Abstract
The gene coding for the filamentous hemagglutinin (FHA), one of the main factors involved in mediating adherence of Bordetella pertussis to ciliated host cells, was cloned in Escherichia coli, and the 3,500-base-pair nucleotide sequence encoding the amino-terminal region was determined. Molecular cloning, together with the characterization of recombinant FHA-related proteins produced in E. coli, revealed that the primary translation product is a protein of about 370 kilodaltons (kDa). The mature 220-kDa FHA polypeptide secreted by B. pertussis is most probably generated by proteolytic processing that eliminates a carboxy-terminal portion of about 150 kDa. The 1,087 amino-terminal residues of the predicted FHA sequence showed a number of remarkable features. Extensive homology to the Serratia marcescens and Proteus mirabilis hemolysin proteins was found between amino acids 91 and 205 of the FHA sequence, suggesting involvement of this FHA domain in host cell binding or secretion of FHA from B. pertussis. In addition, two regions containing repetitive amino acid sequences were identified. One region, extending from residues 382 to 664, was formed by six repeats, and a second, extending from residues 701 to 912, contained three repeats. The reactivities of several recombinant FHA-derived proteins with a panel of monoclonal antibodies identified at least four epitopes composing an immunoreactive domain present in the carboxy-terminal moiety of the mature FHA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbieri J. T., Rappuoli R., Collier R. J. Expression of the S-1 catalytic subunit of pertussis toxin in Escherichia coli. Infect Immun. 