Abstract
Species in the genus Pneumocystis can cause severe pneumonia in immune-compromised hosts. The identification of specific targets present in Pneumocystis species, but lacking in mammalian hosts, is paramount to developing new means to treat this infection. One such potential protein is Rtt109, which is a type of histone acetyltransferase (HAT) required for DNA replication in fungi, but not found in mammals. Sequence orthologues of Rtt109 are present in other fungi, but are absent in mammals, making it a potential pan-specific target against medically relevant fungi. Accordingly, we sought to identify the presence of an Rtt109 in P. carinii. A Pneumocystis carinii (Pc) Rtt109 165-bp partial sequence was initially identified from the incomplete P. carinii genome database. Subsequently, a full-length, 1,128-bp cDNA with homology to Saccharomyces cerevisiae Rtt109 (39% Basic Local Alignment Search Tool (BLASTP)) was cloned and characterized. Sequence analysis of PcRtt109 indicated that the P. carinii molecule contains the putative catalytic aspartate present in yeast. We further demonstrated that the PcRtt109 expressed in rtt109Δ S. cerevisiae cells restored H3-K56 acetylation and the sensitivity toward DNA-damaging agents of rtt109Δ mutant cells. Purified PcRtt109 had the ability to acetylate lysine-56 of histone H3, similar to the ability of Schizosaccharomyces pombe Rtt109 protein. The site-directed mutagenesis of PcRtt109 D84A, a potential regulatory site in the Rtt109 HAT family, abolished H3 acetylation, whereas a DD218/219AA mutation that compromised the activity of ScRtt109 had little effect, demonstrating similarities and differences in Pneumocystis PcRtt109 compared with yeast Saccharomyces cerevisiae Rtt109. These results indicate that P. carinii contains an Rtt109 HAT molecule, and represent the complete identification and characterization of a HAT molecule from this important opportunistic fungal pathogen.
Keywords: Pneumocystis, histone acetyltransferase, Rtt109
CLINICAL RELEVANCE.
Pneumocystis is an important cause of lethal pneumonia in immune-compromised hosts. Pneumocystis contains an active histone transacetylase Pneumocystis carinii (Pc) Rtt109, needed for DNA replication and repair. Inhibitors of this molecule may represent a new drug target for this infection.
In eukaryotic organisms, DNA is assembled into chromatin structures consisting of repeat units called nucleosomes; each wrapped around two copies of core histone octamers. Chromatin exerts substantial regulatory effects on DNA metabolic processes, including DNA transcription and translation, repair, and replication (1–3). Posttranslational modifications of histones play an important role in regulating these processes (4). Modifications of histones were demonstrated to occur mostly on the N-terminus. These modifications play a vital role in both transcriptional regulation and DNA replication (5, 6). One modification to the histone core involves the acetylation of lysine-56 of histone H3 (H3-K56Ac) that occurs on newly synthesized histones (5). The histone acetyltransferase (HAT) responsible for the acetylation of K56 on the H3 histone in fungi is Rtt109 (7, 8). From a functional standpoint, the acetylation by Rtt109 of H3 histone promotes the survival of cells exposed to DNA-damaging agents, at least partly by promoting replication-coupled nucleosome assembly after DNA replication and DNA repair (9, 10).
In Saccharomyces cerevisiae, two chaperone proteins, Asf1 and Vps75, were implicated in the function of Rtt109. In vitro, the acetylation of K56 on the H3 histone is stimulated by both Asf1 and Vps75, and maximal activity requires the presence of both chaperones. However, only Asf1 is required to produce H3-K56Ac in cells. In vitro, both Asf1 and Vps75 are important for the high catalytic activity of Rtt109 in H3-K56Ac (7, 12–15). Mutational studies indicate that aspartic acid at position 89 (D89) is essential for the HAT activity of S. cerevisiae Rtt109 (ScRtt109). D287 was proposed to serve as the catalytic residue (16). However, mutations at D287 had no apparent effect on the enzymatic activity of ScRtt109. Mutations at both D287 and D288 resulted in a severe reduction of the HAT activity of ScRtt109 (17). Therefore, D287 alone is unlikely to serve the catalytic residue. These three aspartate residues are conserved among the Rtt109 molecules of various fungal species. Accordingly, determining whether these aspartate residues are important for the HAT activity of the enzyme will be of value.
Only minor amounts of histone H3 Lys-56 acetylation have been recorded in Drosophila and human cells. Genes encoding proteins orthologous to Rtt109 appear to be restricted to the fungi. However, a domain of Rtt109 shares structural similarity with human p300. Recently, H3K56Ac was shown to be catalyzed by p300 or “General Control Nonderepressible 5” (GCN5) (18–20). An inhibitor against p300 has little effect on Rtt109 catalytic activity, suggesting that Rtt109 uses a distinct mechanism for catalysis compared with p300. Together, these results suggest that Rtt109 may be an attractive target for the development of therapeutic antifungal drugs. Although P. carinii infects rats and Pneumocystis jirovecii infects humans, all drugs currently in clinical use for the treatment in human disease were first developed through studies of P. carinii in rats or Pneumocystis murina in mice, before the initiation of human studies. Thus, studies of potential drug targets in P. carinii are important initial steps in the development of drugs for Pneumocystis infection in humans.
Accordingly, we sought to determine whether histone H3 lysine-56 acetylation is present in P. carinii (21–23). In our investigation, we evaluated the activity and characterization of this protein in Pneumocystis. Here, we demonstrate that P. carinii contains a functional Rtt109 homologue, with extensive homology to both yeast and Schizosaccharomyces pombe Rtt109 proteins. We further confirmed that Pneumocystis carinii (Pc) Rtt109 could confer the H3-K56 acetylation of H3 histone in a deficient yeast rtt109Δ strain, and restore growth to this mutant strain in the presence of genotoxic agents that interfere with DNA replication. Finally, an in-depth biochemical analysis of purified PcRtt109 protein verified the importance of Asf1 and Vps75 in the efficient acetylation of H3-K56. Mutations in amino acid D84 of PcRtt109 resulted in a dramatic decrease in H3K56 acetylation. However, in striking contrast to yeast Rtt109, the mutations at DD218/219 exerted little effect on this catalytic event, indicating unique characteristics of the Pneumocystis enzyme. Thus, we provide the first evidence for the identification and biochemical characterization of an Rtt109 homologue from a pathogenic fungal organism. Because of the Rtt109 protein's importance in maintaining proper DNA replication and lesion repair, and its differentiating features compared with human H3-K56Ac enzymes, PcRtt109 may represent a viable target for future antifungal drug development.
MATERIALS AND METHODS
Strains
In these studies, P. carinii organisms were originally derived from American Type Culture Collection (ATCC, Manassas, VA) stocks and propagated in corticosteroid-treated rats, as reported previously (24, 25). Whole populations of P. carinii containing both trophic forms and cysts were purified from chronically infected rat lungs by homogenization and filtration through 10-μM filters, as we previously described (26, 27). The yeast rtt109Δ strain (YLL002W) and the parental wild-type strain were both obtained from the ATCC. Yeast strains were transformed with the yeast expression plasmid pYES2.1/V5-His-TOPO under the control of the Galactose 1 (GAL1) promoter containing either the complete PcRtt109 cDNA, or with the control vector pYES2.1/V5-His/lacZ. This expression vector has been used extensively to study P. carinii gene transcription and translation (28–30).
