Abstract
Alveolar formation is hallmarked by the transition of distal lung saccules into gas exchange units through the emergence of secondary crests and an exponential increase in surface area. Several cell types are involved in this complex process, including families of epithelial cells that differentiate into alveolar type I and II cells. Subsets of cells expressing Clara cell secretory protein (CCSP) have been identified in both lung and bone marrow compartments, and are described as a progenitor/stem cell pool involved in airway regeneration and alveolar homeostasis. Whether these cells also participate in alveolar formation during postnatal development remains unknown. Based on their regenerative capacity, we asked whether these cells participate in alveogenesis. We used a previously described transgenic mouse model (CCSP-tk) in which Ganciclovir exposure selectively depletes all cells with CCSP promoter activity through intracellular generation of a toxic metabolite of thymidine kinase. Our results showed that Ganciclovir treatment in newborn CCtk mice depleted this cell population in lung airways and bone marrow, and was associated with alveolar hypoplasia and respiratory failure. Hypoplastic lungs had fewer alveolar type I and II cells, with impaired secondary crest formation and decreased vascular endothelial growth factor expression in distal airways. These findings are consistent with a model in which a unique population of cells with CCSP promoter activity that expresses vascular endothelial growth factor participates in alveolar development.
Keywords: alveogenesis, variant Clara cells, bone marrow, alveolar hypoplasia, vascular endothelial growth factor
CLINICAL RELEVANCE.
This research presents novel findings that a subset of cells located in bone marrow and lung compartments are necessary for alveolar development. Depletion of these cells led to alveolar hypoplasia in mice through reduced expression of the paracrine factor, vascular endothelial growth factor. Further identification and isolation of these cells may lead to new strategies to treat or prevent diseases with alveolar hypoplasia, such as bronchopulmonary dysplasia.
Alveolar formation (alveogenesis) is a complex developmental program that describes the transition of lung architecture from terminal saccules to functional gas exchange units within the distal lung. It begins with the formation of secondary crests or “septae” that grow perpendicularly from the saccular walls, and are comprised of a central core containing connective tissue surrounded by capillaries and epithelium. Extension of septae is accompanied by differentiation of cuboidal epithelial cells into surfactant-producing alveolar type II cells and a corresponding increase in surfactant secretion. Alveolar type II cells then undergo terminal differentiation to alveolar type I cells that are largely responsible for gas exchange (reviewed in Ref. 1). Disruptions to this process result in alveolar hypoplasia hallmarked by enlarged and simplified alveoli, and can lead to respiratory compromise, as seen in the chronic lung disease of premature newborns, bronchopulmonary dysplasia (2, 3).
To date, although a considerable amount of literature describes the cell types involved in maintenance of proximal lung epithelium after lung inflammation and injury (4–9), limited data exist on cell types involved in alveolar homeostasis and alveolar regeneration (10, 11). The lung is divided into distinct compartments that comprise (1) proximal and distal airways, and (2) distal lung parenchyma, including alveoli. These regions are further subdivided to include niches known to harbor lung-specific resident stem/progenitor cells that can expand and locally repopulate damaged epithelium (12). These intrapulmonary niches include the submucosal glands of the trachea and main bronchi, bronchus, and main bronchioles, and the bronchoalveolar duct junction (BADJ). A recent report also proposes an extrapulmonary niche within the bone marrow that contains epithelium-like cells that might contribute to repair after lung epithelial injury (13). Although a few studies have attempted to identify lines of cells that may specifically participate in alveolar regeneration using lineage-tracing approaches, the information remains both limited and controversial (9, 10). The current dogma proposes that each individual intrapulmonary niche contains stem/progenitor cells responsible for maintenance of its own local epithelial cell populations. The question of whether specific cell subpopulations or their precursors residing in intra- or extrapulmonary niches retain the ability to regulate alveogenesis, and thereby cross currently accepted compartmental boundaries, remains unknown.
A subset of epithelial cells with Clara cell secretory protein (CCSP) promoter activity was recently described that may function as an airway stem/progenitor cell pool that causes alveolar dysfunction when selectively depleted (5, 11). The cells are termed variant Clara cells, and were identified using a transgenic mouse line (CCSP–herpes simplex thymidine kinase [CCtk]), in which the mouse CCSP promoter is fitted downstream with a cDNA cassette for herpes simplex virus thymidine kinase (HSVtk). Exposure of this mouse line to the antiviral agent, Ganciclovir (GCV), induces an intracellular reaction with HSVtk to form a toxic metabolite of thymidine kinase that results in selective depletion of all cells with an activated CCSP promoter. Previous experiments comparing this model to the naphthalene-induced Clara cell cytotoxicity model have shown that, unlike naphthalene exposure, GCV treatment of adult CCtk transgenic mice leads to depletion of all cells expressing tk, and causes alveolar dysfunction with an inability to regenerate damaged alveoli (11). The investigators concluded that the subpopulation of cells expressing the tk transgene serves a stem/progenitor function in maintaining airway integrity, and their depletion results in alveolar damage as a consequence of unresolved inflammation.
The purpose of the current study was to investigate whether selective depletion of the tk-expressing cell population in newborn animals would impair alveolar formation during postnatal development. Given the evidence that a subset of tk-expressing cells may possess stem/progenitor–like qualities, we asked whether these cells participate in alveogenesis. To answer this question, we treated transgenic CCtk mice and their wild-type littermates with GCV in the immediate newborn period before the start of alveolar development. We found that GCV treatment in CCtk mice induced alveolar hypoplasia by depleting tk-expressing cells in both lung and bone marrow compartments, and was associated with decreased expression of vascular endothelial growth factor (VEGF) in distal airways. This is the first report to implicate a potential role for cells with CCSP promoter activity in alveogenesis during lung development.
MATERIALS AND METHODS
Animals
Transgenic CCtk mice (a generous gift of Dr. Barry Stripp, Duke University) were maintained and bred as a hemizygous pathogen-free colony. See the online supplement for details on animal husbandry, genotyping, dissection, and bronchoalveolar lavage.
