is present at baseline in developing murine lungs. Interestingly, although AB-PAS–positive cells are rare in the bronchi before E15.5, they are abundant in the developing bronchioles and alveolar saccules. The AB-PAS staining seen in these distal regions is distributed among basal and apical intracellular compartments, and is most likely attributable to the high concentrations of glycogen within developing acini (11). Thus, given that histochemical stains such as AB-PAS identify numerous glycosylated macromolecules and are not specific for mucins per se, we tested for changes in the production of respiratory mucin, using specific transcript and protein probes.
Figure 1.
Alcian blue/periodic acid–Schiff (AB-PAS)–positive cells are prominent in prenatal and postnatal developing murine lungs. Tissue sections (5 μm) were removed from whole embryos (E) and isolated lungs of postnatal (PN) wild-type C57/BL6 mice isolated at the indicated time points, and stained with AB-PAS. Large arrows in low-magnification images (top) identify airway sites shown in high magnification (below). Small arrows (below) identify positively stained cells. Scale bar, 2 mm for low magnification, and 30 μm for high magnification.
Muc5ac and Muc5b mRNAs Are Expressed in the Developing Murine Lung
Mucin transcripts were analyzed by quantitative RT-PCR (Figure 2 and Table E3). At the earliest time point studied, E14.5, Muc5b transcripts were abundant (38 mol Muc5b mRNA/mol γ-actin mRNA). By contrast, Muc2 and Muc5ac transcripts were 1,471-fold and 1,992-fold less abundant, respectively. At PN14, Muc5b remained the most abundant gel-forming mucin expressed in the airways (44 mol Muc5b mRNA/mol γ-actin mRNA). The expression of Muc5ac increased 7.7-fold (0.15 mol Muc5ac mRNA/mol γ-actin mRNA), but this was not statistically significant (P = 0.21), and is still approximately 300 times lower than the expression of Muc5b at this time point. Muc2 expression decreased 4.7-fold at this time point to a barely detectable level. Muc19 transcripts were undetectable at all time points in our study (< 10 copies per reaction), consistent with previous findings in adult mice (7, 12). For reference, expression of the airway secretory cell marker Scgb1a1 was also analyzed. The expression of Scgb1a1 was low at prenatal time points (0.14–1.6 mol Scgb1a1 mRNA/mol γ-actin mRNA), but was rapidly and robustly induced postnatally, increasing 6,138-fold to 16,072-fold from PN5 to PN28. At PN5, the expression of Scgb1a1 was approximately 15-fold higher than that of Muc5b (1,537 mol Scgb1a1 mRNA/mol γ-actin mRNA versus 102 mol Muc5b mRNA/mol γ-actin mRNA). Lastly, we measured the expression of the membrane-associated mucins Muc1, Muc4, and Muc16. At the time points examined here, all three were expressed, with Muc1 and Muc4 present at approximately 100-fold and approximately 1,000-fold higher concentrations than Muc16, and approximately 10-fold and approximately 100-fold lower concentrations than Muc5b, respectively (Figure E1).
Figure 2.
Quantitative RT-PCR analysis demonstrates the expression of Muc5ac and Muc5b in the developing murine lung. Gene-specific probes were used to measure concentrations of the mucins Muc2 (solid circles), Muc5ac (solid squares), and Muc5b (solid triangles), and of Clara cell secretory protein (Scgb1a1) (open circles), compared with γ-actin. Data represent means ± standard errors (n = 3–8 animals/time point). Data were analyzed by ANOVA. P < 0.05 was considered significant. **Identifies significance between concentrations of Muc5b mRNA and those of Muc5ac and Muc2. †Identifies significance between Muc5ac and Muc2. ‡Identifies significance between embryonic day (E) 14.5 and later values. Differences in concentrations of Muc5ac and Muc2 were not significant over time. PN, postnatal day.