Media and Growth Conditions
For testing PcRtt109 transcriptional response at the specified pH, P. carinii life forms were purified by homogenization, and filtrated through 10-μM filters to remove lung cells. Total P. carinii life forms were resuspended in Ham's F-12 media plus 10% FCS at the specified pH for a period of 2 hours at 37°C with 5% CO2 (28). For the selection of transformants and the maintenance of plasmids in yeast, we used Complete Synthetic Media (Q·BIOgene, Solon, OH) with the appropriate dropout components. For the analysis of rtt109Δ yeast cells expressing PcRtt109 cDNA, cultures were incubated overnight in the presence of 2% galactose. All yeast cultures were grown overnight at 30°C.
Identification of PcRtt109 cDNA
Upon conducting an initial keyword search for “Rtt109” in the P. carinii Genome Project Database (http://pgp.cchmc.org/), we located a total of eight partial PcRtt109 sequences in the genome database. A 165-bp cDNA sequence was selected, based on its suitability for sequence extension and complete cloning, using the rapid amplification of cDNA ends (RACE) strategy. Initial analysis of the 165-bp cDNA sequence derived from the P. carinii Genome Project Database revealed the sequence to be homologous to the N-terminal region of a family of histone acetyltransferases found in other fungi and yeast. We next obtained the complete cDNA sequence, using a ligase-mediated rapid amplification strategy from the 5′ and 3′ cDNA ends, using the 5′ and 3′ “gene-racing” method (GeneRacer Kit; Invitrogen, Carlsbad, CA) (28, 29). In brief, known sense and antisense primers derived from the partial sequence were combined with 5′ and 3′ anchored PCR primers to obtain the complete full-length gene, which we termed PcRtt109. To accomplish this, 2 μg of total P. carinii RNA were prepared with TRIzol reagent (Invitrogen), according to the manufacturer's protocol, and ligated to the GeneRacer RNA oligo. We next used either the Oligo dT GeneRacer primer or a specific reverse primer from the PcRtt109 sequence as primers to produce the first-strand cDNAs. Both 5′ and 3′ RACE reactions were completed according to the manufacturer's protocol, and aliquots of RACE products were resolved on 2% agarose gels. The complete sequencing of amplified regions generated the complete 1,128-bp cDNA of PcRtt109.
Chromosomal and Southern Hybridization of PcRtt109
To verify that the original P. carinii genome database clone was truly of Pneumocystis origin, and to localize the chromosomal origin of this molecule, two experiments were undertaken. First, the 165-bp sequence obtained from the P. carinii Genome Database was amplified from P. carinii genomic DNA, radiolabeled as a probe, and used to hybridize to BamHI, HindIII, or XhoI P. carinii genomic DNA. As a negative control, the same probe was hybridized against similarly digested uninfected rat-lung genomic DNA. In parallel, the random primer–labeled 165-bp amplicon was further hybridized to P. carinii chromosomes, separated by a contour-clamped homogenous electric field (CHEF) blot to determine its chromosomal localization (28, 31–34). Hybridization and subsequent washes were performed according to the ExpressHyb protocol (Clontech, Mountain View, CA).
Evaluation of PcRtt109 Expression
Previous studies demonstrated the differential expression of Pneumocystis genes under conditions of varying pH (28, 35). Accordingly, the pH-regulated expression of PcRtt109 was evaluated by Northern blotting. Pneumocystis organisms were maintained for 2 hours at various pH levels, and total RNA was isolated. An equal amount of RNA (5.0 μg) was separated through a 1.0% agarose gel in the presence of 2.2 M formaldehyde, transferred to nitrocellulose, and hybridized with the 165-bp probe, labeled with α-32P dATP (Amersham, Inc., Piscataway, NJ). After hybridization, membranes were washed four times, and visualized using autoradiography.
To analyze the potential effects of pH on PcRtt109 expression further, quantitative real-time RT-PCR was also performed. To accomplish this, we used the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). RNA was extracted from P. carinii organisms, using a Qiagen RNA isolation kit (Valencia, CA) after incubation under various pH conditions for 2 hours. To generate cDNA for quantitative PCR analyses, SuperScript III Reverse Transcriptase was used (Invitrogen). The final cDNA PCR products were quantified with the incorporation of the SYBR Green PCR Master Mix (Invitrogen). Conditions for the PCR reactions entailed 50°C for 2 minutes, 95°C for 2 minutes, and 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. During the 72°C condition, analysis of the SYBR fluorophore for quantification was peformed. Primers for the amplification of PcRtt109 cDNA included 5′-ATCTTGACGGGGGTTTTGTAGAG -3′ and 5′-GCTCACTCGCATCACCTTGAAG -3′. A standard curve was generated to determine the relative PcRtt109 mRNA expressed at the respective pH.
Complementation of Rtt109-Deficient Yeast (rtt109Δ) H3-K56 Acetylation Defects through the Heterologous Expression of PcRtt109
Pneumocystis species cannot be maintained using in vitro culture, and cannot yet be genetically manipulated. To overcome this problem, our group and other laboratories have assessed gene function through the use of heterologous expression and complementation in related species such as in S. cerevisiae. Accordingly, to evaluate the potential activities of Pneumocystis PcRtt109 in complementing the H3-K56 acetylation defect, the rtt109Δ yeast strain was transformed with either pYES2.1 alone or the same vector containing the full-length Pneumocystis PcRtt109 cDNA. Exponentially growing yeast cells were induced to express the transgene by culture in the presence of 2% galactose overnight. Finally, cells were harvested and the protein was extracted (17). Approximately 5–10 μg of total proteins were separated on 15% PAGE gels and transferred to nitrocellulose, and the membranes were blocked with 5% milk in Tris-buffered saline Tween-20 (TBST) buffer and subjected to Western blotting with antibodies recognizing acetylated H3-K56Ac. Total H3 histone and proliferating cell nuclear antigen (PCNA) were used as protein loading controls (17).
Genotoxin Sensitivity Assays
The acetylation of H3 histone by Rtt109 proteins promotes the survival of cells exposed to DNA-damaging agents. Accordingly, we assessed the growth of PcRtt109-complemented yeast in the presence or absence of various genotoxins. After the overnight growth of various strains at 30°C in minimal medium with 2% galactose, yeast cells were diluted to approximately 6 × 106 cells/ml. Yeast cells were then serially diluted and plated on minimal media plates containing 2% galactose in the presence of several DNA-damaging agents: camptothecin (CPT; 1 μg/ml), hydroxyurea (HU; 50 mM), and methyl methanesulfonate (MMS; 0.005%) (17). Next, yeasts were cultured for 2 additional days, and growth was assessed.