GCV or Naphthalene Treatment
On Postnatal Day 1, all littermates within a litter received either GCV (0.625 mg/g) or saline (25 μl equivalent volume), or naphthalene (0.3 mg/g) or oil (25 μl equivalent vehicle volume), via intraperitoneal injection. GCV- and saline-treated animals received a corresponding repeat dose on Postnatal Day 4 to ensure adequate delivery and exposure to injection agent.
Immunohistochemistry/Immunofluorescence
Slides were cleared in CitriSolv and rehydrated through a graded series of alcohols. Slides were stained using the Histomouse Streptavidin Peroxidase kit (Zymed Laboratories Inc., South San Francisco, CA) after antigen retrieval, as described by the manufacturer (Dako Inc., Carpinteria, CA). For immunofluorescence, slides underwent antigen retrieval followed by incubation with the primary antibodies overnight. All negative control slides showed no immunostain. See the online supplement for additional details and Hart's elastin (Eln) stain.
Lung Morphometric Analysis
Four random 5-μm paraffin-embedded tissue sections were taken from three different transgenic and wild-type lungs at specified stages, and separately stained with hematoxylin and eosin or immunostained with anti-CCSP and anti-Nkx2.1 (TTF1) antibodies, as described previously here. The sections were photographed using a SPOT Insight QE camera (Sterling Heights, MI) and software and each image file was analyzed in a blinded manner using the NIH ImageJ 1.37v program (National Institutes of Health, Bethesda, MD) to quantify the number of cells stained positively for the specified antibody. See the online supplement for lung morphometry details.
mRNA Analysis
mRNA was extracted from lungs of CCtk transgenic mice and wild-type littermate control animals by snap freezing immediately after dissection and transfer to −80°C. This was followed by homogenization using a hand-held power homogenizer, and by subsequent organic solvent extraction with TRIzol and chloroform (Invitrogen Corp., Carlsbad, CA), as described by the manufacturer. See the online supplement for RT-PCR and quantitative PCR details.
Bone Marrow Harvest and Cytospin Immunofluorescence Analysis
After animals were killed, dual external hemipelvectomy was performed to isolate the femur and tibia of both legs. After removal of muscle and connective tissue, the femur and tibia were severed at the apical points, and each bone individually flushed with 150 μl of fresh, cold bone marrow cell buffer (0.5% BSA, 2 mM EDTA, 1× PBS) and pooled into one 1.5-ml Eppendorf tube and immediately placed on ice. A total cell count was taken, and each tube (representing one animal) was split into eight tubes (to avoid cell overlap on slides), followed by resuspension in fresh bone marrow cell buffer, and cytospun onto glass slides. Slides containing cells were incubated in 4% paraformaldehyde for 1 hour at 4°C. Paraformaldehyde was then gently rinsed off with 1× PBS, and immunofluorescence was performed as described previously here.
Statistical Analysis
Data were analyzed using the Microsoft Excel 2003 statistical package (Microsoft Corp., Redmond, WA). Two-group comparisons were evaluated using the unpaired Student's t test. Data were expressed as means (±SEM) where appropriate.
RESULTS
GCV-Induced tk Activation Causes Alveolar Inflammation and Injury in Adult CCtk Lungs
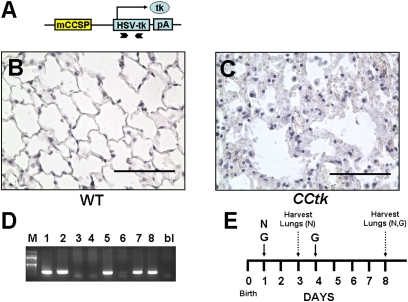
The CCtk transgene contains a fragment of the mouse Clara cell secretory promoter upstream of an HSVtk gene fragment (Figure 1A). Exposure of adult CCtk mice to GCV generates a toxic metabolite of thymidine kinase that permanently depletes all cell populations with CCSP-promoter activity and causes lung inflammation with irreversible alveolar injury (5). Data characterizing the effects of GCV treatment on CCtk mice are currently limited to adult mice receiving chronic GCV exposure extending over 24 hours (7–11) to several days (5). To determine whether a single dose of GCV is capable of inducing similar alveolar effects, 8-week-old adult CCtk mice received 10 mg GCV (0.5 mg/g) intraperitoneally, and lungs were subsequently harvested at 10 days. Results show marked leukocytic infiltrate and alveolar edema in GCV-treated transgenic lungs compared with GCV-treated wild-type control tissue (Figures 1B and 1C). GCV exposure in a survival study uniformly led to animal mortality in adult CCtk mice between 10–14 days as a result of alveolar compromise, as previously described (11). These data demonstrate that a single dose of GCV in adult CCtk mice effectively induces alveolar disruption.
Figure 1.
Ganciclovir (GCV)-induced tk activation causes alveolar inflammation and injury in adult Clara cell secretory protein (CCSP)–herpes simplex thymidine kinase (CCtk) mice. (A) Schematic diagram of CCSP-tk transgene showing a fragment of the mouse CCSP promoter upstream of a herpes simplex virus thymidine kinase cDNA fragment flanked by bovine growth hormone polyadenylation signal. Arrow, transcription start site; chevron arrows, positions of primers for genotyping. Hematoxylin and eosin (H&E)–stained lung sections comparing GCV-treated wild-type (WT) controls animals (B) versus GCV-treated CCtk animals (C) (magnification, 40×; scale bar, 100 μM). (D) Genotyping via PCR identifying transgenic CCtk animals. (E) Schematic timeline of GCV (G) or naphthalene (N) treatment followed by lung harvest in newborn mice.