Muc5ac and Muc5b Proteins Are Expressed in the Developing Murine Lung
Protein expression levels were tested immunohistochemically, using antibodies specific for Muc5ac and Muc5b. Muc5b protein was continuously produced by tracheobronchial epithelial cells from E14.5–PN28, with a steady increase from E16.5–E18.5, after which it remained abundantly detectable postnatally within tracheobronchial surface epithelial cells (Figure 3). Muc5b was highly enriched in cartilaginous airways from E15.5 onward. It was also present in the intrapulmonary axial airways, although often at significantly lower concentrations, and it was absent in the bronchioles (Table E4). Muc5ac was also detectable during murine lung development (Figure 4). In contrast to the persistently strong and significantly more abundant immunolabeling seen for Muc5b (Table E4), immunolabeling for Muc5ac in the airways proved negative at all time points studied, except on PN14 (Table E5).
Figure 3.
Muc5b protein is constitutively produced in prenatal and postnatal developing murine lungs. Immunostaining for Muc5b was performed using polyclonal rabbit anti-mouse Muc5b antibody (1:10,000), and detected with a peroxidase-conjugated goat anti-rabbit antibody (1:200) and 3,3′-diaminobenzidine (DAB). Large arrows in low-magnification images (top) identify airway sites shown in high magnification (below). Small arrows (below) identify positively stained cells. Scale bar, 2 mm for low magnification, and 30 μm for high magnification.
Figure 4.
Muc5ac protein is briefly produced in postnatal developing murine lungs. Immunostaining for Muc5ac was performed using polyclonal chicken anti-mouse Muc5ac peptide chicken polyclonal antibody (1:2,000), and detected with a biotinylated goat anti-chicken antibody (1:500) and DAB. Large arrows in low-magnification images (top) identify airway sites shown in high magnification (below). Small arrows (below) identify positively stained cells. Scale bar, 2 mm for low magnification, and 30 μm for high magnification.
DISCUSSION
This study demonstrates that murine airways express the gel-forming mucins Muc5ac and Muc5b during prenatal and postnatal development. In particular, Muc5b is abundantly expressed at early embryonic time points, and continuously throughout lung development (Figures 2 and 3). It remains abundant under steady-state conditions in the adult, as we showed previously (7, 8). Thus, the production of Muc5b is an early, intrinsic, and constitutive process in the airways. By contrast, although it is markedly and selectively up-regulated during inflammation in numerous models (2, 3), the expression of Muc5ac is much lower at all time points studied here (Figure 2). These data suggest that Muc5b is sufficient for the homeostatic functions of airway mucus, whereas the expression of Muc5ac may take on greater importance under pathophysiological conditions.
Numerous studies from our group and others focused on the association of the up-regulated production of mucin in disease states such as asthma (13, 14), chronic obstructive pulmonary disease (15–17), and cystic fibrosis (18–20), and in animal models of these diseases (7, 21–26). In the present study, however, we focused on the production of airway mucins under nondiseased conditions. The current dogma states that the intrapulmonary airways of mice are lined by Clara cells but lack goblet/mucous cells at baseline (the latter finding was based on traditional staining techniques such as AB-PAS) (27). However, we found that numerous cells do line murine airways, and produce mucin in the baseline state (Figures 1 and 3). Our best explanations for the differences between our findings and those in the literature are twofold. First, the production of Muc5b at baseline occurs in balance with its tonic rate of secretion, keeping intracellular concentrations low. This is supported by its intracellular accumulation at the histochemical level, without any change in its expression at the mRNA level in secretion-deficient Munc13–2 knockout mice (8). Second, the routine AB-PAS chemical labeling techniques have 100–1,000-fold lower sensitivity (28, 29) than those using antibody/lectin labeling followed by enzyme-mediated detection approaches, similar to those used here (8, 29, 30). As such, this study confirms abundant Muc5b production in adult mice (8, 31). Further, we demonstrate that Muc5b gene expression occurs precociously in murine lungs, preceding even that of the eponymous marker Clara cell secretory protein (Scgb1a1).