1987 May;55(5):1321–1323. doi: 10.1128/iai.55.5.1321-1323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. R., Parker C. D. Cloning of the filamentous hemagglutinin of Bordetella pertussis and its expression in Escherichia coli. Infect Immun. 1987 Jan;55(1):154–161. doi: 10.1128/iai.55.1.154-161.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffaud G., Inouye M. Signal peptidases recognize a structural feature at the cleavage site of secretory proteins. J Biol Chem. 1988 Jul 25;263(21):10224–10228. [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Irons L. I., Ashworth L. A., Wilton-Smith P. Heterogeneity of the filamentous haemagglutinin of Bordetella pertussis studied with monoclonal antibodies. J Gen Microbiol. 1983 Sep;129(9):2769–2778. doi: 10.1099/00221287-129-9-2769. [DOI] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locht C., Barstad P. A., Coligan J. E., Mayer L., Munoz J. J., Smith S. G., Keith J. M. Molecular cloning of pertussis toxin genes. Nucleic Acids Res. 1986 Apr 25;14(8):3251–3261. doi: 10.1093/nar/14.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Cieplak W., Marchitto K. S., Sato H., Keith J. M. Activities of complete and truncated forms of pertussis toxin subunits S1 and S2 synthesized by Escherichia coli. Infect Immun. 1987 Nov;55(11):2546–2553. doi: 10.1128/iai.55.11.2546-2553.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Bartoloni A., Perugini M., Rappuoli R. Expression and immunological properties of the five subunits of pertussis toxin. Infect Immun. 1987 Apr;55(4):963–967. doi: 10.1128/iai.55.4.963-967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M., Cowell J. L., Burstyn D. G., Manclark C. R. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J Infect Dis. 1984 Dec;150(6):823–833. doi: 10.1093/infdis/150.6.823. [DOI] [PubMed] [Google Scholar]