GCV-Induced tk Activation Causes Alveolar Hypoplasia in Newborn CCtk Lungs
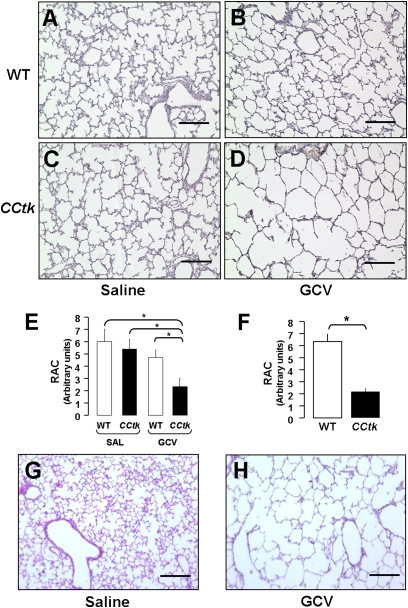
With the observation that GCV treatment of adult CCtk mice disrupts alveolar integrity, we investigated whether GCV treatment of newborn CCtk mice would impair alveolar formation. Female CCtk mice were bred with strain-matched wild-type males (CCtk males are infertile), and pups were allowed to be delivered, with day of birth identified as Postnatal Day 0. CCtk mice are hemizygous, and transgene inheritance follows normal Mendelian ratios to generate approxiamtely 50% transgenic and 50% wild-type progeny, allowing for incorporation of wild-type internal controls within each litter (Figure 1D). On Postnatal Day 1, newborn pups were injected with a single dose of GCV (0.625 mg/g) intraperitoneally, which was repeated on Postnatal Day 4 to ensure adequate exposure before animals were killed on Postnatal Day 8 (Figure 1E). Control litters received corresponding saline injections on Days 1 and 4 of life. Analysis of five independent litters showed marked alveolar hypoplasia characterized by enlarged alveolar airspaces with thinned septae in GCV-treated transgenic lungs as compared with GCV-treated wild-type and saline-treated transgenic and wild-type controls (Figures 2A–2D). Alveolar hypoplasia was quantified by morphometric measurement, showing significantly lower radial alveolar counts, representing increased alveolar airspace size in GCV-treated transgenic lungs (Figure 2E). Alveolar hypoplasia was further pronounced relative to controls at Postnatal Day 16, with radial alveolar counts decreased by nearly 70% (Figures 2F–2H) (17). Importantly, a rigorous analysis of multiple serial histological sections from each GCV-treated transgenic lung at Postnatal Days 8 and 16 showed no evidence of active or resolving inflammation, and no interstitial thickening within the airways or alveoli. Furthermore, no weight loss was noted in either the CCtk or control animals. GCV treatment was uniformly lethal in all CCtk newborns, however, with maximum survival around 16 days. By the day of expiration, animals were noted to be in respiratory distress, with gasping and cyanosis reflecting respiratory insufficiency. These data demonstrate that GCV treatment of newborn CCtk mice permanently arrests alveolar development in the absence of a detectable inflammatory response.
Figure 2.
GCV-induced tk activation causes alveolar hypoplasia in newborn CCtk mice. H&E-stained lung sections comparing saline-treated WT control mice (A), GCV-treated WT control mice (B), saline-treated CCtk control mice (C), and GCV-treated CCtk transgenic mice (D) at 1 week after GCV treatment. Radial alveolar counts in CCtk mice compared with controls at 1 week (E) and 2 weeks (F) after GCV treatment. Representative H&E-stained lung sections in controls (G) versus CCtk mice (H) at 2 weeks after GCV treatment (magnification, 10×; scale bar, 100 μm; *P < 0.05). RAC, radial alveolar count.
GCV-Induced tk Activation Depletes CCSP-Positive Cells in Newborn CCtk Lungs
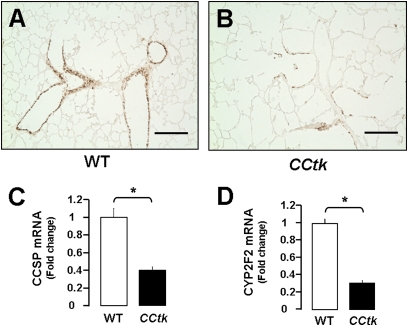
To verify that GCV treatment depletes CCSP-positive cells in newborn CCtk mice, we examined lung CCSP protein and mRNA levels. Immunohistochemical staining showed marked depletion of CCSP-positive cells in proximal and distal airways after GCV treatment in transgenic newborn animals (Figures 3A and 3B). Similarly, quantitative real-time PCR showed significant decreases in total lung mRNA levels of CCSP and cytochrome p450 (Cyp2f2, a marker of secretory Clara cells) (Figures 3C and 3D) (18). These data validate that GCV depletes CCSP-positive cells in our newborn CCtk model.
Figure 3.
GCV-induced tk activation depletes CCSP-positive cells in newborn CCtk mice. Immunohistochemistry for CCSP showing depleted CCSP-positive cells after GCV treatment in WT control mice (A) versus GCV-treated CCtk mice (B) (magnification, 10×; scale bar, 100 μm). Real-time quantitative PCR analysis comparing total lung mRNA after GCV treatment in CCtk mice compared with GCV-treated WT controls for CCSP mRNA expression (C) and CYP2F2 mRNA expression (D) (*P < 0.05).
Alveolar Hypoplasia Is Hallmarked by Fewer Alveolar Type I and II Cells
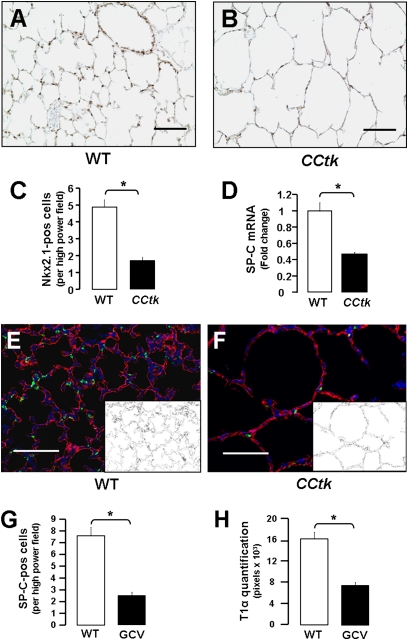
With the finding that GCV treatment arrests alveolar development in newborn CCtk mice, we investigated which alveolar cell type(s) were responsible for the altered phenotype. Immunohistochemical staining for Nkx2.1 (expressed in alveolar type II cells postnatally) showed lower alveolar type II cell numbers in transgenic mice compared with control animals (Figures 4A–4C), and quantitative real-time PCR similarly showed a corresponding decrease in mRNA levels of surfactant protein (SP)–C (alveolar type II–specific protein) (Figure 4D). Quantification of alveolar type I cells was estimated through immunofluorescence for T1α (alveolar type I–specific protein), followed by binary image conversion and summation of pixel numbers corresponding to alveolar type I cell distribution (Figures 4E and 4F). Results show proportional decreases in SP-C–positive cells and T1α distribution after GCV treatment in CCtk mice (Figures 4G and 4H). These data demonstrate that alveolar hypoplasia after GCV treatment of newborn CCtk animals is associated with fewer alveolar type I and II cells.