The increased production of mucin in the intrapulmonary airways of adult mice during mucous metaplasia localizes to a subset of Scgb1a1-expressing secretory cells in the bronchial (central) airways, but it is absent in Scgb1a1-expressing cells of the bronchiolar (peripheral) airways (9). This phenomenon of spatially restricted mucin production in adult mice is also present in the baseline uninflamed state (8). In the present study, we found that this was also the case in developing lungs in utero. Thus, the specification program that determines the mucin-producing potential of secretory cells in murine airways occurs embryonically. In addition to Muc5ac and Muc5b, numerous other conducting airway-specific genes demonstrate spatially and temporally restricted gene expression patterns. These include Scgb1a1 and the related secretaglobin genes Scgb3A1 and Scgb3A2 (32), the adenosine A3 receptor (33), and the chitinases (34). Notably, nascent Muc5b expression at E14.5 precedes that of Scgb1a1 and its relatives at E16.5 and later (Figure 2) (32). Given that discrete spatiotemporal gene regulatory programs are activated at each step of lung development, understanding which program functions at the initial time of airway secretory cell differentiation could provide novel insights into the regulation of secretory product synthesis in adult lungs.
In addition to the novel identification of mucin expression patterns in embryonic murine lungs, our study also identified a brief period of mucous metaplasia during postnatal development. At PN14, the airway epithelium has a mucous phenotype, similar to that seen in antigen-sensitized and antigen-challenged mice with a prominent mucous phenotype and induction of Muc5ac (9). This early postnatal phenomenon was also reported by others, with allergic inflammatory (35, 36) and Foxa2 transcription factor (35) regulatory pathways being strongly implicated. Notably, our study is novel because it distinguished between the abundance of Muc5ac, Muc5b, and other mucins. At the protein level, Muc5ac was significantly increased at PN14, whereas the production of Muc5b remained stable (Figures 3 and 4, and Tables E3 and E4). In our study, however, the 7.7-fold increase in the detection of Muc5ac mRNA was not statistically significant (Figure 2). This discordance is likely related to either a missed point of transcriptional up-regulation at some discrete time after PN5 and before PN14, or to the tonic activation of the Muc5ac promoter at moderately low concentrations throughout this period. Because this phenotype occurs during postnatal alveologenesis, that is, a period of high cell proliferation and alveolar multiplication in murine lungs (37–39), a persistently up-regulated mucous phenotype may be induced to facilitate the clearance of materials from peripheral compartments during distal lung morphogenesis.
In humans, gel-forming mucins are also expressed during lung development (40, 41). Although the gestational periods of mice and humans differ greatly (3 weeks versus 40 weeks, respectively), the phases of lung development are quite similar. The embryonic, pseudoglandular, and canalicular phases occur at 3–7, 5–17, and 16–26 weeks in humans, and on E9–E11.5, E11.5–E16.5, and E16.5–E17.5 in mice. The patterns of MUC5AC/Muc5ac and MUC5B/Muc5b expression during these stages correlate across species (MUC in humans/Muc in mice). Before E14.5 in mice, the bronchial epithelium is a poorly differentiated, pseudostratified layer, and very little Muc5ac or Muc5b is evident. This is also the case for MUC5AC and MUC5B expression in human embryos before 5 weeks of gestational age. During the pseudoglandular and canalicular phases of lung development, a transformation of the epithelium to a cuboidal appearance occurs, coinciding with the increasing expression of MUC5B/Muc5b in the bronchial submucosal glands (humans) and the surface epithelium (humans and mice) (40, 41) (Figure 3 and Table E3). Indeed, significant differences are evident in the lungs of mice and humans, involving total lung capacity (TLC), conducting airway volume relative to TLC, the abundance and location of smooth muscle and cartilage, and the expression and localization of secretory products such as Scgb1a1. However, because mice lack intrapulmonary submucosal glands, the abundant expression of Muc5b by surface cells is likely to substitute for homeostatic functions that are supplied by Muc5b-rich submucosal gland secretions in larger mammals and humans from development onward. We thus propose that even in the presence of strong differences in lung anatomy among species, the phenotypic continuity of baseline MUC5B/Muc5b expression underlies an important functional role for that expression in homeostatic functions such as mucociliary clearance.