Expression and Purification of Recombinant Rtt109 Proteins
To purify S. pombe Rtt109 (SpRtt109p) and PcRtt109p proteins, both full-length cDNAs were amplified from cDNA, using Pfu DNA polymerase (Stratagene, Santa Clara, CA). Both genes were further cloned into the pGEX-4T1 vector, sequenced, and used to transform bacteria strain BL21 (DE3)pLys-S. The expression of Glutathione S-transferase (GST)-tagged proteins was performed overnight at 18°C, using 0.1 mM Isopropyl B-D-1-thiogalactopyranoside (IPTG). Approximately 1.0-L aliquots of overnight culture broth were collected by centrifugation, and lysates were obtained by passing cells through a French press in lysis buffer (17). Lysates were clarified by centrifugation, and the respective proteins were purified over glutathione-Sepharose, using standard techniques. To analyze the role of potential known catalytic amino-acid residues in PcRtt109 protein further, site-directed mutagenesis (QuikChange reagent; Stratagene) was performed on PcRtt109 aspartic acid 84 and aspartic acids 218 and 219. Both amino-acid regions were converted to alanine residues. Both regions were previously implicated in the possible acetylation of H3-K56 in yeast (17).
Purification of Recombinant Histone H3/H4 Tetramers and the Formation of Asf1–H3/H4 Complexes
Purified Drosophila H3/H4 tetramers were obtained as previously described (36). After purifying yeast Asf1 protein over glutathione-sepharose beads, Drosophila H3/H4 tetramers were incubated with purified Asf1 protein overnight at 4°C, and the Asf1–H3/H4 complex was separated by gel filtration chromatography (7).
HAT Assays
HAT activity was measured as previously reported, with minimal modifications (17, 37). Briefly, samples were incubated at 30°C for 30 minutes in 15-μl reaction mixtures that contained 50 mM Tris-HCl, pH 8.0, 5% (wt/vol) glycerol, 0.1 mM EDTA, 1 mM dithiothreitol, 5 mM phenylmethylsulfonyl fluoride, and 6 pmol of [3H]acetyl-coenzyme A (CoA). Recombinant Drosophila H3/H4 tetramers (1 μg; dH3/H4) or Asf1-dH3/H4 was added as substrate. One half of each reaction mixture (7.5 μl) was then spotted onto P-81 phosphocellulose paper filters and air-dried. Paper filters were washed five times for 5 minutes each with 100 ml of 50 mM NaHCO3 buffer, pH 9.0, and once with 50 ml of acetone. The radioactivity of air-dried filters was measured using a liquid scintillation counter. To detect which protein was acetylated, proteins from each reaction mixture were resolved using 15% SDS-PAGE, and then the gels were dried and exposed to film. To detect whether H3–lysine-56 was acetylated, samples were incubated with unlabeled acetyl-CoA at 30°C for 30 minutes, and Western blot analysis, using antibodies against H3-K56Ac, was performed. For HAT assays, purified regulatory alpha (REGα), a proteosome-binding protein, was used as a negative control (17).
Statistical Analysis
All data are expressed as mean ± SEM. Differences between groups were determined using one-way ANOVA and two-tailed Student t tests. Statistical testing was performed using the GraphPad Prism version 5.0b software program (La Jolla, CA), with statistical differences considered significant if P < 0.05.
RESULTS
Pneumocystis carinii Contains a Putative Rtt109 Homologue Gene, PcRtt109
A partial Rtt109-related sequence was initially identified by conducting a keyword search in the incomplete Pneumocystis carinii genome database. This search revealed an incomplete DNA fragment of 165 bp, that when translated by Basic Local Alignment Search Tool (BLASTX) algorithms exhibited similarities to the S. cerevisiae Rtt109 protein in the region of amino acids 47–255. Therefore, to characterize this molecule further, we isolated the remaining portions of PcRtt109, using the RACE strategy. Analysis of the complete PcRtt109 cDNA sequence revealed an open reading frame encoding a predicted 1,128-bp amino-acid protein with a theoretical mass of 43,482 Da (GenBank accession number FJ588486). Protein sequence alignments of PcRtt109 with other fungal Rtt109 orthologues are shown in Figure 1. In addition to S. pombe, S. cerevisiae, and Candida albicans, BLASTX analyses revealed homology with other fungal Rtt109 orthologues that included Ajellomyces dermatitidis (GenBank accession number EEQ89207), Aspergillus nidulans (GenBank accession number CBF77983), Aspergillus fumigatus (GenBank accession number XP_753685), and Penicillium marneffei (GenBank accession number XP_002147821), among others. PcRtt109 was predicted to contain the conserved aspartate residue at amino-acid position 84. Mutations of corresponding residues of the budding yeast Rtt109 results in the complete abolishment of its ability to acetylate H3-K56 in vitro and in cells (16, 17). In addition, at amino acid 221, PcRtt109 also appears to exhibit a conserved lysine residue, which was shown in yeast to be the autoacetylation site of Rtt109.
Figure 1.
Pneumocystis carinii (PcRtt109) is homologous to yeast and other potential fungal Rtt109 proteins involved in the acetylation of histone H3 at lysine 56. Multiple-sequence alignments of Rtt109-related histone acetyltransferases were performed using CLUSTALW (MacVector 8.1.2). *Similar amino acids. *Similarly charged amino acids. PNECA, P. carinii; SCHPO, Saccharomyces pombe; YEAST, Saccharomyces cerevisiae; CANAL, C. albicans.
Characterization of Pneumocystis PcRtt109
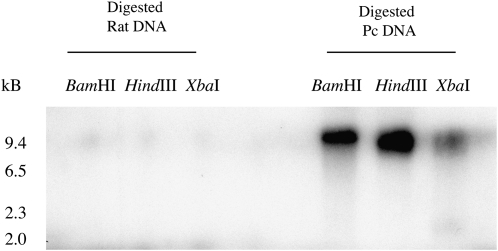
Upon obtaining the complete 1,128-bp Pneumocystis PcRtt109 sequence, the original 165-bp sequence was used as a radiolabeled probe and hybridized to restriction endonuclease–digested Pneumocystis carinii genomic DNA, demonstrating strong localization to a single band after application of the representative digests (Figure 2). Furthermore, the 165-bp PcRtt109 probe failed to hybridize to digested, uninfected rat-lung DNA, indicating that the amplification product was specifically represented within the P. carinii genome. We further mapped the PcRtt109 165-bp probe to a single P. carinii chromosome (chromosome 6) by CHEF blotting (Figure 3). Taken together, these data strongly indicate that PcRtt109 is present as a single-copy gene within the organism.
Figure 2.
A 165-bp Pneumocystis PcRtt109 gene fragment specifically hybridizes to P. carinii genomic DNA. P. carinii organisms were freshly isolated, and genomic DNA was isolated and digested with the indicated restriction endonucleases. The digestion products were separated by electrophoresis and transferred to nitrocellulose. The 165-bp PcRtt109 amplicon was labeled and hybridized to the membrane indicating specific interaction with Pneumocystis nucleic acids. Furthermore, the amplicon failed to hybridize to rat genomic DNA. These data strongly suggest that Pneumocystis PcRtt109 is represented as a single locus within the P. carinii genome.
Figure 3.
The PcRtt109 165-bp amplicon hybridizes to a single chromosome. Left: Ethidium-stained P. carinii chromosomes were separated by contour-clamped homogenous electrophoresis. Lane 1 represents reference markers. Lanes 2–4 each represent chromosomes derived from the P. carinii isolate purified from an individually isolated infected rat lung (56). Right: Lanes 6–8 represent the separated chromosomes transferred to nitrocellulose and hybridized with the radiolabeled 165-bp probe. Arrow marks the hybridization of the PcRtt109 probe that interacted with a single Pneumocystis chromosome. Lane 5 represents the transferred reference markers that did not hybridize to the PcRtt109 probe. The nucleic loading of Lanes 2 and 6 were substantially lower and thus less able to demonstrate the robust hybridization of PcRtt109 evident in Lanes 7 and 8.