Figure 4.
Alveolar hypoplasia is hallmarked by fewer alveolar type I and II cells. Immunohistochemistry for Nkx2.1 after GCV treatment in WT control (A) versus GCV-treated CCtk mice (B) (magnification, 20×; scale bar, 100 μm). (C) Corresponding quantification of Nkx2.1-positive cells. (D) Real-time quantitative PCR analysis comparing total lung mRNA for surfactant protein (SP)–C mRNA expression after GCV treatment in CCtk mice compared with GCV-treated WT control animals (*P < 0.05). Immunofluorescence for SP-C and T1α after GCV treatment in control (E) versus CCtk mice (F) (magnification, 20×; scale bar, 100 μm; insets represent binary image of T1α-positive pixels). Quantitation of SP-C–positive cells (G) and T1α pixels (H) (*P < 0.05).
Alveolar Hypoplasia Is a Result of Impaired Secondary Crest Formation
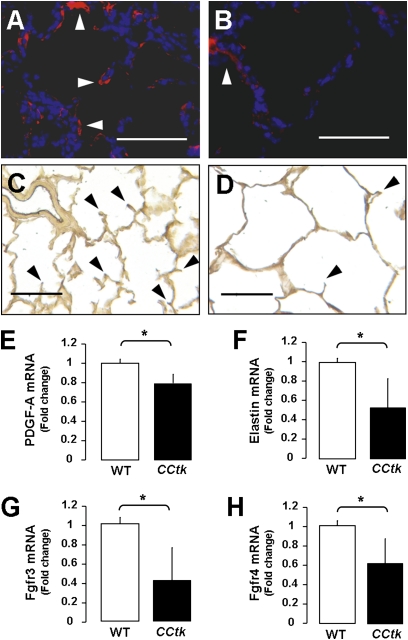
To determine the mechanisms responsible for arrested alveolar formation, we examined several genes known to be involved in alveolar secondary crest formation. Relevant gene products include α–smooth muscle actin, Eln, platelet-derived growth factor–A, and fibroblast growth factor (FGF) 3 and FGF4, and their receptors, Fgfr3 and -4 (1). Immunofluorescence staining showed that α–smooth muscle actin signal was markedly decreased, and Hart's Eln stain similarly showed fewer secondary crests in GCV-treated transgenic lungs (Figures 5A–5D) (16). Quantitative real-time PCR analysis confirmed decreased levels of Eln and platelet-derived growth factor–A (necessary for elastic fiber stability during septal formation), as well as decreases in Fgfr3 and -4 (products expressed on the surface of developing lung epithelium) (Figures 5E–5H). Taken together, these data demonstrate that GCV treatment of CCtk newborns alters the expression of genes involved in secondary crest formation.
Figure 5.
Alveolar hypoplasia is a result of impaired secondary crest formation. Immunofluorescence for α–smooth muscle actin (α-SMA) after GCV treatment in WT control (A) versus CCtk mice (B). Hart's elastin (Eln) stain after GCV treatment in WT control (C) versus CCtk mice (D). Arrowheads indicate α-SMA ([A and B]: magnification, 40×; scale bar, 100 μm) and Eln in ([C and D]: magnification, 20×; scale bar, 100 μm). Real-time quantitative PCR analysis comparing total lung mRNA after GCV treatment in CCtk mice compared with GCV-treated WT control animals for mRNA expression of platelet-derived growth factor (PDGF)–A (E), Eln (F), and fibroblast growth factor receptor (Fgfr) 3 and 4 (G and H) (*P < 0.05).
Naphthalene-Sensitive Clara Cell Depletion Does Not Impair Alveolar Formation
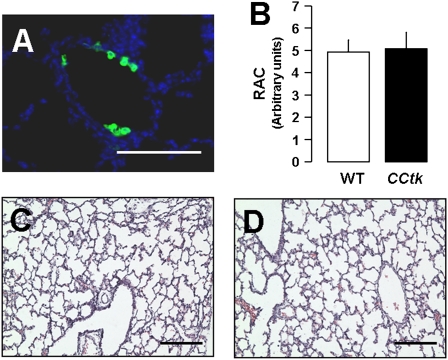
Previous studies have shown that Clara cells in 7-day-old mice are sensitive to cytotoxicity induced by the volatile aromatic hydrocarbon, naphthalene. Despite low levels of cytochrome p450, differentiating Clara cells in newborn mice are more susceptible to injury by naphthalene than differentiated Clara cells in adults (19). Naphthalene injection leads to loss of epithelial cell mass, with areas of denudement and squamation of remaining epithelial cells lining terminal bronchioles. Given our observation that GCV treatment depletes CCSP-positive cells in lung airways, we investigated whether isolated depletion of airway Clara cells via naphthalene would also impair alveolar formation. In studies paralleling GCV treatment, newborn animals received a single dose of naphthalene (0.3 mg/g) intraperitoneally on Postnatal Day 1 followed by killing at either 48 hours or 1 week later, on Postnatal Day 8 (Figure 1E). Although lungs of naphthalene-treated CCtk transgenic and wild-type animals showed evidence of epithelial cytotoxicity through loss of CCSP-positive cells at 48 hours (Figure 6A), no detectable differences were noted in alveolar morphometry or phenotype at Postnatal Day 8 (Figures 6B–6D). Levels of CCSP-positive cells had also recovered to normal by Postnatal Day 8 (data not shown). These results demonstrate that depletion of naphthalene-sensitive Clara cells alone does not impair alveolar formation, and suggest that alveolar impairment from GCV treatment in CCtk mice may be due to an impact on a more distinct cell population.