The expression of gel-forming mucins by the airway epithelium is presumed to have an important function in regulating lung health and disease. The 11p15 locus (where MUC5AC and MUC5B lie) is linked to bronchial hyperreactivity in asthma (42), and polymorphisms in the MUC5B promoter region were linked to the development of diffuse panbronchiolitis (43) and idiopathic interstitial pneumonias (44). Confirmation that these variations are based on deleterious mutations will implicate impaired homeostatic and pathophysiological mucous functions, and in particular the importance of the gel-forming mucins MUC5AC and MUC5B, in the development and prevention of lung pathology. In addition to chronic lung diseases in which the production of mucins may be etiological, diseases in which airway mucin overproduction and mucous hypersecretion are prominent in perinatal or early postnatal life, such as bronchopulmonary dysplasia and cystic fibrosis, may be affected by the gestational production of airway mucins. Notably, childbirth can result in the exposure of newborn infants to environmental microbial agents such as group B streptococci (45). We speculate that the production of Muc5b is important in preventing colonization and infection by these and other potential pathogens. However, in the absence of empirical tests of causality, the assertion of essential roles for these respiratory mucins remains hypothetical. Findings in Muc2 mutant mice serve as precedents that provide strong support for a protective function of gel-forming mucin in the intestinal tract. The loss or mutation of Muc2, the sole gel-forming mucin produced in the colon at baseline, causes persistent colitis and the development of colorectal tumors in mice (46–48). Future studies investigating the roles of the respiratory mucins Muc5ac and Muc5b may demonstrate their significance in health and disease.
This work was supported in part by National Institutes of Health grant HL080396 (C.M.E.) and American Lung Association grant RG-22720.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0020OC on March 18, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 2000;92:55–81. [DOI] [PubMed] [Google Scholar]
- 2.Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther 2009;121:332–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 4.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 2008;70:459–486. [DOI] [PubMed] [Google Scholar]
- 5.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 2002;347:2141–2148. [DOI] [PubMed] [Google Scholar]
- 6.Perl AK, Whitsett JA. Molecular mechanisms controlling lung morphogenesis. Clin Genet 1999;56:14–27. [DOI] [PubMed] [Google Scholar]
- 7.Young HW, Williams OW, Chandra D, Bellinghausen LK, Perez G, Suarez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol 2007;37:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13–2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 2008;586:1977–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shekels LL, Lyftogt C, Kieliszewski M, Filie JD, Kozak CA, Ho SB. Mouse gastric mucin: cloning and chromosomal localization. Biochem J 1995;311:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ten Have-Opbroek AA, Dubbeldam JA, Otto-Verberne CJ. Ultrastructural features of Type II alveolar epithelial cells in early embryonic mouse lung. Anat Rec 1988;221:846–853. [DOI] [PubMed] [Google Scholar]
- 12.Das B, Cash MN, Hand AR, Shivazad A, Grieshaber SS, Robinson B, Culp DJ. Tissue distribution of murine Muc19/Smgc gene products. J Histochem Cytochem 2010;58:141–156. [DOI] [PMC free article] [PubMed]
- 13.Groneberg DA, Eynott PR, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson AG, Harrison BD, et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 2002;40:367–373. [DOI] [PubMed] [Google Scholar]
- 14.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523. [DOI] [PubMed] [Google Scholar]
- 15.Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology 2004;45:477–484. [DOI] [PubMed] [Google Scholar]
- 16.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 2006;130:1102–1108. [DOI] [PubMed] [Google Scholar]
- 17.Hovenberg HW, Davies JR, Herrmann A, Linden CJ, Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj J 1996;13:839–847. [DOI] [PubMed] [Google Scholar]
- 18.Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax 2007;62:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med 2002;96:81–86. [DOI] [PubMed] [Google Scholar]
- 20.Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med 2007;175:816–821. [DOI] [PubMed] [Google Scholar]