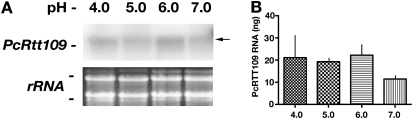
Previous observations from our laboratory indicated that the transcriptional expression of key life-cycle–regulatory molecules, including the Pneumocystis cell wall integrity gene PcPhr1 and the cell wall biosynthesis gene PcCbk1, are both controlled by environmental pH level (28, 35). More recently, we observed that another Pneumocystis gene, the transcription factor PcFlo8 required for downstream activation in yeast of the major adhesin, Flo11, is also pH-regulated in Pneumocystis (38). All three genes in Pneumocystis are expressed at their greatest level at pH 6–8, a physiologic pH present in the lung (28, 35, 38, 39). Accordingly, we sought to address whether PcRtt109 expression was also regulated by environmental pH during 2 hours in tissue culture medium with a pH in the range of 4–7. Unlike the other three Pneumocystis genes tested previously, no difference was evident in the expression levels of the PcRtt109 transcript according to Northern blot analysis (Figure 4A). To quantify these observations further, quantitative real-time RT-PCR was performed under these pH conditions (Figure 4B). Again, in contrast to our previous studies of pH-responsive 1 (PcPhr1) and cell wall kinase 1 (PcCbk1), we saw no reduction of the suppression of PcRtt109 expression at pH 4.0, 5.0, or 6.0. Although the expression of PcRtt109 may be elevated at a pH below 7.0, these values were not statistically different from the expression at a lung pH of approximately 7.0 (P = 0.569, according to ANOVA).
Figure 4.
Pneumocystis PcRtt109 mRNA is equally expressed over a wide range of environmental pH. (A) To examine whether PcRtt109 expression is regulated over various levels of environmental pH, as we observed with certain other life-cycle regulation genes, total P. carinii life forms were isolated and resuspended in Ham's F-12 medium with 10% FCS at pH 4.0–7.0 at 37°C for 2 hours. Transcript levels were detected according to Northern blotting with the 165-bp amplicon. Top: Hybridization of PcRtt109 probe to Pneumocystis RNA adherent to the nylon membrane, with arrow indicating the transcript of approximately 1.1 kb. Bottom: Photograph of the two major ribosomal subunits, visualized by ethidium bromide staining, demonstrating equal RNA loading. (B) To analyze the further potential effects of pH on the expression of PcRtt109, quantitative real-time RT-PCR was also performed. P. carinii PcRtt109 mRNA (ng) was expressed for 2 hours at the respective pH in tissue culture media, with pH in the range of 4–7. No significant alteration of expression was evident over the pH range tested.
Pneumocystis PcRtt109 Can Restore H3-K56Ac in Budding Yeast Lacking RTT109
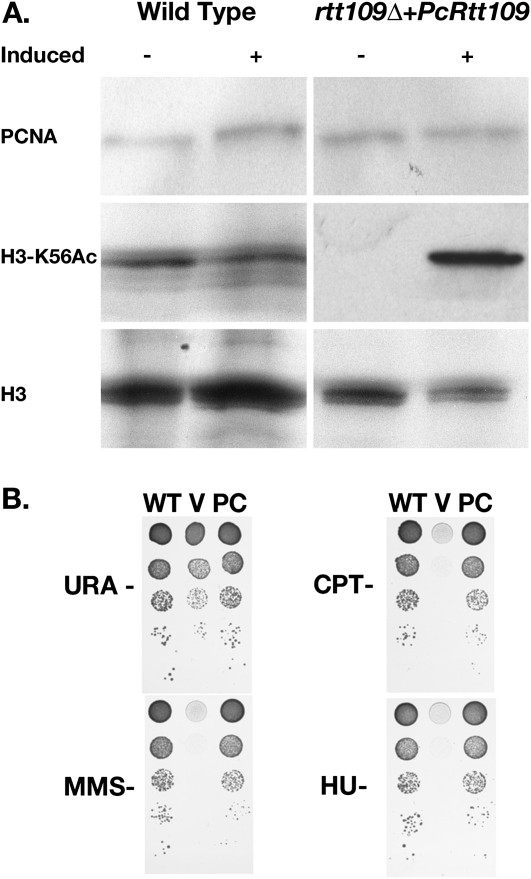
To assess the potential function of PcRtt109; studies were performed to determine whether PcRtt109 cDNA encoded a protein possessing HAT activity. Because of an inability to culture and transform Pneumocystis, conventional methods used to study gene function in this organism are not currently possible. However, recent investigations used the heterologous expression and complementation of genetic knockouts in either S. cerevisiae or S. pombe to analyze the potential function of Pneumocystis genes (39–41). Using this approach, the biological activity of PcRtt109 was assessed by determining its ability to complement budding yeast rtt109Δ cells. The budding yeast rtt109Δ cells were transformed with a construct for the expression of PcRtt109 under inducible conditions, and the protein was isolated for Western blotting. In liquid culture, H3-K56Ac was detectable in rtt109Δ cells expressing PcRtt109 under inducing conditions, whereas H3-K56Ac was not detectable in cells under conditions in which PcRtt109 was not induced. Similarly, rtt109Δ cells transformed with vector alone exhibited minimal HAT activity (data not shown). Western blots of both total H3 histone and PCNA were performed to demonstrate equal protein loading (Figure 5A). These data strongly indicate that Pneumocystis-derived PcRtt109 can function in promoting H3-K56 acetylation in budding yeast.
Figure 5.
Pneumocystis PcRtt109 can restore the ability of deficient rtt109Δ yeast to acetylate H3-K56 and hence improves the sensitivity of this strain to DNA-damaging agents. (A) Acetylation of H3-K56 is detected in mutant rtt109 yeast cells in the presence of the Pneumocystis PcRtt109 during overnight expression with 2% galactose. Western blotting was performed to examine total proteins from yeast cells as shown, with antibodies against proliferating cell nuclear antigen (PCNA), H3-K56Ac, and histone H3 (H3). (B) Next, the inability to acetylate H3-K56 was shown to correlate with sensitivity to DNA-damaging agents. WT, wild-type + control vector; V, rtt109Δ + control vector; PC, rtt109Δ + PcRtt109 cDNA. Tenfold serial dilutions of yeast cells were spotted onto minimal medium (uracil [URA], 2% galactose) containing methyl methanesulfonate (MMS), camptothecin (CPT), or hydroxyurea (HU). Pneumocystis PcRtt109 restores the ability of rtt109Δ-deficient yeast to acetylate H3-K56, and thereby improves the sensitivity of this strain to DNA-damaging agents.