Figure 6.
Naphthalene-sensitive Clara cell depletion does not impair alveolar formation. (A) Immunofluorescence for CCSP at 48 hours after naphthalene injection showing depletion of Clara cells (magnification, 40×; scale bar, 100 μm). (B) Radial alveolar counts and representative H&E-stained lung sections at 1 week after GCV treatment in WT control (C) versus CCtk mice (D) (magnification, 10×; scale bar, 100 μm).
GCV-Induced tk Activation Permanently Depletes tk-Positive Cells in Newborn CCtk Lungs
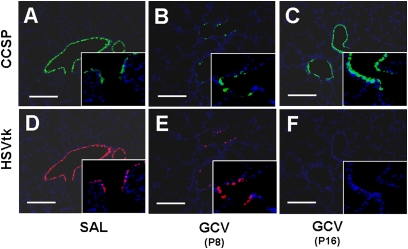
Given the finding that alveolar hypoplasia after GCV treatment does not result from depletion of Clara cells alone, we examined the effects of GCV on the tk-positive cell population in the lung. Results show that tk-positive cells are significantly depleted in parallel with CCSP-positive cells at 1 week after GCV treatment when compared with saline-treated CCtk mice (Figures 7A, 7B, 7D, and 7E). Interestingly, whereas tk-positive cells were completely and permanently depleted by Postnatal Day 16, CCSP-positive Clara cells reappeared in proximal and distal lung airways (Figures 7C and 7F). These data suggest that the tk-positive subpopulation is permanently depleted after GCV treatment, and raise the possibility that these cells may be a key participant in alveolar formation.
Figure 7.
GCV-induced tk activation permanently depletes tk-positive cells in newborn CCtk mouse lungs. Immunofluorescence for CCSP (A–C) and HSVtk (D–F) at 1 week after GCV treatment (B and E) or saline treatment (A and D) and at 2 weeks after GCV treatment (C and F) in CCtk mice (magnification, 10× [insets, 40×]; scale bar, 100 μm).
Potential Mechanisms of GCV-Induced Alveolar Hypoplasia in CCtk Lungs
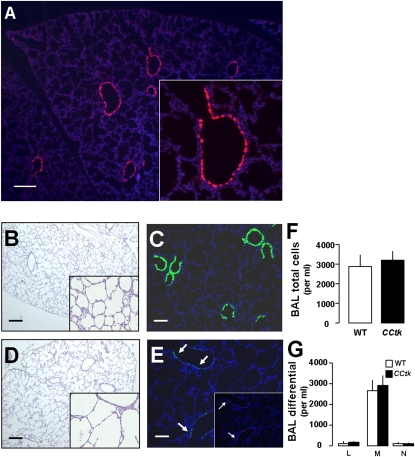
To identify the origin of tk-positive cells, we first examined their distribution within the lung in our naive (untreated) CCtk mice. Immunofluorescence staining showed that tk-positive cells were limited to proximal and distal airways, with no detectable expression in the distal lung. The distribution of tk-positive cells included the BADJ, but did not extend into the alveoli (Figure 8A). Because it is possible that low, undetectable levels of tk expression in alveoli might incite an early inflammatory response and lead to alveolar hypoplasia, we examined the lungs of CCtk newborns and their wild-type progeny via bronchoalveolar lavage and histological analysis at 48 hours after GCV injection. Results show that, although GCV-treated CCtk lungs already had evidence of alveolar impairment, no signs of alveolar inflammation were detectable via bronchoalveolar lavage (14, 15) or via detailed histological analysis (Figures 8B–8G). These data demonstrate that the alveolar impact of GCV treatment in CCtk transgenic lungs begins early, and does not result from alveolar injury. The alveolar changes may be a reflection of effects on tk-positive cells located outside of the alveolar compartment—namely, in the airways, BADJ, or extrapulmonary niche.
Figure 8.
Potential mechanisms of GCV-induced alveolar hypoplasia. Immunofluorescence for HSVtk in 1-week-old, naive CCtk mouse lung (A). Representative lung photomicrographs of H&E-staining (B, D) and immunofluorescence (A, C, E) for CCSP (green) and HSVtk (red) lungs at 48 hours after GCV treatment in WT (B and C) versus CCtk mice (D and E), with corresponding total bronchoalveolar lavage (BAL) cells (F) and BAL differential (G) (magnification, 4× in [A–E] [insets, 40×]; scale bar, 100 μm). L, lymphocytes; M, macrophages; N, neutrophils.