- 21.Kouznetsova I, Chwieralski CE, Balder R, Hinz M, Braun A, Krug N, Hoffmann W. Induced trefoil factor family 1 expression by trans-differentiating Clara cells in a murine asthma model. Am J Respir Cell Mol Biol 2007;36:286–295. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhao YH, Wu R. In silico cloning of mouse Muc5b gene and upregulation of its expression in mouse asthma model. Am J Respir Crit Care Med 2001;164:1059–1066. [DOI] [PubMed] [Google Scholar]
- 23.Zudhi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol 2000;22:253–260. [DOI] [PubMed] [Google Scholar]
- 24.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol 2007;36:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med 2008;177:730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 2004;10:487–493. [DOI] [PubMed] [Google Scholar]
- 27.Pack RJ, Al Ugaily LH, Morris G, Widdicombe JG. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res 1980;208:65–84. [DOI] [PubMed] [Google Scholar]
- 28.Pon DJ, van Staden CJ, Rodger IW. Hypertrophic and hyperplastic changes of mucus-secreting epithelial cells in rat airways: assessment using a novel, rapid, and simple technique. Am J Respir Cell Mol Biol 1994;10:625–634. [DOI] [PubMed] [Google Scholar]
- 29.Thornton DJ, Carlstedt I, Sheehan JK. Identification of glycoproteins on nitrocellulose membranes and gels. Mol Biotechnol 1996;5:171–176. [DOI] [PubMed] [Google Scholar]
- 30.Lin H, Carlson DM, St George JA, Plopper CG, Wu R. An ELISA method for the quantitation of tracheal mucins from human and nonhuman primates. Am J Respir Cell Mol Biol 1989;1:41–48. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, Ammar-Aouchiche Z, Ho SB, Ehre C, Kesimer M, Knoll BJ, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol 2008;38:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, Stripp BR. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med 2002;166:1498–1509. [DOI] [PubMed] [Google Scholar]
- 33.Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol 2006;35:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, Elias JA. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol 2006;291:L502–L511. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Wan H, Luo F, Zhang L, Xu Y, Lewkowich I, Wills-Karp M, Whitsett JA. FoxA2 programs Th2 cell–mediated innate immunity in the developing lung. J Immunol 2010;184:6133–6141. [DOI] [PubMed] [Google Scholar]
- 36.Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, O'Neal WK, Boucher RC, Randell SH. Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na+ channel–overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol 2009;182:4357–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee SK, Young HW, Barczak A, Erle DJ, Blackburn MR. Abnormal alveolar development associated with elevated adenine nucleosides. Am J Respir Cell Mol Biol 2004;30:38–50. [DOI] [PubMed] [Google Scholar]
- 38.Hind M, Corcoran J, Maden M. Alveolar proliferation, retinoid synthesizing enzymes, and endogenous retinoids in the postnatal mouse lung: different roles for Aldh-1 and Raldh-2. Am J Respir Cell Mol Biol 2002;26:67–73. [DOI] [PubMed] [Google Scholar]
- 39.Thurlbeck WM. Lung growth and alveolar multiplication. Pathobiol Annu 1975;5:1–34. [PubMed] [Google Scholar]
- 40.Buisine MP, Devisme L, Copin MC, Durand-Reville M, Gosselin B, Aubert JP, Porchet N. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol 1999;20:209–218. [DOI] [PubMed] [Google Scholar]
- 41.Reid CJ, Gould S, Harris A. Developmental expression of mucin genes in the human respiratory tract. Am J Respir Cell Mol Biol 1997;17:592–598. [DOI] [PubMed] [Google Scholar]
- 42.Collaborative Study on the Genetics of Asthma. A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nat Genet 1997;15:389–395. [DOI] [PubMed] [Google Scholar]
- 43.Kamio K, Matsushita I, Hijikata M, Kobashi Y, Tanaka G, Nakata K, Ishida T, Tokunaga K, Taguchi Y, Homma S, et al. Promoter analysis and aberrant expression of the MUC5B gene in diffuse panbronchiolitis. Am J Respir Crit Care Med 2005;171:949–957. [DOI] [PubMed] [Google Scholar]
- 44.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. A common MUC5B promoter polymorphism is associated with pulmonary fibrosis. N Engl J Med 2011;364:1503–1512. [DOI] [PMC free article] [PubMed]
- 45.Larsen JW, Sever JL, Group B. Streptococcus and pregnancy: a review. Am J Obstet Gynecol 2008;198:440–448. [DOI] [PubMed] [Google Scholar]
- 46.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008;5:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006;131:117–129. [DOI] [PubMed] [Google Scholar]
- 48.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002;295:1726–1729. [DOI] [PubMed] [Google Scholar]