- Poole K., Schiebel E., Braun V. Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol. 1988 Jul;170(7):3177–3188. doi: 10.1128/jb.170.7.3177-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
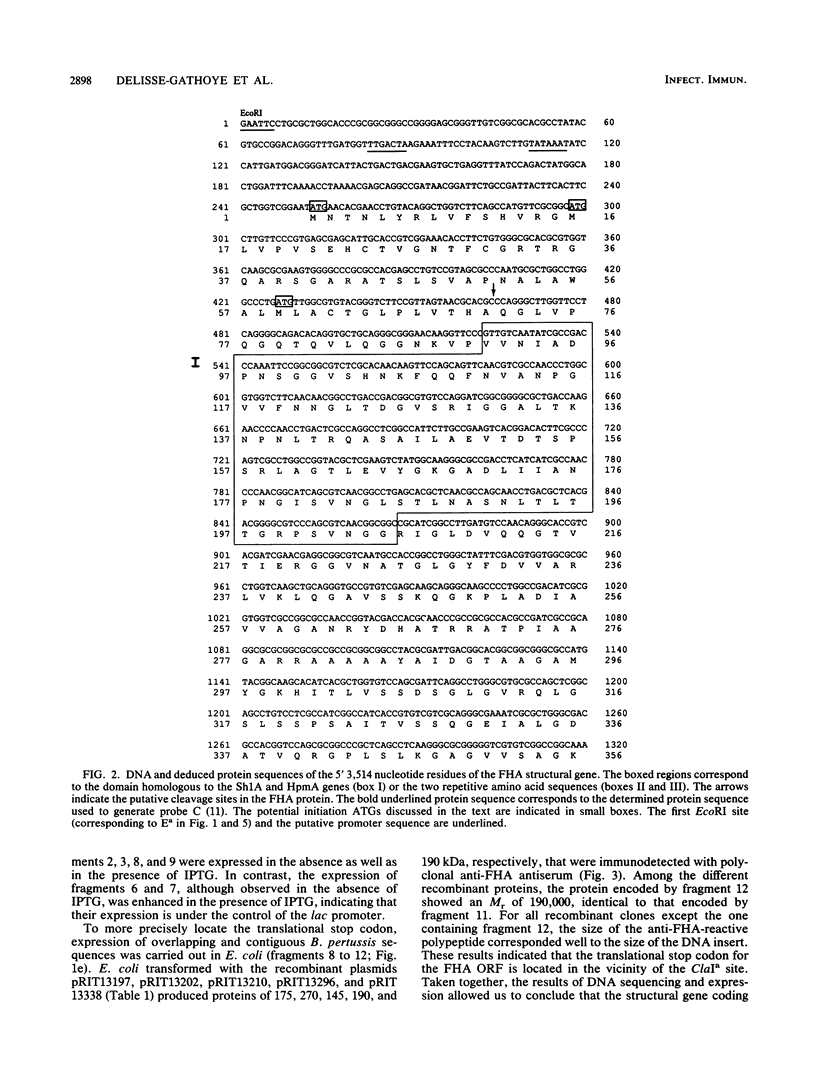
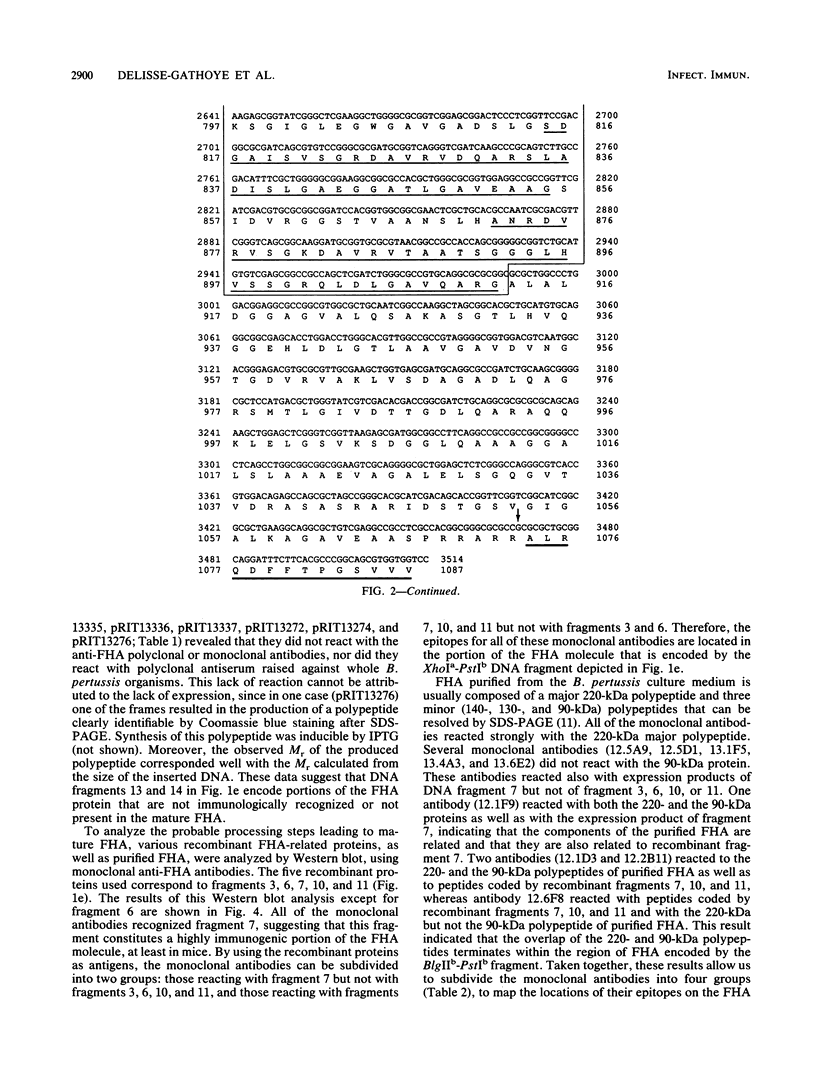
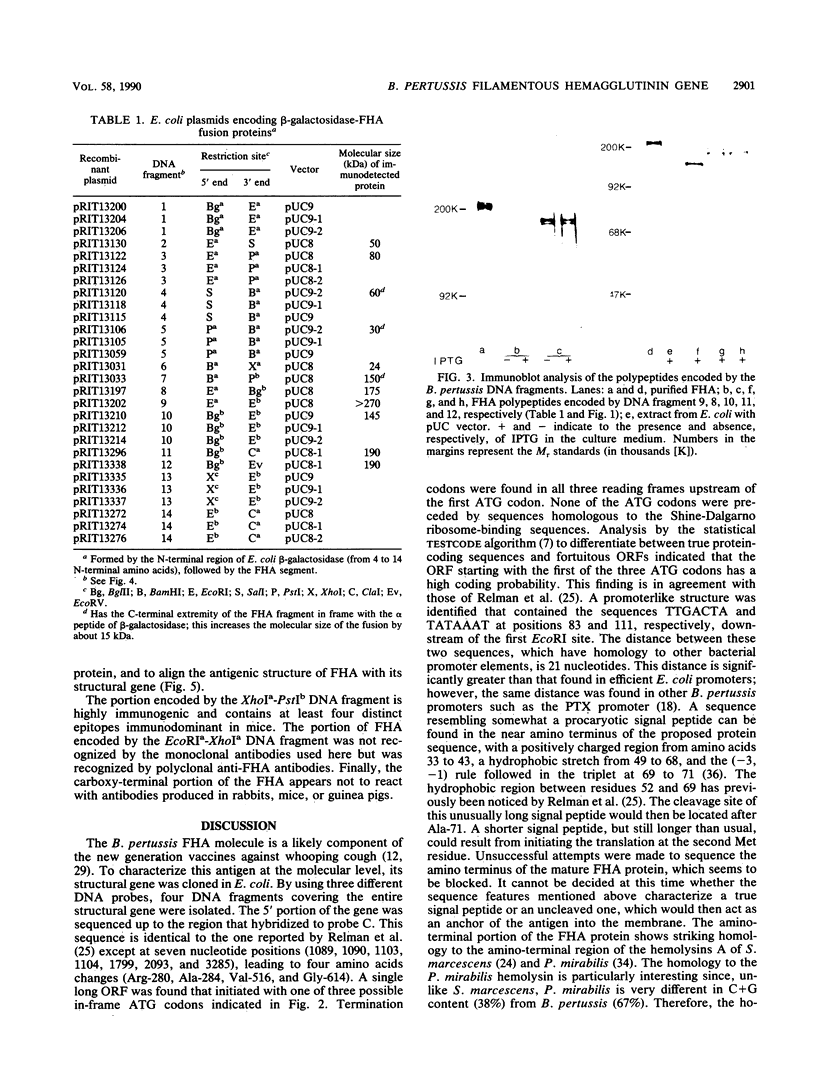
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeberg-Nyman K., Engström O., Löfdahl S., Ylöstalo S., Sarvas M. Expression and secretion of pertussis toxin subunit S1 in Bacillus subtilis. Microb Pathog. 1987 Dec;3(6):461–468. doi: 10.1016/0882-4010(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Sato H., Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect Immun. 1984 Nov;46(2):415–421. doi: 10.1128/iai.46.2.415-421.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Braun V. Integration of the Serratia marcescens haemolysin into human erythrocyte membranes. Mol Microbiol. 1989 Mar;3(3):445–453. doi: 10.1111/j.1365-2958.1989.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Schwarz H., Braun V. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J Biol Chem. 1989 Sep 25;264(27):16311–16320. [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- Uphoff T. S., Welch R. A. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J Bacteriol. 1990 Mar;172(3):1206–1216. doi: 10.1128/jb.172.3.1206-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]