tk-Positive Cells Are also Located in the Bone Marrow
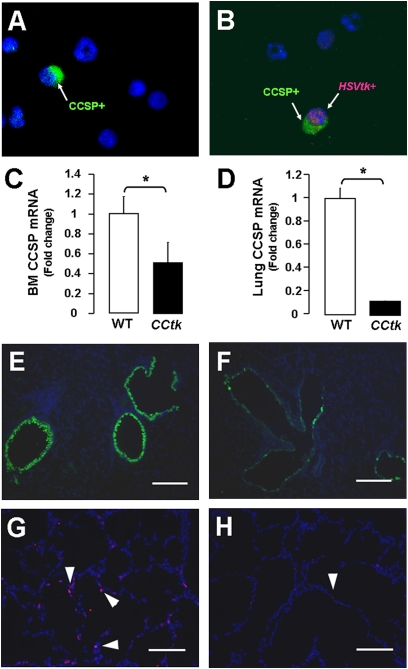
Two recent reports describe the identification of bone marrow–derived epithelium-like cells capable of repopulating injured mouse airway epithelium (13, 20). The investigators showed that CCSP-positive cells reside in the bone marrow, and can specifically home to the lung after naphthalene-induced epithelial injury. The studies provide compelling evidence that bone marrow–derived cells may contribute to lung regeneration. Based on these findings, we investigated whether the bone marrow compartment of CCtk mice contains tk-positive cells. Bone marrow and lungs from adult CCtk mice were harvested at 1 week after treatment with either 10 mg GCV (0.5 mg/g) or saline control intraperitoneally, and processed for cytospin, immunofluorescence, and mRNA analysis. Results from cytospins stained with immunofluorescent antibodies to detect CCSP-positive and tk-positive cells confirmed that CCSP-positive cells are found in bone marrow from wild-type mice treated with saline or GCV, and, more importantly, that CCtk mice harbor tk/CCSP dual-positive cells in the bone marrow (Figures 9A and 9B). Bone marrow from CCtk mice after treatment with GCV showed no detectable levels of tk/CCSP dual-positive cells (data not shown). These findings were confirmed by mRNA extraction of cell pellets from bone marrow cytospins, showing significantly decreased CCSP expression in the GCV-treated CCtk mice compared with GCV-treated wild-type control animals (Figure 9C). Lung tissue analysis further verified that GCV treatment of CCtk mice decreased CCSP expression via quantitative real-time PCR and immunofluorescence (Figures 9D–9F). Finally, lungs of GCV-treated CCtk mice had a significant decrease in mitotic index, as assessed by immunofluorescence for the proliferation marker, Ki67 (Figures 9G and 9H), but showed no change in cell apoptosis via terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling assay (data not shown). These data raise the novel possibility that bone marrow–derived epithelium-like cells may participate in lung growth and alveolar formation.
Figure 9.
tk-positive cells are also located in the bone marrow. Immunofluorescence from bone marrow cytospins showing CCSP-positive cells in WT mice (A) and tk/CCSP dual-positive cells in CCtk mice (B) (magnification, 40×). (C) Real-time quantitative PCR analysis after GCV treatment in CCtk mice compared with GCV-treated WT control mice for bone marrow CCSP mRNA and (D) lung CCSP mRNA expression (*P < 0.05). Immunofluorescence showing corresponding decrease in CCSP-positive cells in lungs after GCV treatment in WT control (E) versus CCtk mice (F) (magnification, 20×; scale bar, 100 μm). Ki67-positive cells (arrowheads) after GCV treatment in newborn WT control (G) versus CCtk mice (H) (magnification 20×; scale bar, 100 μm).
Alveolar Hypoplasia Is Associated with Decreased VEGF Expression
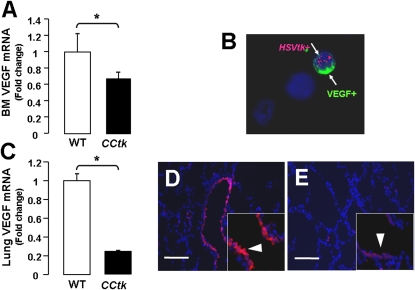
To determine whether alveolar hypoplasia is mediated by loss of a paracrine factor, we measured VEGF expression in lungs and bone marrow after GCV treatment of CCtk mice. Results show that mRNA expression of VEGF was significantly decreased in bone marrow and whole-lung homogenates (Figures 10A and 10C), and that VEGF protein expression was significantly decreased in distal airways (Figures 10D and 10E). Immunofluorescence analysis of bone marrow cytospins further demonstrated the presence of tk/VEGF dual-positive cells (Figure 10B). In summary, these findings imply that alveolar hypoplasia resulting from depletion of tk-positive cells may be due to reduced expression of the paracrine factor, VEGF.
Figure 10.
Alveolar hypoplasia is associated with decreased vascular endothelial growth factor (VEGF) expression. Real-time quantitative PCR analysis after GCV treatment in CCtk mice compared with GCV-treated WT control animals for VEGF mRNA in bone marrow (A) and lung (C) (*P < 0.05). (B) Immunofluorescence from bone marrow cytospins showing tk/VEGF dual-positive cells (arrows) in CCtk mice (magnification, 40×). Immunofluorescence of VEGF expression (arrowheads) after GCV treatment in WT control (D) versus CCtk mice (E) (magnification, 40×; scale bar, 100 μm).
DISCUSSION
Alveolar formation involves the coordination of numerous cell types during lung morphogenesis. Our results show that selective depletion of a subpopulation of cells with an active CCSP promoter during alveolar development results in alveolar hypoplasia. To our knowledge, the present study is the first to propose that this unique subpopulation of cells participates in alveolar formation. These findings support a model in which manipulation of epithelial cells located within the airway and/or bone marrow compartment may affect alveolar formation in the distal lung compartment.
Results of the current study demonstrate that acute dosing of GCV in newborn CCtk mice results in marked alveolar hypoplasia through decreased alveolar type I and II cells, impaired secondary crest formation, and decreased lung VEGF expression. These phenotypic changes occurred in the absence of any detectable inflammation. This experimental strategy was compared head-to-head against the effects of naphthalene exposure showing that isolated Clara cell depletion alone does not cause alveolar hypoplasia. The naphthalene injury model in newborns has been well described by Plopper and colleagues (19), who have characterized the relationship between naphthalene-induced airway injury in neonatal and adult mice. Their data demonstrate that naphthalene increases susceptibility of Clara cells to injury in newborn mice compared with adults, despite lower levels of cytochrome P450. The naphthalene results are consistent with previous observations that CCSP−/− knockout mice develop normal alveoli (21). Interestingly, although all tk-positive cells were completely and permanently deleted after GCV treatment, cells exclusively positive for CCSP (and negative for tk) soon reappeared. Although the significance of these findings remains unknown, a possible explanation for this observation may be that this cell subtype originates from local progenitors, such as basal cells that differentiate into CCSP-positive Clara cells (9, 22). Taken together, these findings imply that GCV treatment of CCtk mice depletes a unique subpopulation of cells, independent from naphthalene-sensitive Clara cells, that participates in alveogenesis.