Previous studies further demonstrated that budding yeast cells deficient in Rtt109 HAT protein are sensitive to low concentrations of DNA-damaging agents, primarily because of the loss of H3-K56Ac. Indeed, yeast cells lacking Rtt109 protein exhibit significantly less growth at higher dilutions in the presence of these agents (8, 17). Therefore, to characterize PcRtt109 further, we also tested the protein's ability to complement a yeast deletion mutant of Rtt109 in the presence of various DNA-damaging components. A variety of agents were selected to represent different mechanisms of DNA damage. For instance, CPT binds to the topoisomerase I–DNA complex, stabilizing the complex and preventing DNA re-ligation, resulting in DNA damage. MMS methylates DNA on N7-deoxyguanine and N3-deoxyadenine residues, stalling replication fork progression, and perhaps inducing DNA breaks. HU acts as a ribonucleotide reductase inhibitor that depletes deoxyribonucleotides, thereby inhibiting DNA replication fork generation (42). The wild-type parent strain and rtt109Δ mutant strains containing the control empty vector, along with rtt109Δ yeast cells containing PcRtt109 cDNA, were cultured overnight under inducing conditions, and were subsequently plated on media containing 2% galactose with serial dilutions of these respective DNA toxins. Under these conditions, the presence of PcRtt109 cDNA efficiently complemented the Rtt109 knockout mutant to levels identical to the growth of wild-type yeast, further indicating that PcRtt109 exhibits HAT activity (Figure 5B).
Pneumocystis PcRtt109 Is Able to Acetylate H3-K56 Histone in a Fashion Similar to That of S. pombe Rtt109
Recently, our group and others identified and characterized the fission yeast S. pombe Rtt109 homologue, termed SpRtt109 (7, 11, 36). Similar to its budding yeast partner, SpRtt109 can acetylate H3-K56 histone both in vitro and in vivo. Likewise, SpRtt109 promotes cell survival in response to genotoxic agents that perturb DNA replication (11). Therefore, in our initial assays to determine the acetylation of Drosophila H3/H4 tetramers, we compared equal amounts of purified GST-tagged PcRtt109 and SpRtt109, respectively. Upon recovery of the acetylated H3/H4 tetramers on filters, we demonstrated that PcRtt109 exhibited a similar ability to incorporate 3H acetate into proteins, compared with SpRtt109 (Figure 6A). Upon separating these reaction mixtures on SDS-PAGE gels, we further determined that the acetylation event catalyzed by PcRtt109p was specific to the H3 and not H4 histone, as shown by the specific H3 bands evident on autoradiography and the minimal acetylation in the location of H4, as detected by Coomassie brilliant blue staining of the tetrameric complexes (Figure 6B). We next questioned whether Asf1 was involved in the regulation of the Pneumocystis PcRtt109 acetylation of H3-K56 by comparing the ability of PcRtt109 to acetylate H3 in Asf1–H3/H4 to the ability of control H3/H4 tetramers alone. Interestingly, PcRtt109 acetylated H3-K56 much more efficiently in the presence of the Asf1–H3/H4 complexes than with H3/H4 tetramers alone (Figure 6C). Taken together, our results indicate that PcRtt109 functions as an H3-K56 histone acetyltransferase, and that this reaction is strongly regulated by Asf1.
Figure 6.
Pneumocystis PcRtt109p is a histone acetyltransferase that acetylates histone H3-K56. (A) Pneumocystis PcRtt109, expressed as a glutathione S-transferase fusion protein (GST-PcRtt109), displays histone acetyl transferase (HAT) activity comparable to that of the previously characterized Saccharomyces pombe Rtt109 (spRtt109) protein. *P < 0.001, compared with histone acetyltransferase activity of the negative control proteosome-binding protein regulatory alpha (REGα). (B) Similar to S. pombe spRtt109 HAT protein, GST-PcRtt109 protein acetylates histone H3, but not H4. Samples from HAT assays were further separated on SDS-PAGE gels, followed by Coomassie brilliant blue staining (CBB) to demonstrate equal substrate content. Autoradiography was performed to detect [3H]-acetylated labeled proteins. (C) Histone chaperone Asf1 protein (Asf1) stimulates acetylation of histone H3-K56 by PcRtt109p. Experiments were performed as already described, but with the incorporation of unlabeled acetyl-coenzyme A. Western blots were performed with antibodies recognizing H3 acetylated at lysine 56 or against total H3 histone. (D) Mutation of the PcRtt109p aspartate-84 residue results in the abolishment of HAT activity, whereas conversion of aspartates 218 and 219 to alanines causes a minimal decrease in HAT activity. *P < 0.001, compared with histone acetyltransferase activity of the REGα negative control protein.
Finally, previous authors demonstrated the importance of single as well as specific amino-acid regions important in the S. cerevisiae Rtt109 protein for the acetylation of H3-K56 (11, 17, 43, 44). Accordingly, two regions in PcRtt109 were subjected to site-directed mutagenesis, to determine whether the acetylation event would be affected. Specifically, mutations in which either the PcRtt109 D84 or a double mutation at the tandem D218/D219 region were converted to alanine. The PcRtt109 D84 to A84 mutation resulted in a greatly reduced ability of PcRtt109 to acetylate the target. However, in contrast to the budding yeast Rtt109, the tandem DD218/219 to AA218/219 mutation caused little defect in acetylation activity (Figure 6D). Western blot analysis confirmed that equal amounts of total histone H3 were used. These observations demonstrate the importance of the aspartic acid (D) amino-acid residue in the conserved Serine-Lysine-Alanine-Aspartic acid (SKAD) motif of Rtt109 fungal/yeast proteins that have been characterized so far. Our data support enzymatic differences in the roles of the other previously characterized aspartyl–aspartyl motif, which is important for activity in the budding yeast Rtt109 proteins, but of little apparent role in maintaining PcRtt109 HAT catalytic activity.
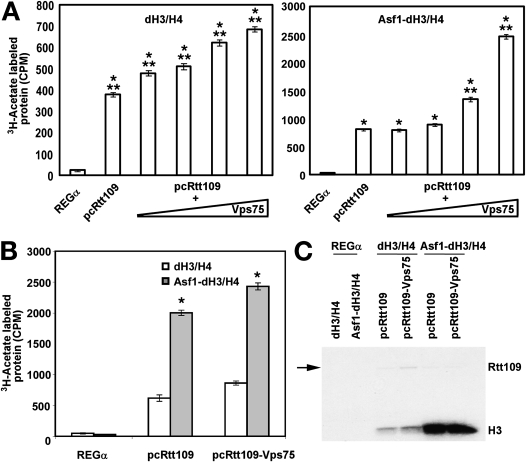
Vps75 Greatly Enhances the Ability of PcRtt109 for H3-K56 Acetylation In Vitro
Recently, our group demonstrated that Vps75 complexes with SpRtt109, resulting in a dramatic increase in the ability of HAT to acetylate H3/H4 tetramers complexed with Asf1–H3/H4 (12, 45, 46). We therefore sought to delineate the potential role of Vps75 in the HAT activity of PcRtt109. Upon the addition of Vps75, we observed strong stimulation (2–3-fold) of PcRtt109 HAT activity toward H3 (Figure 7A). To verify that H3 histone was acetylated, the reaction mixtures were separated by SDS-PAGE. After autoradiography, we confirmed that the PcRtt109p–Vps75 complex specifically acetylates H3 histone. Histone acetylation was most efficient in the presence of Asf1, as measured by acetylated H3 counts (Figure 7B) or by autoradiography of the acetylated H3 (Figure 7C). Thus, Asf1 potently stimulates the activity of Pneumocystis PcRtt109p–Vps75 complexes in vitro. We also observed some low-level autophosphorylation of PcRtt109 (Figure 7C). In yeast, the autoacetylation of lysine-290 within the activation domain is required for stabilizing the protein, and is needed for enzymatic activity (47).