Previous reports have established that the lung possesses distinct populations of progenitor cells responsible for epithelial regeneration after lung injury in adult mice (11). These cell populations reside in niches throughout the proximal–distal airway axis, and contribute to repopulation of specific cell type(s) within each locale. Although the identification of stem/progenitor populations capable of reconstituting airway epithelium is receiving increasing attention, considerable controversy still exists regarding which cells can actually participate in alveolar regeneration. Two such cell types, the bronchioalveolar stem cell (BASC) and Oct-4–positive stem/progenitor cell, are thought to contribute descendants to alveoli (10, 23). In addition, the subpopulation of Clara cells termed variant Clara cells, identified by CCSP promoter activity, may also possess stem/progenitor cell qualities responsible for bronchiolar regeneration, with a potential impact on alveolar homeostasis (5, 11). Finally, an epithelium-like population of bone marrow cells expressing CCSP has recently been shown to repopulate airway epithelium after injury (13, 20). Our report extends these findings and proposes that a subset of cells identified by CCSP promoter activity may play a role in alveogenesis.
The CCtk transgenic mouse model provides a useful tool to study the mechanisms regulating alveogenesis. Most work to date on alveolar regeneration has been limited to the description of the BASC, which is thought to coexpress CCSP and SP-C (10). Although BASCs were thought to give rise to both bronchiolar and alveolar cells based on work performed in vitro, the ability of these cells to contribute to alveolar descendants was recently challenged using lineage tracing analysis. Hogan and colleagues (9) recently showed that a cross between Scgb1a1-CreER mice (Scgb1a1 = CCSP) and the Rosa26R-eYFP reporter line resulted in labeling of only 1–2% of total alveolar cells in the steady state when the promoter was activated after a single injection of tamoxifen to activate the Cre recombinase–modified estrogen receptor fusion protein. A parallel experiment after hyperoxia challenge demonstrated no difference in the percentage of cells in these alveolar regions containing lineage-labeled cells in hyperoxia-exposed versus room air–exposed control mice. The investigators thus concluded that bronchiolar CCSP-positive cells do not contribute to alveolar repair. Interestingly, although studies in rabbits and rats describe a proportion of alveolar type II cells throughout the alveoli that express low levels of CCSP (24, 25), no expression of CCSP- or tk-positive cells was detectable in alveoli of CCtk transgenic mice from our study. In addition to BASCs, the Oct-4–positive cell population has been introduced as a putative neonatal lung stem/progenitor cell that is capable of being cultured in vitro for weeks and induced to differentiate into alveolar type II and type I cells. Though a small number of Oct-4–expressing cells have been described at the bronchoalveolar junction of neonatal mouse lungs, no clear evidence of Oct-4–positive cells was detectable in our study.
Given the lack of evidence that alveolar cell precursors reside in intrapulmonary conducting airways, the possibility that an extrapulmonary niche, such as bone marrow, may harbor these precursors remains plausible. Two recent papers provide evidence that epithelium-like cells that express CCSP within the bone marrow can incorporate into airways and alveoli after transtracheal delivery after naphthalene injury (13, 20). These cells can be isolated and propagated in air–liquid interface culture to differentiate into both airway and alveolar lineages. Furthermore, lethally irradiated CCSP−/− knockout animals that received tagged wild-type bone marrow showed donor-derived epithelium in both normal and naphthalene-damaged airways. It is intriguing to consider the possibility that these cells may not only contribute to lung regeneration after injury, but may also participate in alveolar formation during postnatal development. We thus asked whether CCtk mice harbor tk-positive cells in the bone marrow, and whether these cells are depleted after GCV treatment. Our results confirmed that bone marrow from CCtk mice contains tk/CCSP dual-positive cells that are depleted after GCV treatment. Although there is currently no demonstrable evidence through lineage labeling that CCSP-positive cells participate in alveolar formation by incorporation into the alveoli (9), we propose that these cells may participate in alveolar formation through indirect mechanisms.
Several recent examples in the literature support the theory that bone marrow–derived progenitor cells may exert their effects on alveolar growth indirectly through paracrine-mediated activity (26–30). Ortiz and colleagues (28) showed that conditioned media from mesenchymal stem cells inhibited TNF-α production by activated macrophages, and inhibited proliferation of IL-1α–dependent T cells in vitro. Xu and colleagues (29) similarly showed that bone marrow–derived mesenchymal stem cells decreased inflammatory responses after endotoxin exposure in mice, independent of lung engraftment. Balasubramaniam and colleagues (27), van Haaften and colleagues (26), and Aslam and colleagues (30) have suggested that paracrine mechanisms likely account for the beneficial effects seen in rodent models of hyperoxia-induced alveolar hypoplasia after the instillation of bone marrow–derived angiogenic cells, bone marrow–derived mesenchymal stem cells, or conditioned media from bone marrow stromal cells, respectively. These observations are further supported by our data that show that VEGF expression in lungs and bone marrow is decreased after GCV treatment in CCtk mice, and by evidence that tk-positive cells coexpress VEGF. Thus, because VEGF plays an important role in lung morphogenesis (31, 32) and is expressed in bone marrow progenitor cells (33–35), our findings support a model in which depletion of tk-positive cells may impair alveolar formation through decreased VEGF expression.
A few alternative possibilities are important to consider. First, it is possible that, although we did not detect any evidence of acute or resolving inflammatory cell infiltrate at 48 hours, 1 week, or 2 weeks after GCV treatment, a transient inflammatory response could have been elicited between those time points. This scenario seems unlikely, however, given the kinetics of GCV-induced inflammation in adult mice, showing that alveolar leukocyte infiltration is a late phenomenon (Day 14) (11), and by our finding that alveolar changes had already begun by 48 hours. Second, we cannot rule out that there may be some low-level ectopic expression throughout the alveoli of the endogenous CCSP promoter or the 2.1-kb fragment of the mouse CCSP promoter used in the transgenic construct, as previously described in the rat CCSP promoter (36). Ablation of tk-positive alveolar cells induced by GCV could thus lead to fewer alveoli in transgenic mice, although no alveolar tk-expressing cells were detectable in our study or in the original description of the adult CCtk model (5). If ectopic alveolar expression of CCSP promoter were present, we would expect to see evidence of inflammation or injury, which was not detected at any time point examined. Third, it should be noted that our studies examining the effects of GCV treatment on bone marrow responses were conducted in adult mice. A complete analysis of bone marrow obtained from newborn animals treated with GCV needs further examination. Finally, it is important to recognize that the current approach to studying the potential effects of depleting tk-positive cells focuses exclusively on the epithelial compartment. Analysis of potential effects on the developing endothelium, implicating a “vascular hypothesis,” also needs further examination.
In summary, this study proposes a novel concept that a unique population of tk-expressing cells with stem/progenitor–like properties participates in alveolar development. Current evidence suggests that these cells may contribute to alveogenesis indirectly through secretion of VEGF. These findings may be of high clinical relevance to lung disorders, such as bronchopulmonary dysplasia, where injury or ablation of this critical population of cells may be responsible for alveolar hypoplasia. Strategies to isolate and culture these cells for future rescue experiments are currently under development, and may ultimately be used to enhance or promote alveogenesis.
Supplementary Material
Acknowledgments
The authors thank Dr. Bo-Chul Shin and the Devaskar Laboratory, Neonatal Research Center, University of California Los Angeles (UCLA) for use of and assistance with the immunofluorescence microscope. They also thank Dr. John Belperio in the Division of Pulmonary and Critical Care at UCLA for use of the cytospin and TaqMan PCR machines.
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant KO8 HL076538 (V.A.L.), University of California Los Angeles Faculty Research Grant (V.A.L.), NIH/NHLBI grant RO1 HL60231 (P.M.), and by the Hastings Foundation (P.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0429OC on August 6, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Galambos C, Demello DE. Regulation of alveologenesis: clinical implications of impaired growth. Pathology 2008;40:124–140. [DOI] [PubMed] [Google Scholar]
- 2.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641–643. [DOI] [PubMed] [Google Scholar]
- 4.Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995;269:L791–L799. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional Clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L1256–L1263. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001;24:671–681. [DOI] [PubMed] [Google Scholar]
- 8.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 2002;161:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. Airway injury in lung disease pathophysiology: selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am J Physiol Lung Cell Mol Physiol 2004;287:L1256–L1265. [DOI] [PubMed] [Google Scholar]
- 12.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 2006;133:2455–2465. [DOI] [PubMed] [Google Scholar]
- 13.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow–derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest 2009;119:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Londhe VA, Belperio JA, Keane MP, Burdick MD, Xue YY, Strieter RM. CXCR2/CXCR2 ligand biological axis impairs alveologenesis during dsRNA-induced lung inflammation in mice. Pediatr Res 2005;58:919–926. [DOI] [PubMed] [Google Scholar]
- 15.Londhe VA, Belperio JA, Keane MP, Burdick MD, Xue YY, Strieter RM. CXCR2 is critical for dsRNA-induced lung injury: relevance to viral lung infection. J Inflamm (Lond) 2005;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice: prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol 2008;294:L3–L14. [DOI] [PubMed] [Google Scholar]
- 17.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child 1960;35:544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Londhe VA, Nguyen HT, Jeng JM, Li X, Li C, Tiozzo C, Zhu N, Minoo P. NF-kb induces lung maturation during mouse lung morphogenesis. Dev Dyn 2008;237:328–338. [DOI] [PubMed] [Google Scholar]
- 19.Fanucchi MV, Buckpitt AR, Murphy ME, Plopper CG. Naphthalene cytotoxicity of differentiating Clara cells in neonatal mice. Toxicol Appl Pharmacol 1997;144:96–104. [DOI] [PubMed] [Google Scholar]
- 20.Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M, Keshavjee S, Hu J, Waddell TK. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol 2007;293:L740–L752. [DOI] [PubMed] [Google Scholar]
- 21.Stripp BR, Lund J, Mango GW, Doyen KC, Johnston C, Hultenby K, Nord M, Whitsett JA. Clara cell secretory protein: a determinant of PCB bioaccumulation in mammals. Am J Physiol 1996;271:L656–L664. [DOI] [PubMed] [Google Scholar]
- 22.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling TY, Kuo MD, Li CL, Yu AL, Huang YH, Wu TJ, Lin YC, Chen SH, Yu J. Identification of pulmonary OCT-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-COV) infection in vitro. Proc Natl Acad Sci USA 2006;103:9530–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patton SE, Gupta RP, Nishio S, Eddy EM, Jetten AM, Plopper CG, Nettesheim P, Hook GE. Ultrastructural immunohistochemical localization of Clara cell secretory protein in pulmonary epithelium of rabbits. Environ Health Perspect 1991;93:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strum JM, Compton RS, Katyal SL, Singh G. The regulated expression of mRNA for Clara cell protein in the developing airways of the rat, as revealed by tissue in situ hybridization. Tissue Cell 1992;24:461–471. [DOI] [PubMed] [Google Scholar]
- 26.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009;180:1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow–derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol 2010;298:L315–L323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow–derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 2007;293:L131–L141. [DOI] [PubMed] [Google Scholar]
- 30.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 2009;180:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Wang K, Ferrara N, Vu TH. Vascular endothelial growth factor co-ordinates proper development of lung epithelium and vasculature. Mech Dev 2005;122:877–886. [DOI] [PubMed] [Google Scholar]
- 32.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006;290:L209–L221. [DOI] [PubMed] [Google Scholar]
- 33.Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem 2005;95:827–839. [DOI] [PubMed] [Google Scholar]
- 34.Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y, Zhang Z. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 2010;12:605–614. [DOI] [PubMed] [Google Scholar]
- 35.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 2006;98:1414–1421. [DOI] [PubMed] [Google Scholar]
- 36.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol 2005;33:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.