Figure 7.
Increased concentrations of yeast Vps75 complexes along with Asf1 protein presence enhance PcRtt109p histone acetyl transferase activity of H3-K56. (A) To discern the role of Vps75 in activating acetylation of the histone target protein by PcRtt109, we mixed PcRtt109 (1.0 μg) with varying amounts of purified Vps75 protein (1.45 μg, 2.9 μg, 4.35 μg, or 5.8 μg of Vps75), and incubated it on ice for 30 minutes before performing the HAT assay, as described in Materials and Methods. Increased concentrations of yeast Vps75 protein caused increased acetylation of H3-K56 in the presence of [3H] acetyl-CoA (left). Yeast Asf1 greatly increased H3-K56 acetylation, and ws Vps75 concentration–dependent (right). *P < 0.001, comparing incorporation of 3H-acetate to that observed in REGα control. **P < 0.001, comparing incorporation of 3H-acetate to that observed in PcRtt109 without Vps75. (B) To document whether Asf1 stimulates the acetylation of H3-K56 by PcRtt109p–Vps75 complexes, PcRtt109–Vps75 complex (1 μg) was mixed with Asf1 (5.8 μg) and tested in the HAT assay. As already described, the incorporation of [3H] acetate was determined according to scintillation counting. *P < 0.001, comparing the incorporation of 3H-acetate with each substrate set in the presence and absence of added Asf1 (i.e., open versus shaded bar for each condition, respectively). (C) Reaction mixtures similar to those depicted in B were resolved using SDS-PAGE, followed by autoradiography to detect [3H]-acetylated proteins. PcRtt109p also exhibited autoacetylation activity. In all experiments, recombinant REGα, a proteosome-binding protein, was used as a negative control. Low-level autophosphorylation PcRtt109 was also evident (arrow).
DISCUSSION
The continued importance of Pneumocystis pneumonia in immune-compromised patients (e.g., patients with AIDS, malignancies, and organ transplantation) makes efforts to understand this infection's life cycle and to develop additional antifungal agents for combating this infection of vital importance. Even current treatment regimens are not completely effective, insofar as the mortality for Pneumocystis pneumonia remains as high as 40% in immune-compromised patients without AIDS (48, 49). Furthermore, emerging drug resistance is becoming an increasing concern with this organism (50–52). Therefore, the identification and exploitation of novel targets, present in this fungus but apparently lacking in the host, such as PcRtt109, are of critical importance in future therapeutic developments.
In the present investigation, we gained additional insights into the regulation of the Pneumocystis life cycle. We demonstrated that Pneumocystis expresses PcRtt109 histone H3 lysine-56 acetyltransferase. We further confirmed the activity of PcRtt109 through its heterologous expression in rtt109Δ yeast cells, which restored cell survival in the presence of genotoxic agents that interfere with DNA replication. The molecular cloning and modeling of the PcRtt109 gene indicate a mature protein with amino-acid similarity to both S. cerevisiae and S. pombe Rtt109 proteins. In addition, we demonstrated that the PcRtt109 HAT appears to be equally expressed over a wide range of environmental pH levels. This is the first complete characterization of an Rtt109 HAT protein in a pathogenic fungal organism.
Moreover, upon scanning the incomplete P. carinii Genome Database, a Pneumocystis H3 histone homologue was detected, possessing significant homology to S. pombe H3 histone (BLASTX, 97%). Furthermore, the yeast K56 acetylation site on Pneumocystis histone H3 was evident in this sequence, providing further support that these studies of Pneumocystis H3-K56Ac biology are relevant to the nuclear biology of this organism.
Moreover, as expected, the PcRtt109 HAT molecule exhibits several features similar to both S. cerevisiae and S. pombe Rtt109 proteins. First, we proved that PcRtt109 can acetylate Drosophila H3/H4 tetramers to a degree similar to that of recombinant S. pombe Rtt109 protein. In addition, in the presence of the yeast histone chaperone protein Asf1, we demonstrated a requirement for the efficient acetylation of H3-K56 by PcRtt109–Vps75 HAT complexes in vitro. Related to this finding, upon analysis of the Pneumocystis Genome Database, we were also able to detect the presence of an Asf1 histone chaperone homologue. In the future, we plan to isolate Pneumocystis Vps75 and determine if it to can also directly stimulate the Pneumocystis PcRtt109 acetylation of H3-K56. A scan of the available Pneumocystis carinii database revealed no Vps75 homologue, although we did discover a Vps4 yeast homolog that was implicated in multivesicular body sorting and the regulation of cellular sterol metabolism (53, 54). The P. carinii Genome Database is only partly (∼ 50%) complete. Therefore, a Vps75 homologue may yet be discovered within Pneumocystis.
To characterize the PcRtt109 protein further biochemically, specific site-directed mutagenesis experiments were performed on amino acid D84 alone, and amino acids D218 and D219 in tandem. Both these regions were previously implicated as important for Rtt109 HAT catalytic activity (17). Although there is some debate on the role of the common glutamate residue (D89) in yeast as the deprotonation site (16), our results nonetheless add to the data on the Rtt109 HAT family, specifically, that the equivalent D84 amino acid in PcRtt109 is critical for H3K56Ac activity to occur. Furthermore, in contrast to what we previously observed in yeast Rtt109, a double mutant in PcRtt109 at DD218/219 to consecutive alanines exerted little overall effect on H3 histone acetylation activity. This finding is extremely fascinating, because our group showed previously that this double mutant in the budding yeast Rtt109 causes the complete abolishment of H3-K56Ac activity both in vitro and in vivo (17). Thus, Pneumocystis PcRtt109 exhibits some significant differences from yeast-derived ScRtt109. Some conflicting data exist regarding the importance of these two amino acids and regions when mutated in a single fashion. For instance, our previous work showed that mutating yeast Rtt109 287 and 288 from amino acids D to N individually caused a modest decrease in the effect of H3-K56Ac activity, as measured by whole-cell lysate Western blot or growth in the presence of DNA-damaging agents (17). Contrary to these findings, a D288 to A288 mutation obliterated yeast Rtt109 downstream H3K56Ac activity, but a D287 to A287 mutation did not (16). Therefore, our site-directed mutagenesis data suggest that this amino-acid motif in Rtt109 is complex and not yet fully understood, and the possibility remains that this site may not participate in catalytic activity, as previously suggested (16). Further studies in our laboratory will evaluate the role of these double mutants in the ability of PcRtt109 protein to complement yeast rtt109Δ mutants in the genotoxic assay as well as over all H3-K56Ac ability.
In the past, budding yeast Rtt109 was proposed to be activated upon the autoacetylation of lysine-290 (43, 47). However, we observed that mutations at lysine 290 exert little effect on H3-K56Ac in cells. Others suggest that an active and inactive state exist, where the active domain is attached to its own protein acetyltransferase (PAT) domain. Upon autoacetylation, the PAT domain comes into contact with the acetylated lysine-290, creating a tight complex between activation and PAT domains. Vps75 is believed to enhance this activity upon binding a Vps75 “loop” present in yeast Rtt109 (47). In our analysis of PcRtt109, we observed the presence of a lysine residue at position 221. Furthermore, as shown in Figure 7C, we demonstrate that PcRtt109 can be autoacetylated, presumably leading to H3-K56 acetylation activity. Further mutational studies are required to determine whether PcRtt109p residue K221 becomes autoacetylated, leading to a significant downstream functional role in H3K56 acetylation.
Mammalian hosts do not possess an apparent sequence homologue of Rtt109. Moreover, p300, the functional equivalent of yeast Rtt109, appears to adopt distinct mechanisms for catalysis, differing from those of Rtt109. Therefore, the inhibition of this enzyme represents a particularly attractive target for the treatment of fungal infections. As noted, partial and full-length Rtt109 orthologues are present in a number of fungi. Specific Rtt109 inhibitors may increase our arsenal for combating fungal infections, including those organisms resistant to standard agents (51). Although yeast rtt109Δ cells are viable, in vivo evidence suggests that they are highly susceptible to very small amounts of chemotherapeutic agents such as CPT, whereas wild-type cells are not (8, 17). Therefore, inhibitors against Rtt109 may provide novel agents to combat fungal infection during cancer chemotherapy. Another potential therapeutic strategy would involve designing specific inhibitors of Rtt109 that could be used in conjunction with other antifungal agents such as β-1,3-glucan synthesis inhibitors, to target multiple biochemical pathways, thereby decreasing the incidence of antifungal resistance and potentially providing therapeutic synergy. Such synergistic strategies have proved essential in combating other infectious diseases (55).
Studies of the actual effects on gene knockouts in Pneumocystis have not been performed because of the lack of a reliable culture system. Although determining the role of PcRtt109 in DNA replication and histone H3-K56Ac directly in Pneumocystis PcRtt109Δ strains would be interesting, the transformation and culture systems for this organism do not yet exist. Nevertheless, the information provided here strongly indicates the presence of a PcRtt109p functional histone acetyltransferase in P. carinii, thereby providing a potential new therapeutic target for this intractable and important pathogenic fungus.
Acknowledgments
We thank Zvezdana Vuk-Pavlovic and Joshua Burgess for many helpful discussions. We further acknowledge the efforts of Deanne Hebrink in the generation of the P. carinii organisms used in this study, and thank Dr. Melanie T. Cushion at the University of Cincinnati for her generous gift of CHEF blots.
This work was supported by the Minnesota Partnership for Biotechnology and Medical Genomics (Z.Z. and Judith Berman), the Mayo Foundation, and National Institutes of Health grants R01-HL62150 and R01-HL55934 (A.H.L.) and GM81838 and GM71729 (Z.Z.). J.H. is the recipient of a Kendall-Mayo Postdoctoral Fellowship.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0443OC on July 23, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell 2007;128:707–719. [DOI] [PubMed] [Google Scholar]
- 2.Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell 2007;128:721–733. [DOI] [PubMed] [Google Scholar]
- 3.Kouzarides T. Chromatin modifications and their function. Cell 2007;128:693–705. [DOI] [PubMed] [Google Scholar]
- 4.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol 2007;8:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell-cycle–regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 2005;436:294–298. [DOI] [PubMed] [Google Scholar]
- 6.Peterson CL. Genome integrity: a HAT needs a chaperone. Curr Biol 2007;17:R324–R326. [DOI] [PubMed] [Google Scholar]
- 7.Han J, Zhou H, Li Z, Xu RM, Zhang Z. Acetylation of lysine 56 of histone H3 catalyzed by Rtt109 and regulated by Asf1 is required for replisome integrity. J Biol Chem 2007;282:28587–28596. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science 2007;315:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CC, Carson JJ, Feser J, Tamburini B, Zabaronick S, Linger J, Tyler JK. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell 2008;134:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Zhou H, Wurtele H, Davies B, Horazdovsky B, Verreault A, Zhang Z. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 2008;134:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xhemalce B, Miller KM, Driscoll R, Masumoto H, Jackson SP, Kouzarides T, Verreault A, Arcangioli B. Regulation of histone H3 lysine 56 acetylation in Schizosaccharomyces pombe. J Biol Chem 2007;282:15040–15047. [DOI] [PubMed] [Google Scholar]
- 12.Han J, Zhou H, Li Z, Xu RM, Zhang Z. The Rtt109–Vps75 histone acetyltransferase complex acetylates non-nucleosomal histone H3. J Biol Chem 2007;282:14158–14164. [DOI] [PubMed] [Google Scholar]
- 13.Park YJ, Sudhoff KB, Andrews AJ, Stargell LA, Luger K. Histone chaperone specificity in Rtt109 activation. Nat Struct Mol Biol 2008;15:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessulat M, Alamgir M, Salsali H, Greenblatt J, Xu J, Golshani A. Interacting proteins Rtt109 and Vps75 affect the efficiency of non-homologous end-joining in Saccharomyces cerevisiae. Arch Biochem Biophys 2008;469:157–164. [DOI] [PubMed] [Google Scholar]
- 15.Berndsen CE, Tsubota T, Lindner SE, Lee S, Holton JM, Kaufman PD, Keck JL, Denu JM. Molecular functions of the histone acetyltransferase chaperone complex Rtt109–Vps75. Nat Struct Mol Biol 2008;15:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin C, Yuan YA. Structural insights into histone H3 lysine 56 acetylation by Rtt109. Structure 2008;16:1503–1510. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Zhou H, Horazdovsky B, Zhang K, Xu RM, Zhang Z. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 2007;315:653–655. [DOI] [PubMed] [Google Scholar]
- 18.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 2009;459:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paolinelli R, Mendoza-Maldonado R, Cereseto A, Giacca M. Acetylation by Gcn5 regulates CDC6 phosphorylation in the S phase of the cell cycle. Nat Struct Mol Biol 2009;16:412–420. [DOI] [PubMed] [Google Scholar]
- 20.Tjeertes JV, Miller KM, Jackson SP. Screen for DNA-damage–responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J 2009;28:1878–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas CF Jr, Leof EB, Limper AH. Analysis of Pneumocystis carinii introns. Infect Immun 1999;67:6157–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Leigh JW, Brinkmann H, Cushion MT, Rodriguez-Ezpeleta N, Philippe H, Lang BF. Phylogenomic analyses support the monophyly of taphrinomycotina, including Schizosaccharomyces fission yeasts. Mol Biol Evol 2008. 26(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edman JC, Kovacs JA, Masur H, Santi DV, Elwood HJ, Sogin ML. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature 1988;334:519–522. [DOI] [PubMed] [Google Scholar]
- 24.Limper AH, Hoyte JS, Standing JE. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Invest 1997;99:2110–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limper AH, Edens M, Anders RA, Leof EB. Pneumocystis carinii inhibits cyclin-dependent kinase activity in lung epithelial cells. J Clin Invest 1998;101:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limper AH, Martin WJ II. Pneumocystis carinii: inhibition of lung cell growth mediated by parasite attachment. J Clin Invest 1990;85:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durkin MM, Shaw MM, Bartlett MS, Smith JW. Culture and filtration methods for obtaining Pneumocystis carinii trophozoites and cysts. J Protozool 1991;38:210S–212S. [PubMed] [Google Scholar]
- 28.Kottom TJ, Limper AH. Pneumocystis carinii cell wall biosynthesis kinase gene CBK1 is an environmentally responsive gene that complements cell wall defects of CBK-deficient yeast. Infect Immun 2004;72:4628–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kottom TJ, Kennedy CC, Limper AH. Pneumocystis PCINT1, a molecule with integrin-like features that mediates organism adhesion to fibronectin. Mol Microbiol 2008;67:747–761. [DOI] [PubMed] [Google Scholar]
- 30.Burgess JW, Kottom TJ, Limper AH. Pneumocystis carinii exhibits a conserved meiotic control pathway. Infect Immun 2008;76:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vohra PK, Kottom TJ, Limper AH, Thomas CF Jr. Pneumocystis carinii BCK1 complements the saccharomyces cerevisiae cell wall integrity pathway. J Eukaryot Microbiol 2003;50:676–677. [DOI] [PubMed] [Google Scholar]
- 32.Kottom TJ, Limper AH. Cell wall assembly by Pneumocystis carinii: evidence for a unique GSC-1 subunit mediating beta-1,3-glucan deposition. J Biol Chem 2000;275:40628–40634. [DOI] [PubMed] [Google Scholar]
- 33.Thomas CF Jr, Kottom TJ, Leof EB, Limper AH. Characterization of a mitogen-activated protein kinase from Pneumocystis carinii. Am J Physiol 1998;275:L193–L199. [DOI] [PubMed] [Google Scholar]
- 34.Kottom TJ, Thomas CF Jr, Mubarak KK, Leof EB, Limper AH. Pneumocystis carinii uses a functional CDC13 B-type cyclin complex during its life cycle. Am J Respir Cell Mol Biol 2000;22:722–731. [DOI] [PubMed] [Google Scholar]
- 35.Kottom TJ, Thomas CF Jr, Limper AH. Characterization of Pneumocystis carinii PHR1, a pH-regulated gene important for cell wall integrity. J Bacteriol 2001;183:6740–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levenstein ME, Kadonaga JT. Biochemical analysis of chromatin containing recombinant Drosophila core histones. J Biol Chem 2002;277:8749–8754. [DOI] [PubMed] [Google Scholar]
- 37.Tanner KG, Langer MR, Kim Y, Denu JM. Kinetic mechanism of the histone acetyltransferase GCN5 from yeast. J Biol Chem 2000;275:22048–22055. [DOI] [PubMed] [Google Scholar]
- 38.Halme A, Bumgarner S, Styles C, Fink GR. Genetic and epigenetic regulation of the Flo gene family generates cell-surface variation in yeast. Cell 2004;116:405–415. [DOI] [PubMed] [Google Scholar]
- 39.Forster HV, Dempsey JA, Chosy LW. Incomplete compensation of CSF [H+] in man during acclimatization to high altitude (48300 m). J Appl Physiol 1975;38:1067–1072. [DOI] [PubMed] [Google Scholar]
- 40.Hauser PM, Lo Presti L, Cockell M, Cerutti L, Simanis V. Analysis of Pneumocystis carinii gene function by complementation in yeast mutants. J Eukaryot Microbiol 2006;53:S149–S150. [DOI] [PubMed] [Google Scholar]
- 41.Grigore D, Meade JC. Functional complementation of the yeast P-type H-ATPase, PMA1, by the Pneumocystis carinii P-type H-ATPase, PCA1. J Eukaryot Microbiol 2006;53:157–164. [DOI] [PubMed] [Google Scholar]
- 42.Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 2001;412:557–561. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y, Holbert MA, Wurtele H, Meeth K, Rocha W, Gharib M, Jiang E, Thibault P, Verrault A, Cole PA, et al. Fungal Rtt109 histone acetyltransferase is an unexpected structural homolog of metazoan p300/CBP. Nat Struct Mol Biol 2008;15:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fillingham J, Recht J, Silva AC, Suter B, Emili A, Stagljar I, Krogan NJ, Allis CD, Keogh MC, Greenblatt JF. Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol Cell Biol 2008;28:4342–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams SK, Truong D, Tyler JK. Acetylation in the globular core of histone H3 on lysine-56 promotes chromatin disassembly during transcriptional activation. Proc Natl Acad Sci USA 2008;105:9000–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berndsen CE, Tsubota T, Lindner SE, Lee S, Holton JM, Kaufman PD, Keck JL, Denu JM. Molecular functions of the histone acetyltransferase chaperone complex Rtt109–Vps75. Nat Struct Mol Biol 2008. 15(9):948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stavropoulos P, Nagy V, Blobel G, Hoelz A. Molecular basis for the autoregulation of the protein acetyl transferase Rtt109. Proc Natl Acad Sci USA 2008;105:12236–12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004;350:2487–2498. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan JE, Hanson DL, Navin TR, Jones JL. Risk factors for primary Pneumocystis carinii pneumonia in human immunodeficiency virus–infected adolescents and adults in the United States: reassessment of indications for chemoprophylaxis. J Infect Dis 1998;178:1126–1132. [DOI] [PubMed] [Google Scholar]
- 50.Walker DJ, Wakefield AE, Dohn MN, Miller RF, Baughman RP, Hossler PA, Bartlett MS, Smith JW, Kazanjian P, Meshnick SR. Sequence polymorphisms in the Pneumocystis carinii cytochrome B gene and their association with atovaquone prophylaxis failure. J Infect Dis 1998;178:1767–1775. [DOI] [PubMed] [Google Scholar]
- 51.Walker DJ, Meshnick SR. Drug resistance in Pneumocystis carinii: an emerging problem. Drug Resist Updat 1998;1:201–204. [DOI] [PubMed] [Google Scholar]
- 52.Vale N, Collins MS, Gut J, Ferraz R, Rosenthal PJ, Cushion MT, Moreira R, Gomes P. Anti-Pneumocystis carinii and antiplasmodial activities of primaquine-derived imidazolidin-4-ones. Bioorg Med Chem Lett 2008;18:485–488. [DOI] [PubMed] [Google Scholar]
- 53.Vajjhala PR, Catchpoole E, Nguyen CH, Kistler C, Munn AL. Vps4 regulates a subset of protein interactions at the multivesicular endosome. FEBS J 2007;274:1894–1907. [DOI] [PubMed] [Google Scholar]
- 54.Xiao J, Xia H, Zhou J, Azmi IF, Davies BA, Katzmann DJ, Xu Z. Structural basis of VTA1 function in the multivesicular body sorting pathway. Dev Cell 2008;14:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmeira VF, Kneipp LF, Rozental S, Alviano CS, Santos AL. Beneficial effects of HIV peptidase inhibitors on Fonsecaea pedrosoi: promising compounds to arrest key fungal biological processes and virulence. PLoS ONE 2008;3:e3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rebholz SL, Cushion MT. Three new karyotype forms of Pneumocystis carinii f. sp. Carinii identified by contoured clamped homogeneous electrical field (CHEF) electrophoresis. J Eukaryot Microbiol 2001;109S–110S. [DOI] [PubMed]